Desensitization of G-protein-coupled receptors induces vascular hypocontractility in response to norepinephrine in the mesenteric arteries of cirrhotic patients and rats
Shanghai, China
Desensitization of G-protein-coupled receptors induces vascular hypocontractility in response to norepinephrine in the mesenteric arteries of cirrhotic patients and rats
Wei Chen, Jiang-Yong Sang, De-Jun Liu, Jun Qin, Yan-Miao Huo, Jia Xu and Zhi-Yong Wu
Shanghai, China
BACKGROUND:The increased β-arrestin-2 and its combination with G-protein-coupled receptors (GPCRs) lead to GPCRs desensitization. The latter may be responsible for decreased contractile reactivity in the mesenteric arteries of cirrhotic patients and rats. The present study is to investigate the machinery changes of α-adrenergic receptors and G proteins and their roles in the contractility of mesenteric arteries of cirrhotic patients and animal models.
METHODS:Patients with cirrhosis due to hepatitis B and cirrhotic rats induced by CCl4were studied. Mesenteric artery contractility in response to norepinephrine was determined by a vessel perfusion system. The contractile effect of G protein-coupled receptor kinase-2 (GRK-2) inhibitor on the mesenteric artery was evaluated. The protein expression of the α1adrenergic receptor, G proteins, β-arrestin-2, GRK-2 as well as the activity of Rho associated coiled-coil forming protein kinase-1 (ROCK-1) were measured by Western blot. In addition, the interaction of α1adrenergic receptor with β-arrestin-2 was assessed by co-immunoprecipitation.
RESULTS:The portal vein pressure of cirrhotic patients and rats was signif i cantly higher than that of controls. The doseresponse curve to norepinephrine in mesenteric arteriole was shifted to the right, and EC50was signif i cantly increased in cirrhotic patients and rats. There were no signif i cant differences in the expressions of the α1adrenergic receptor and G proteins in the cirrhotic group compared with the controls. However, theprotein expressions of GRK-2 and β-arrestin-2 were signif i cantly elevated in cirrhotic patients and rats compared with those of the controls. The interaction of the α1adrenergic receptor and β-arrestin-2 was signif i cantly aggravated. This interaction was signif i cantly reversed by GRK-2 inhibitor. Both the protein expression and activity of ROCK-1 were signif i cantly decreased in the mesenteric artery in patients with cirrhosis compared with those of the controls, and this phenomenon was not shown in the cirrhotic rats. Norepinephrine signif i cantly increased the activity of ROCK-1 in normal rats but not in cirrhotic ones. Norepinephrine signif i cantly increased ROCK-1 activity in cirrhotic rats when GRK-2 inhibitor was used.
CONCLUSIONS:β-arrestin-2 expression and its interaction with GPCRs are signif i cantly upregulated in the mesenteric arteries in patients and rats with cirrhosis. These upregulations result in GPCR desensitization, G-protein dysfunction and ROCK inhibition. These may explain the decreased contractility of the mesenteric artery in response to vasoconstrictors.
(Hepatobiliary Pancreat Dis Int 2013;12:295-304)
portal hypertension; desensitization; G-protein-coupled receptors; β-arrestin-2; Rho associated coiled-coil forming protein kinase
Introduction
Liver cirrhosis is associated with the development of hyperdynamic circulation and portal hypertension. The increase in portal pressure is triggered by persistent splanchnic vasodilation and increased intrahepatic vascular resistance. Although the levels of vasoconstrictors such as norepinephrine and angiotensin- II (AT-II) increase in the blood circulation and are accompanied by enhanced sympathetic excitability in portal hypertension, the splanchnic arteryremains dilated. Hyporeactivity of this artery in response to vasoconstrictors plays a key role in blood vessel dilation and hyperdynamic circulation.[1,2]
Contraction of arterial smooth muscle cells is mainly mediated by the phosphorylation of myosin light chains (MLC), which is subject to the dual regulation of MLC kinase (MLCK) and MLC phosphatase (MLCP), and the contractile force increases with increasing phosphorylation of MLC (P-MLC) content.[3]Contractile pathways in smooth muscle cells are activated after stimulation of G-protein-coupled receptors (GPCRs) by vasoconstrictors. Two of the best characterized and most ubiquitous vasopressor receptors are the α1adrenoceptor and the AT-II type 1 receptor. Both are coupled to heterotrimeric G proteins containing Gαq/11, Gα12 and Gα13 subunits. After receptor activation by catecholamines or AT-II, these Gα proteins dissociate from their β/γ-subunits and the receptor, and subsequently regulate MLCK and MLCP through calcium-dependent and calcium sensitization pathways. The latter is also known as the RhoA/Rho associated coiled-coil forming protein kinase (ROCK) pathway.[4,5]
Under normal circumstances, GPCRs are activated by vasoconstrictors. The activated GPCRs recruit more β-arrestin-2 protein under the inf l uence of G proteincoupled receptor kinase 2 (GRK-2), thereby hindering the transmission of contractile signals and bringing about desensitization.[6-8]β-arrestin-2-mediated receptor desensitization is due to high-intensity and prolonged stimulation in the circulation. Signif i cantly high levels of endogenous vasoconstrictor such as catecholamine and increased aff i nity between GPCRs and β-arrestin-2, which enhances receptor desensitization and decreases receptor resensitization, may partially result in arterial hyporesponsiveness.[4,9]
In the present study, patients with cirrhosis due to hepatitis B and cirrhotic rat models induced by CCl4were used to investigate the machinery changes of α-adrenergic receptors and G proteins and their roles in the contractility of mesenteric arteries of cirrhotic patients and animal models.
Methods
Materials
Healthy male Sprague-Dawley rats weighing 120 to 130 g were provided by the Experimental Animal Center of the School of Medicine of Shanghai Jiaotong University. The following materials were used in the study: GRK-2 inhibitor methyl ([5-nitro-2-furyl]vinyl)-2-furoate (Calbiochem, USA), rabbit anti-ROCK-1 antibody (CST, USA), normal rabbit IgG (sc-2027), rabbit anti-moesin antibody (sc-6410), rabbit anti-phospho-moesin (Thr 558) antibody (sc-12895), mouse anti-β-arrestin-2 antibody (sc-6387), mouse anti-GRK2 antibody (sc-166284), rabbit anti-α1adrenergic receptor antibody (sc-31358), rabbit anti-Gαq/11 antibody (sc-392), rabbit anti-Gα12 antibody (sc-409), and rabbit anti-Gα13 antibody (sc-410). The antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, USA).
Patients and animals
Patients
The patients with cirrhosis due to hepatitis B were three males and two females, with an age range of 33-60 years. All these patients were conf i rmed with histories of hematemesis and melena but without complications in the cardiovascular system, lung and brain. Surgical treatment was suggested by gastroscope examination and spiral computed tomography angiography which conf i rmed the cirrhosis and esophagus fundus ventriculi varicosity. Some of these patients only received antihepatitis B virus treatment. The control group included three males and two females, with an age range of 30-58 years. Two patients were diagnosed with small calculus in the common bile duct, and three patients with a benign choledochal cyst. These patients did not take any drugs one month before operation and were conf i rmed without abnormalities of the liver and other organs in terms of function and morphology through computed tomography or magnetic resonance imaging. The fi brosis or cirrhosis was eliminated by liver biopsy. The present study was approved by the local ethics committee.
Animals
A 60% CCl4oil solution was injected intramuscularly at a dose of 0.4 mL per 100 g body weight, twice a week, combined with 5% ethanol as drinking water. Within 14 to 16 weeks, the rats developed cirrhotic nodules along with portal hypertension. When the rats presented with ascites, exposure to CCl4and alcohol was stopped for 7 days. Age-matched, same volume of oil injected rats served as controls.
Protocol for single injection of GRK-2 inhibitor in rats
The groups of cirrhotic and control rats were randomized into the control and treatment subgroups: CS group: control rats treated with saline; CG group: control rats treated with GRK-2 inhibitor; PS group: cirrhotic rats treated with saline; and PG group: cirrhotic rats treated with GRK-2 inhibitor. Animals in the treatment groups received a single injection of GRK-2 inhibitor methyl([5-nitro-2-furyl]vinyl)-2-furoate(200 μg/ kg, i.v.), while their controls received an equal volume of saline. It was proved by previous studies that this dosage was effective in inhibiting the activity of GRK-2.[10,11]Then, at 60 minutes after the treatment with GRK-2 inhibitor or saline injection, the animals were anesthetized and the experiments were performed subsequently.
Determination of portal vein pressure
Patients
Before operation, all of the patients received inhalation anesthesia and equilibrium liquid for rehydration. All these patients did not take any kind of vasoactive agents before the collection of specimens. During the operation, heart rate was controlled between 60-100/min, mean arterial pressure was kept between 80-100 mmHg and oxygen saturation was above 98%. Moreover, artery blood gas was monitored regularly in order to make sure all the indices were in normal range during the operation. Portal vein pressure was measured as described in our previous study.[12]
Animals
The rats were anesthetized with ketamine through intramuscular injection (250 mg/kg) and then fastened. An incision was made at the midline of the abdomen. After exposure of the portal vein, a 22G catheter fi lled with heparin saline was inserted directly into the portal vein and the pressure was measured.
Sampling and stimulation
Following the measurement of the portal vein pressure, we removed the mesenteric arteries as well as the mesentery. All of the specimens from patients were separated from above the fi rst-order mesenteric arteries and the mesentery beside the jejunum one meter away from the suspensory ligament of the duodenum, which would be sure not to impact local blood supply.
All of the specimens were divided into two groups. For the fi rst group, we preserved them in 3-(N-morpholino) propanesulfonic acid (MOPS)-buffered physiological salt solution (MOPS-PSS) (0-4 ℃; pH 7.4) contained NaCl (145 mmol/L), KCl (5 mmol/L), CaCl2(2 mmol/L), MgSO4(1 mmol/L), NaH2PO4(1 mmol/L), dextrose (5 mmol/L), pyruvate (2 mmol/L), EDTA (0.02 mmol/L), and MOPS (3 mmol/L).[13]These specimens were used to dissect the third-order arterioles. For the second group, all of the mesenteric arteries were drawn out and frozen in liquid nitrogen immediately or transferred to MOPS-PSS and incubated at 37 ℃ for 30 minutes. Norepinephrine was then added to the MOPS-PSS at a fi nal concentration of 10 μmol/L. The samples were incubated for another 20 minutes and then stored in liquid nitrogen for later use. Alternatively, the specimens were incubated in MOPS-PSS for 20 minutes at 37 ℃without stimulation by norepinephrine and then frozen in liquid nitrogen.
Determination of mesenteric arteriole reactivity to norepinephrine
The third-order arterioles in the mesentery were sharply dissected with a dissecting microscope and transferred to a vascular perfusion system containing MOPS-PSS (37 ℃; pH 7.4). A glass micropipette containing MOPS-PSS (top diameter, 50 μm) was inserted into one end of an arteriole and fi xed with 11-0 singlestrands. Blood was fl ushed out at a perfusion pressure of 8 mmHg and the other end of the blood vessel was kept open. We then inserted another glass micropipette into the other end of the lumen and tied it with 11-0 suture.
The vascular diameter was measured with a microscope camera system and displayed on a computer screen. The diameter change was determined by arteriole imaging and the data were recorded in a computer. The diameter of each blood vessel under a pressure of 80 mmHg for 30 minutes was recorded as the baseline. According to the cumulative concentration method, norepinephrine was added to the vascular tank to a concentration ranging from 10-7to 10-5mol/L, and the contraction rate of the blood vessel was recorded under different concentrations. Cumulative doseresponse curves for norepinephrine were drawn on the basis of the contraction rate, i.e., the ratio of intravascular diameter change to maximum diameter. The vasoconstriction rate and the logarithm of the norepinephrine concentration were used as the vertical axis and the abscissa, respectively. We compared the differences in the curves among the groups and recorded the concentration of norepinephrine when the contraction rate was 50% (i.e., EC50).
Western blot
The expression of the following proteins in the mesenteric artery was detected by Western blot: α1adrenergic receptor, Gαq/11, Gα12, Gα13, β-arrestin-2, ROCK-1, moesin and p-moesin. To determine the change in ROCK-1 activity, phosphorylation of the ROCK substrate moesin was detected by Western blot. Since ROCK is capable of phosphorylating threonine at position 558 in moesin, its activity change can be examined with a site-specif i c antibody via Western blot[9,14,15]as described in our previous studies.[16,17]In brief, frozen mesenteric arteries were homogenized in1 mL of tissue lysis buffer. The homogenate was moved to Eppendorf tubes and centrifuged at 4 ℃, 10 000 g for 15 minutes. The protein concentration was determined by using a BCA Protein Assay Kit (Pierce, USA). Forty micrograms of total protein was loaded into each well of a 10% SDS-polyacrylamide gel after being denatured at 100 ℃ for 5 minutes. After electrophoresis, the proteins were transferred to a nitrocellulose membrane at 250 mA for 30 minutes. The membrane was blocked in 5% milk in Tris-buffered saline (TBS) for 1 hour at room temperature. The primary antibody was added and incubated with the membrane over night at room temperature, and then the membrane was washed 3 times with TBS/Tween 20. A secondary antibody was then added to the membrane and it was incubated at room temperature with gentle agitation. Two hours later, the membrane was washed three times with TBS/Tween 20 for 10 minutes per wash. The bands were visualized using an enhanced chemiluminescence kit after being exposed on X-ray fi lm. β-actin was used as an internal reference, then protein quantitative analysis was performed by employing the digital imaging software (Kodak, USA).
Co-immunoprecipitation
Protein extracts were incubated with normal rabbit IgG (50 μL) in an ice bath for 1 hour, pre-cleared by incubation with protein A-agarose beads (Bio-Rad, USA) at 4 ℃ for 30 minutes, and centrifuged at 12 000 g at 4 ℃ for 10 minutes. Then, 80 μL of the supernatant (about 50 μg protein) was incubated with the adrenergic receptor antibody at 4 ℃ for 2 to 4 hours, and then washed in lysis buffer following centrifugation at 1000 g for 5 minutes. The precipitates were then stored at -80 ℃or used for Western blot to detect β-arrestin-2 .
Statistical analysis
Data were presented as mean±SEM and analyzed using SPSS 16.0. The variables were compared by oneway ANOVA or Student'sttest. APvalue of <0.05 was considered to be statistically signif i cant. The change in the reactivity of the mesenteric arteriole in response to norepinephrine was presented as a dose-response curve, which was fi tted by nonlinear regression analysis (Graph Pad Software Inc., San Diego, CA., USA), and EC50values were calculated from the fi tted curve.
Results
Portal vein pressure in rats
The portal vein pressure increased signif i cantly in the cirrhotic rats compared to that in the controls (15.1± 1.6 vs 5.9±0.9 mmHg,n=7-8,P<0.01), and there was no signif i cant difference between GRK-2 inhibitor-treated and untreated rats group (cirrhosis or control).
Portal vein pressure in human beings
The portal vein pressure was signif i cantly increased in the cirrhotic patients compared with the controls (25.6 ±1.8 vs 13.7±2.0 mmHg,n=5,P<0.01).
Hypocontractility of isolated rat mesenteric arterioles in response to norepinephrine
To conf i rm the arteriole hypocontractility to norepinephrine of cirrhotic rats and the effect of GRK-2 inhibitor, the contractility of isolated arteriole to norepinephrine was assessed in the control and cirrhotic rats with or without treated with GRK-2 inhibitor. As shown in Fig. 1, compared with the CS and CG groups, the dose-response curve of the mesenteric arteriole in response to norepinephrine shifted to the right and the EC50increased in the cirrhotic rats. However, in mesenteric arterioles from the cirrhotic rats treated with GRK-2 inhibitor, the dose-response curve shifted toward the control group and EC50was decreased (P<0.01).
Hypocontractility of isolated human mesenteric arterioles in response to norepinephrine
Compared with the control group, the doseresponse curve of the mesenteric arteriole in response to norepinephrine shifted to the right and the EC50was increased in the patients with cirrhosis (P<0.01; Fig. 2).
Expressions of the α1adrenergic receptor, G proteins, and GPCR-desensitizing proteins in rat mesenteric arteries
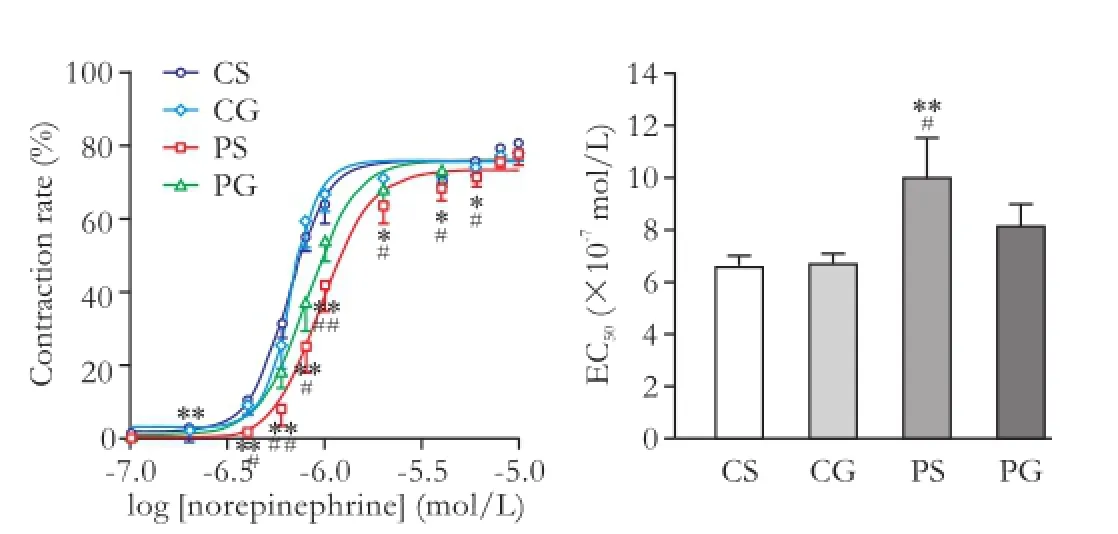
Fig. 1.Dose-response curves and norepinephrine concentrations (EC50) for 50% contractility in isolated rat mesenteric arterioles. Values are mean±SEM;n=6. *:P<0.05; **:P<0.01, compared with the CS group; #:P<0.05; ##:P<0.01, compared with the PG group.

Fig. 2.Dose-response curves and norepinephrine concentrations (EC50) for 50% contractility in isolated human mesenteric arterioles. Values are mean±SEM;n=5. *:P<0.05, compared with the control group; **:P<0.01, compared with the control group.
The expressions of α1adrenergic receptor, G proteins, GRK-2, and β-arrestin-2 in rat mesenteric arteries were compared among the four groups by Western blot. There were no signif i cant differences in protein expressions of the α1adrenergic receptor, Gαq/11, Gα12, and Gα13 (P>0.05; Fig. 3). However, GRK-2 and β-arrestin-2 were signif i cantly increased in the cirrhotic group compared with those in the control group with or without treatment by GRK-2 inhibitor. GRK-2 inhibitor did not change the expression of GRK-2 in the mesenteric artery; GRK-2 inhibitor signif i cantly decreased the expression of β-arrestin-2 (P<0.01; Fig. 3).
Expressions of the α1adrenergic receptor, G proteins, and GPCR-desensitizing proteins in human mesenteric arteries
There were no signif i cant differences in expressions of the α1adrenergic receptor, Gαq/11, Gα12, and Gα13 (P>0.05; Fig. 4). Protein GRK-2 and β-arrestin-2 were signif i cantly increased in the cirrhotic group compared with the control group (P<0.01; Fig. 4).
Co-immunoprecipitation of the α1adrenergic receptor with β-arrestin-2 in mesenteric arteries
The interaction between the α1adrenergic receptor and β-arrestin-2 in rat and human mesenteric arteries was investigated by co-immunoprecipitation, and the results showed that the adrenergic receptor coimmunoprecipitated with β-arrestin-2. Moreover, the interaction of the α1adrenergic receptor and β-arrestin-2 was much stronger in the cirrhotic rats than in the control group. However, in the cirrhotic rats, GRK-2 inhibitor interrupted the interaction between the α1adrenergic receptor and β-arrestin-2 (P<0.01; Fig. 5). Very similar fi ndings were obtained in human mesenteric arteries. The binding of β-arrestin-2 to the α1adrenergic receptor was higher in mesenteric arteries from patients with cirrhosis than that in mesenteric arteries of the control group (P<0.01; Fig. 5).

Fig. 3.Protein expressions of the α1adrenergic receptor, G proteins, and GPCR-desensitizing proteins in rat mesenteric arteries. The bar graphs show the relative expression levels of indicated proteins after normalization to β-actin. The levels of α1-adrenergic receptor, G proteins and GPCR-desensitizing proteins that were found in the mesenteric arteries of the CS group were set to 100%. Values are mean±SEM;n=6. **:P<0.01, compared with the CS group; ##:P<0.01, compared with the CG group; αα:P<0.01, compared with the PG group.
Expression and activity of ROCK-1 in rat mesenteric arteries
The protein expressions of ROCK-1, moesin, and p-moesin were compared among the four groups in rats. Moesin is a substrate of ROCK and its phosphorylation was analyzed in order to detect ROCK activity. Westernblot showed that the expression of ROCK-1, moesin, and p-moesin were not signif i cantly different in the four groups (P>0.05; Fig. 6).
Expression and activity of ROCK-1 in human mesenteric arteries
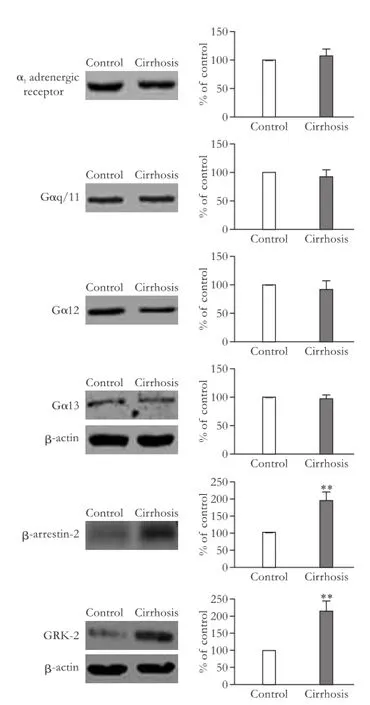
Fig. 4.Protein expressions of the α1adrenergic receptor, G proteins, and GPCR-desensitizing proteins in human mesenteric arteries. The bar graphs show the relative expression levels of indicated proteins after normalization to β-actin. The levels of α1-adrenergic receptor, G proteins and GPCR-desensitizing proteins that were found in the mesenteric arteries of the control group were set to 100%. Values are mean±SEM;n=5. **:P<0.01, compared with the control group.
We also detected the expressions of ROCK-1, moesin and p-moesin in human mesenteric arteries with Western blot. Interestingly, the expression levels of ROCK-1 and p-moesin were signif i cantly decreased in mesenteric arteries in patients with cirrhosis compared to those in the control group (P<0.01), while the expression level of mosein was not signif i cantly changed (P>0.05; Fig. 7).
Norepinephrine activated ROCK-1 in rat mesenteric arteries

Fig. 5.Interaction between the α1adrenergic receptor and β-arrestin-2 in rat and human mesenteric arteries. Values are mean±SEM;n=5-6 for rat mesenteric arteries,n=5 for human mesenteric arteries. The level found in mesenteric arteries from the CS or control group was set to 100%. **:P<0.01, compared with the CS or control group; ##:P<0.01, compared with the CG group; αα:P<0.01, compared with the PG group.
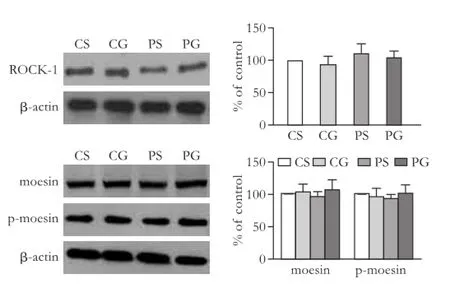
Fig. 6.Comparison of the protein expression and activity of ROCK-1 in rat mesenteric arteries. The bar graphs show the relative expression levels of indicated proteins after normalization to β-actin. The levels of ROCK-1, moesin, and p-moesin found in rat mesenteric arteries from the CS group were set to 100%. Values are mean±SEM;n=6.
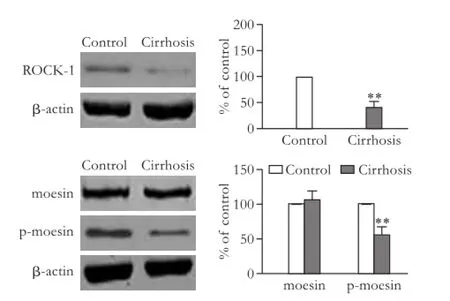
Fig. 7.Comparison of the protein expression and activity of ROCK-1 in human mesenteric arteries. The bar graphs show the relative expression levels of indicated proteins after normalization to β-actin. The levels of proteins found in arteries from control group were set to 100%. Values are mean±SEM;n=5. **:P<0.01, compared with the control group.
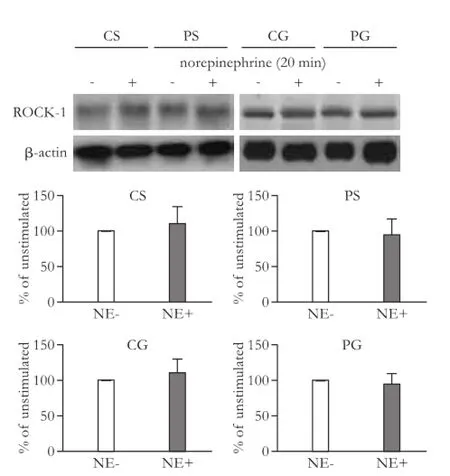
Fig. 8.ROCK-1 protein expression among the four groups with or without stimulation by norepinephrine. The bar graphs show the relative expression levels of indicated proteins after normalization to β-actin. The levels of ROCK-1 found in mesenteric arteries from rats without norepinephrine stimulation were set to 100%. Values are mean±SEM;n=5-6. NE: norepinephrine.
We compared the protein expression and activity of ROCK-1 among the four groups with or without stimulation by norepinephrine. The results suggested that the expression of ROCK-1 was not signif i cantly changed after stimulation with norepinephrine (P>0.05; Fig. 8). The p-moesin but not moesin increased signif i cantly after norepinephrine stimulation in CS and CG rats (P<0.01; Fig. 9). The cirrhotic rats showed no changes in the expression of moesin and p-moesin after stimulation with norepinephrine (P>0.05). However, GRK-2 inhibitor sensitized the response of p-moesin to norepinephrine (P<0.01; Fig. 9).
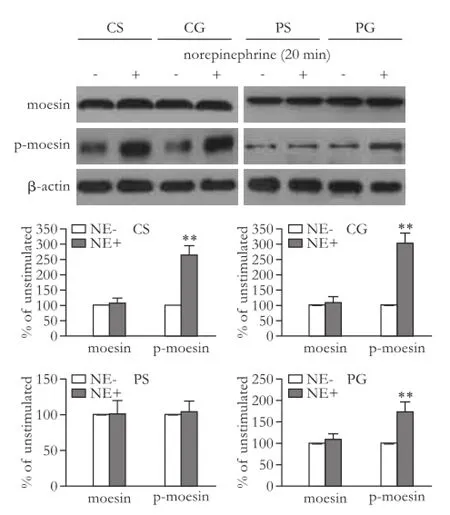
Fig. 9.Change in activity of ROCK-1 among the four groups with or without stimulation by norepinephrine. The bar graphs show the relative expression levels of indicated proteins after normalization to β-actin. The levels of moesin and p-moesin found in mesenteric arteries from rats without norepinephrine stimulation were set to 100%. Values are mean±SEM;n=5-6. NE: norepinephrine. **:P<0.01, compared with the group without norepinephrine stimulation.
Discussion
The complexity of the body's environment including neural, humoral, and mechanical factors is related to vascular reactivity. Therefore, studies on vascular reactivity should be carried out by eliminating interfering factors as much as possible. In the present study, mesenteric arterioles were isolated and fi xed in a perfusion tank that was fi lled with MOPS-PSS to provide a stable environment for vascular metabolism. This also helped to maintain the intravascular pressure and thereby eliminate the inf l uence of fl uid fl ow on the vascular response. The intravascular pressure remained at 80 mmHg for two reasons. First, the vascular diameter increases with the elevation of pressure but remains unchanged when the pressure is kept at 80 mmHg.[18]Second, 80 mmHg is close to the mean arterial blood pressure of humans and rats.
In previous studies on the mechanism that is responsible for vascular reactivity, aortic rings hypocontractility was investigated both in bile duct ligation (BDL) or CCl4induced cirrhotic models.[9,19,20]Ferlitsch et al[21]reported that the responses of forearm arteriesto norepinephrine and AT-II were decreased in patients with cirrhosis. The mean portal vein pressure of our cirrhotic rats was around 15 mmHg, which was in line with the diagnostic standard of portal hypertension in rats. Our data suggested that mesenteric arteriole sensitivity and contractility in response to norepinephrine were decreased in both cirrhotic patients and rats, indicating the hyporesponsiveness of splanchnic and peripheral vessels to vasoconstrictors. Although the hyporeactivity of aortic rings to vasoconstrictor has been will documented,[9,19,20,22,23]the splanchnic vasculature exhibits more hyporeactivity to vasoconstrictor. It is a microvessel with a diameter of 10 to 150 μm that is essential in regulating vascular resistance, not the conduit vessel like aorta. In our study, the third-order mesenteric artery was used to study vascular reactivity, the diameter of which was just inside this range. In addition, a microscopic amplif i cation system was applied in order to precisely observe the small changes in the splanchnic capillary bed that occurs under the effect of a vasoconstrictor.
GPCRs comprise a large protein family and more than 1000 members have been discovered in recent years, including the adrenergic receptor, the AT-II receptor and the M-type acetylcholine receptor. These proteins connect to their corresponding ligands and couple with G proteins to transmit extracellular signals into cells and produce specif i c effects. The persistent existence of an agonist will result in receptor desensitization, which is mainly mediated by two major proteins: GRK-2 and β-arrestins. GRK-2, which is actually a group of protein kinases that specif i cally recognize and phosphorylate agonist-activated GPCRs, has attracted the interest of researchers. As a ubiquitous GRK family member, GRK-2 appears to play a central, integrative role in signal transduction pathways known to modulate intracellular effectors involved in the function of blood vessels. The phosphorylation of GPCR mediated by GRK-2 promotes the binding of β-arrestin-2 to GPCR. β-arrestin-2 is ubiquitously expressed and uncouples the receptor from G proteins, leading to interruption of various signal transduction pathways such as RhoA/ROCK.
In the current study, the protein levels of the α1adrenergic receptor and related G-protein subunits showed no signif i cant differences in the cirrhotic patients and rats compared with the controls. However, the expression of β-arrestin-2, which is closely related to α1adrenergic receptor desensitization,[8,24]increased signif i cantly, whereas the corresponding G proteins exhibited no change. This suggests that the decreased reactivity of the mesenteric artery to contractors was the desensitization and not the quantity change of receptor and G proteins. In addition, the interaction between the α1adrenergic receptor and β-arrestin-2 has been reported in a previous study by co-immunoprecipitation.[25]We found that in the mesenteric arteries in rats with cirrhosis, the binding capacity increased, which indicates a stronger interaction between the two proteins. This phenomenon was also consistent with the fi nding of the studies focusing on human mesenteric arteries. The increased binding of β-arrestin-2 to GPCRs such as the α1adrenergic receptor should inhibit the dissociation of Gα, Gβ, and Gγ, thereby leading to the uncoupling of receptors from G proteins and resulting in desensitization. GRK-2 causes phosphorylation and activation in GPCRs, β-arrestin-2 bound with GPCRs neutralizes GPCRs over activation due to GRK-2 stimulation.[6]Such phenomenon was observed not only in isolated arteries but also in cytological studies. However, the exact mechanism of this phenomenon remains to be elucidated.[26,27]
Studies[28,29]have shown that ROCK is a critical mediator of vasocontraction. Our study did not show signif i cant difference in both ROCK protein expression and reactivity among the four groups of rats. To further investigate the effects of enhanced desensitization on the calcium sensitization pathway, the changes in ROCK protein expression and its activity in rats were detected upon stimulation with norepinephrine (10 μmol/L).
In our study, norepinephrine stimulation did not change the protein expression of ROCK and moesin but the stimulation increased p-moesin signif i cantly. This fi nding suggests that it was the activity, not the expression of ROCK, that was affected by norepinephrine. As mentioned above, ROCK activity can be determined by the level of threonine phosphorylation at position 558. In this study, p-moesin expression was signif i cantly increased in the control group after norepinephrine stimulation but not in the cirrhotic group. Therefore reduced smooth muscle contractility may be a result of the increased β-arrestin-2 expression and its stronger interaction with the α1adrenergic receptor, which hindered G-proteindependent signaling and prevented the vasoconstriction due to the activation of ROCK. In this study, a highly selective GRK-2 inhibitor was applied to inhibit GRK-2 activity but not the expression level.[11]When the activity of GRK-2 was inhibited, the expression of β-arrestin-2 protein was signif i cantly decreased in rat mesenteric arteries and therefore, its interaction with the α1adrenergic receptor was decreased, which released the inhibition of smooth muscle contractility, and recovered the sensitivity of smooth muscle to norepinephrine. This further conf i rmed the role of receptor desensitizationby β-arrestin-2 in inhibiting the transmission of contracting signals.
In the present study, increased expression of β-arrestin-2 and decreased activity of ROCK protein were all observed in the cirrhotic patients and rat models induced by CCl4, but the exact mechanisms of vascular hypocontractility were not exactly the same. The expression and activity of ROCK in mesenteric arteries were the same in rats, whereas ROCK expression and activity were signif i cantly decreased in the patients with cirrhosis compared to those in the controls. A down-regulation of ROCK also contributes to vascular hypocontractility in the cirrhotic rats induced by BDL.[19]Overexpression of β-arrestin-2 might impair contractile signaling in vascular smooth muscle via different mechanisms (Fig. 10). Firstly, binding of β-arrestin-2 to GPCRs causes desensitisation of the G-protein-dependent signaling pathway, including the calcium sensitization pathway. Secondly, enhanced interaction between GPCRs and β-arrestin-2 might result in overactivation of the β-arrestin-2-dependent signaling pathway, and subsequently exaggerated β-arrestin-2-mediated extracellular signal-regulated kinase (ERK) activation may be responsible for posttranscriptional downregulation of ROCK expression. Indeed, pharmacological ERK inhibition such as sorafenib in BDL rats results in up-regulation of ROCK expression and contractility.[30]The lack in CCl4rats of ROCK expression down-regulation found in BDL rats and patients with cirrhosis may demonstrate differences in the β-arrestin-2/ROCK regulation in arteries. Although the existence of apparently very similar features of β-arrestin-2 expression increasing in cirrhosis, the β-arrestin-2 mediated ERK pathway was not overstimulated in CCl4rats, whereas the over-activation of ERK pathway signif i cantly inhibited the expression of ROCK in BDL rats and patients with cirrhosis. This fi nding remains to be further investigated.

Fig. 10.Assumed β-arrestin-2-mediated switching of the α1adrenergic receptor in cirrhosis.
In conclusion, we found that β-arrestin-2 expression and its interaction with the α1adrenergic receptor are upregulated in the mesenteric arteries in cirrhotic patients and rats. These upregulations result in GPCR desensitization, G-protein dysfunction and ROCK inhibition. These results may explain the decreased contractility of the mesenteric artery in response to vasoconstrictors.
Acknowledgement:We thank Associate Professor Dr. Dong Sun (Department of Physiology, New York Medical College, Valhalla, New York, USA) and Professor Chang-Dong Yan (Department of Physiology, Xuzhou Medical College, Xuzhou, China) for technical support and providing us with the vessel perfusion system.
Contributors:XJ and WZY proposed the study. CW, SJY, LDJ, QJ and HYM performed the research. CW wrote the fi rst draft. All authors contributed to the design and interpretation of the study and to further drafts. WZY is the guarantor.
Funding:This study was supported by a grant from the National Natural Science Foundation of China (30972920).
Ethical approval:This study was approved by the Ethic Committee of the Renji Hospital, School of Medicine, Shanghai Jiaotong University.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Hadoke PW. Cirrhosis of the liver and receptor-mediated function in vascular smooth muscle. Pharmacol Ther 2001; 89:233-254.
2 Schepke M, Heller J, Paschke S, Thomas J, Wolff M, Neef M, et al. Contractile hyporesponsiveness of hepatic arteries in humans with cirrhosis: evidence for a receptor-specif i c mechanism. Hepatology 2001;34:884-888.
3 Somlyo AP, Wu X, Walker LA, Somlyo AV. Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases. Rev Physiol Biochem Pharmacol 1999;134: 201-234.
4 Heller J, Trebicka J, Shiozawa T, Schepke M, Neef M, Hennenberg M, et al. Vascular, hemodynamic and renal effects of low-dose losartan in rats with secondary biliary cirrhosis. Liver Int 2005;25:657-666.
5 Mizuno Y, Isotani E, Huang J, Ding H, Stull JT, Kamm KE. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol 2008;295:C358-364.
6 Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 2001;53:1-24.
7 Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 2002;115:455-465.
8 Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor traff i cking and signalling. Biochem J 2003;375:503-515.
9 Hennenberg M, Trebicka J, Biecker E, Schepke M, SauerbruchT, Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology 2007;45:495-506.
10 Taguchi K, Matsumoto T, Kamata K, Kobayashi T. Suppressed G-protein-coupled receptor kinase 2 activity protects female diabetic-mouse aorta against endothelial dysfunction. Acta Physiol (Oxf) 2013;207:142-155.
11 Taguchi K, Matsumoto T, Kamata K, Kobayashi T. Inhibitor of G protein-coupled receptor kinase 2 normalizes vascular endothelial function in type 2 diabetic mice by improving β-arrestin 2 translocation and ameliorating Akt/eNOS signal dysfunction. Endocrinology 2012;153:2985-2996.
12 Sun YW, Chen W, Luo M, Hua R, Liu W, Huo YM, et al. Evaluation of surgical procedure selection based on intraoperative free portal pressure measurement in patients with portal hypertension. Hepatobiliary Pancreat Dis Int 2010;9:269-274.
13 Falcone JC, Kuo L, Meininger GA. Endothelial cell calcium increases during fl ow-induced dilation in isolated arterioles. Am J Physiol 1993;264:H653-659.
14 Trebicka J, Leifeld L, Hennenberg M, Biecker E, Eckhardt A, Fischer N, et al. Hemodynamic effects of urotensin II and its specif i c receptor antagonist palosuran in cirrhotic rats. Hepatology 2008;47:1264-1276.
15 Zhou Q, Hennenberg M, Trebicka J, Jochem K, Leifeld L, Biecker E, et al. Intrahepatic upregulation of RhoA and Rhokinase signalling contributes to increased hepatic vascular resistance in rats with secondary biliary cirrhosis. Gut 2006; 55:1296-1305.
16 Wang Z, Xu JP, Zheng YC, Chen W, Sun YW, Wu ZY, et al. Peroxisome proliferator-activated receptor gamma inhibits hepatic fi brosis in rats. Hepatobiliary Pancreat Dis Int 2011; 10:64-71.
17 Zhang ZQ, Qiu JF, Luo M, Sun YW, Zhao G, Chen W, et al. Liposome-mediated gene transfer of endothelial nitric oxide synthase to cirrhotic rat liver decreases intrahepatic vascular resistance. J Gastroenterol Hepatol 2008;23:e487-493.
18 Sun D, Messina EJ, Kaley G, Koller A. Characteristics and origin of myogenic response in isolated mesenteric arterioles. Am J Physiol 1992;263:H1486-1491.
19 Hennenberg M, Biecker E, Trebicka J, Jochem K, Zhou Q, Schmidt M, et al. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology 2006;130:838-854.
20 Hennenberg M, Trebicka J, Kohistani AZ, Heller J, Sauerbruch T. Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur J Clin Invest 2009;39:906-913.
21 Ferlitsch A, Pleiner J, Mittermayer F, Schaller G, Homoncik M, Peck-Radosavljevic M, et al. Vasoconstrictor hyporeactivity can be reversed by antioxidants in patients with advanced alcoholic cirrhosis of the liver and ascites. Crit Care Med 2005;33:2028-2033.
22 Blanco-Rivero J, Aller MA, Arias J, Ferrer M, Balfagón G. Long-term portal hypertension increases the vasodilator response to acetylcholine in rat aorta: role of prostaglandin I2. Clin Sci (Lond) 2009;117:365-374.
23 Xu J, Cao H, Liu H, Wu ZY. Role of nitric oxide synthase and cyclooxygenase in hyperdynamic splanchnic circulation of portal hypertension. Hepatobiliary Pancreat Dis Int 2008;7: 503-508.
24 Cotecchia S. The α1-adrenergic receptors: diversity of signaling networks and regulation. J Recept Signal Transduct Res 2010;30:410-419.
25 Stanasila L, Abuin L, Dey J, Cotecchia S. Different internalization properties of the alpha1a- and alpha1badrenergic receptor subtypes: the potential role of receptor interaction with beta-arrestins and AP50. Mol Pharmacol 2008;74:562-573.
26 Brown MD, Sacks DB. Compartmentalised MAPK pathways. Handb Exp Pharmacol 2008:205-235.
27 Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 2007;292:C82-97.
28 Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 2000;522:177-185.
29 Somlyo AP, Somlyo AV. Ca2+sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 2003;83:1325-1358.
30 Hennenberg M, Trebicka J, Stark C, Kohistani AZ, Heller J, Sauerbruch T. Sorafenib targets dysregulated Rho kinase expression and portal hypertension in rats with secondary biliary cirrhosis. Br J Pharmacol 2009;157:258-270.
Received September 16, 2012
Accepted after revision April 9, 2013
AuthorAff i liations:Department of Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200127, China (Chen W, Liu DJ, Qin J, Huo YM, Xu J and Wu ZY); Department of Surgery, Xinjiang Kashi District Second People's Hospital, Xinjiang 844000, China (Sang JY)
Zhi-Yong Wu, MD, PhD, Department of Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200127, China (Tel: 86-21-68383752; Email: zhiyongwu@gmail.com)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60047-8
 Hepatobiliary & Pancreatic Diseases International2013年3期
Hepatobiliary & Pancreatic Diseases International2013年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Clinical features and outcomes of patients with severe acute pancreatitis complicated with gangrenous cholecystitis
- Survival outcomes of right-lobe living donor liver transplantation for patients with high Model for End-stage Liver Disease scores
- Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression
- Double-blind randomized sham controlled trial of intraperitoneal bupivacaine during emergency laparoscopic cholecystectomy
- Pancreatic Castleman disease treated with laparoscopic distal pancreatectomy
- Pancreatic duct disruption and nonoperative management: the SEALANTS approach
