Changes in physicochemical properties of proteins in Kayserian Pastirma made from the M.semimembranosus muscle of cows during traditional processing
Aultef Mrghni Ahhme Gen Kneko Hieki Ushio Tomo Inomt Hsn YetimSf Krmn Mihio Mugurum Ryoihi Skt
a Graduate School of Agricultural and Life Sciences,The University of Tokyo,Tokyo 113-8657,Japan
b School of Veterinary Medicine,Azabu University,Sagamihara 252-5201,Japan
c Food Engineering Department,Faculty of Engineering,Erciyes University,Kayseri 38039,Turkey
d Interdisciplinary Graduate School of Agriculture and Engineering,University of Miyazaki,Miyazaki 889-2192,Japan
Abstract In the current study,we examined the effects of beef processing to produce pastirma on the physicochemical properties of proteins in M.semimembranosus(SEM)muscle.Protein concentrations significantl increased in pastirma muscles(P <0.01),as a result of the salting and curing process.The surface hydrophobicity values of processed samples were higher than those without processing at all guanidine-HCl concentrations,suggesting hydrophobicity increased,which may attribute to the new generated peptides during the traditional pastirma-making process.The metmyoglobin content greatly increased (by as much as 89%) in pastirma samples compared with the unprocessed samples.The images of histology also demonstrate that the pastirma processing had no negative impact on the structure of the muscle.The results from this study suggest that the traditional pastirma-making process catalyzed the enzymatic digestion of muscle proteins,and the differences in some physicochemical parameters between the control and pastirma samples were thus likely to be contributable to protein digestion.Thus,the traditional pastirma-making process results in the degradation of many proteins into peptides,which might then be obtainable as functional components to treat human dietand lifestyle-related diseases such as hypertension,hyperglycemia syndromes or to be used as nutraceuticals.
Keywords:Pastirma;Protein digestion;Meat processing;Physicochemical properties;M.semimembranosus
1.Introduction
The Anatolian region has a long,outstanding history and a splendid eating and nutritive dietary culture.Some traditional meat products have been consumed in Turkey,among which Kayserian Pastirma is the most famous.Pastirma is a popular dry-cured beef product made from whole muscle[1].This sort of cured meat has an attractive exterior and interior appearance,delicious taste,unique smell and muscle-like shape.It is a popular meat product coming from the region of Kayseri in central Turkey,and consumers have recently shown considerable interest in related sliced meat products[2].Although pastirma used to be produced and consumed only in the Kayseri region,it is now available in retail markets throughout Turkey[3].
Cattle normally are slaughtered according to local standard and the meat made into pastirma at meat factories in the suburbs of Kayseri city.The firs stage of pastirma processing is dry curing,during which the meat strips are rubbed and covered with a curing mixture (NaCl+KNO3),with several incisions made in the meat to facilitate penetration of the salt and nitrate mixture [4].The beef cuts then undergo a series of processes and treatments lasting for a month.Because of the length of time during the process,the muscle structure and proteins undergo many physicochemical changes.During this period,muscle proteins and fat are hydrolyzed mainly by endogenous enzymes,which results in increased amounts of peptides,free amino acids and free fatty acids[5].The salting and drying procedure affects the structure of the proteins and the enzyme mechanisms,potentially increasing the nutritional and sensory values of pastirma.Few studies focused on some enzymes whose optimum pH is consistent with the Muscle’s pH,that from chilling phase to fully matured ham.Those peptides in which the pH ranged from 5.3 to 6.5,include cathepsin B,cathepsin H,cathepsin D,dipeptidyl-peptidase I or cathepsin C,dipeptidyl-peptidase II,aminopeptidase puromycin-sensitive,aminopeptidase B.Fresh to aged muscle properties together with dry-curing manufacturing practices allowed these proteolytic enzymes to keep partly their activity up to the end of maturing [6].Proteases existence and activities such as calpain and capstatin have been intensively studied for tenderness and flvor and color promotion in both fresh and processed aged meat products.However,in the case of dry-cured beef products like pastirma,the key role that activates endogenous enzymes,which act on muscle proteins,has not been explained yet.
During the long pastirma-making process,muscle protein and fat are hydrolyzed to some extent,by internal enzymes,which eventually produce many small peptides,free amino acids,free fatty acids and volatiles.Those newly developed compounds,directly contribute to the unique flvor of Turkish pastirma.The muscles undergo physicochemical changes during the course of the traditional dry-curing process.We emphasized on the changes occurred to the water-soluble proteins and myofibrilla proteins.These variations occur especially during the ripening period,contributing to the development of an adequate texture and the characteristic flvor[7].Many factors contribute to these changes:the salting,curing and dehydration processes,temperature,spices,time,ripening-chamber type,and oxygen abundance are most important factors affecting the physicochemical properties of the proteins and lipids.However,little information is available regarding the chemical changes in proteins nature during the traditional pastirma-making process.
Because the local consumers wish to understand the functionality and health benefit of pastirma components,including information about its protein content and its beneficia effects on some chronic diseases.The main objective of the whole research to identify and isolate some bioactive peptides that would be used as nutraceuticals to treat some life-style related diseases such as hypertension.However,the aims of this current work were (1) to estimate the degradation capacity of proteins and (2) to determine some physicochemical properties of pastirma made from theM.semimembranosus(SEM)muscle.In future this will assist us to evaluate the generation of new bioactive peptides,which perhaps will be resulted from the pastirma cuts,in light of their subsequent bioavailability of those peptides in terms of their effects on lifestyle-related diseases.Results from undergoing research (not accomplished yet) indicate that bioactive peptides are available as we could measure their IC50,which suggests that the newly generated peptides might act as nutraceuticals to treat some hypertension diseases[8].
2.Materials and methods
2.1.Meat cuts and pastirma-manufacturing
Cuts manufactured about 1 month prior to the experiment were obtained from a local retailer.The pastirma was produced in a factory in Kayseri city using the traditional process.Top (inside) round muscles were sourced from male cows at 30 months old.The pH values of the muscles before processing ranged from 5.6 to 6.0.SEM muscles were used 48 h post-mortem.Two groups of muscles were prepared for each experiment:one group was analyzed as fresh meat,the other group was processed into pastirma.Fresh and processed meat samples were sourced from the same animals,and all fresh samples were kept at-30°C until the experiment.
Meat cuts were processed by removing most of the subcutaneous fat layers and visible connective tissue from the surface[3,9],followed by cutting into rod-shapes,as preferred by consumers.Muscles were placed on a curing mixture(crystallized salt 1000 g+15 g nitrate/kg of meat)at room temperature.Muscles were salted on each side for about 24 h.After curing,the muscles were washed thoroughly using fresh water to remove excess salt from the surface.The cuts were then dried in the open air for a period of 10 d,depending on the weather.Muscles were further processed by squeezing at room temperature for 24 h.Furthermore,the cuts were hanged for extra drying process in the shade for 4-6 d at 15-20°C.The cuts were subsequently covered with a paste of ground spices known as cemen(chemen),as described by Kaban[4].The cured and dried strips were put in a bowl of seasoning mixture containing 12%,20%,13%and 55%of milled fenugreek seeds,crushed garlic,red pepper and water,respectively.Later the cuts were left to cure for 12-24 h in hot weather,and an extra 1-2 d in cooler weather.The excess cemen was removed to leave a thin layer(about 3 mm),and the product was dried again at a moderate temperature for 2 d.Finally the pastirma was wrapped in a paper sheet ready to be sent to the market for consumers.The cemen paste covering the pastirma is an important element in terms of both flvor and preservation.The mixture known as cemen in Turkey is reported to improve the appearance,color,texture,taste,and flvor of pastirma,and is also effective against microbial contamination and helps to prevent excessive drying of the pastirma[10].
2.2.Protein extraction
Proteins were extracted from the fresh and processed(pastirma) samples by adding 28 mL of solution to 2 g of the samples.Proteins were extracted using three different solutions.Distilled water was used to examine the differences in most proteins(H2O P-ex),enzymes and small peptides,which can be extracted in water.The second solution was a low-ionic-strength solution,which extracted proteins define as water-soluble proteins (WSP) (50 mmol/L imidazole-HCl,pH 6.0,2 mmol/L EDTA).The third solution was a high-ionic-strength solution,Guba-Straub-adenosine triphosphate solution (GS-ATP)(0.09 mol/L KH2PO4,0.06 mol/L K2HPO4,0.3 mol/L KCl,1 mmol/L ATP,pH 6.5) [11].The solutions along with the samples were homogenized three times for 30 s,at 10-s intervals,using a polytron homogenizer (Kinematica Co.,Littau,Switzerland) at setting 4.The mixtures were then centrifuged at 12,000 rpm for 30 min at 4°C in a Himac CR 20E centrifuge(Hitachi,Tokyo,Japan).The supernatants were removed and fil tered using filte paper no.5A(Advantec Toyo K.Ltd.,Tokyo,Japan),and the fina solution was used as the extracted-protein solution.The protein concentration of the extracted solution was determined using the biuret method [12],with bovine serum albumin as a standard.The absorbance of the samples was measured at 540 nmusing a Model Bio Spec 1600 spectrophotometer(Shimadzu Co.,Kyoto,Japan),with each sample evaluated in triplicate.
2.3.pH values
The pH values of the aqueous solutions of extracted proteins and meat cuts were measured to determine the qualities of the meat and protein solutions.pH values were measured using a HM-30R Model HM-30R pH Meter.
2.4.Fluorescence intensity
Surface hydrophobicity of the actual proteins in samples while still fresh and proteins with the newly developed peptides in meat samples after processed into pastirma were determined by the 8-anilino-1-naphthalenesulfonic acids (ANS) method[13,14].In view ofits capacity to monitor subtle changes in chemical and physical state of protein,hydrophobicity can be a suitable parameter to estimate protein denaturation [15].ANS 50 mL (8 in 10 mmol/L phosphate buffer,pH 7.0) was added to 4 mL of protein solution.The fluorescenc intensity of the ANS-protein conjugate was measured in a Model RF-1500 spectrofluoromete (Shimadzu Co.,Kyoto,Japan) at an excitation wavelength of 365 nm and an emission wavelength of 470 nm.Quinine sulphate (1 mg/L) was used as the standard solution[16].
2.5.Sodium dodecyl sulphate-polyacrylamide gel electrophoresis(SDS-PAGE)
SDS-PAGE was used to separate the proteins according to their sizes after extraction in the different ionic-strength solutions[17,18].Electrophoresis was carried out using two different acrylamide gel gradients:7.5%(to visualize high-MW proteins),and 7.5%-17.5% (to visualize all proteins and peptides),with 2-mercaptoethanol at 20 mA/gel,according to the discontinuous buffer system Laemmli[19].
2.6.Metmyoglobin measurements
Metmyoglobin concentrations were evaluated using a modificatio of the procedures described by Krzywicki [20]and Saito [21].Samples were blended with fie volumes of cold 0.04 mol/L phosphate buffer at pH 6.8 for 10 s in a homogenizer(Kinematica Co.,Littau,Switzerland).After standing at 1°C for 20 h,the mixture was centrifuged at 2700 r/min for 30 min.The supernatants were then removed and filtere using filte paper no.5A (Advantec Toyo K.Ltd.,Tokyo,Japan).The fil trate was measured at absorbance of 525,572,700 nm using a Model Bio Spec 1600 spectrophotometer(Shimadzu Co.,Kyoto,Japan).The percent of metmyoglobin was calculated using the following formula:

whereA=absorbance atλnm.
2.7.Histology
Histological imaging was carried out with slight modification as described in earlier publication [22].A cut(10 mm×12 mm×12 mm) was removed from each pastirma and fresh muscle sample (positive and/or control) and soaked for 14 d in 10%(V/V)formalin buffer.The fixatio process was designed to preserve the shapes of the cells and tissues,and involved successive immersions in different concentrations of ethanol,followed by three different concentrations of toluene for 8 h each.Finally,the samples were incubated in three different paraffi solutions for 8 h each and refrigerated.The stained slices(hematoxylin and eosin-safran)were mounted on glass microscope slides,visualized and photographed at 20×magnificatio under a transmission electron microscope (Nikon microphot-FXA) connected to a computer with the appropriate software(Nikon-ACT-2u),to obtain the clearest images.
2.8.Statistical analysis
All data are expressed as the mean±SEM of at least fie independent experiments,unless indicated otherwise.Two-way ANOVA was used to determine the significanc of the muscle type(fresh and processed)and the effect of treatment of experiment.Statistical analyses were conducted by SASsoftware using Duncan multiple comparison method.
3.Results and discussion
3.1.Protein extractability and concentration
Samples from pastirma muscles showed significantl increased concentrations of extracted proteins in the three solutions(P<0.01),as a result of the salting process(Fig.1).The salting treatment and curing process was possibly to have a potential effect on the extractability of muscle proteins,which could be due to the disassociation of some proteins from each other especially myofibrils
In both fresh(control)and processed(pastirma)muscles,the extractability of WSPs was lower than that of proteins extracted in H2OP-ex and GS-ATPsolution.Proteins extracted in GS-ATP solution increased as a result of the degradation of large proteins,and ATP was likely to play a crucial role by cleaving the strong bonds,especially that connecting myosin and actin.Interestingly,more proteins were extracted from pastirma in H2O P-ex compared with fresh cuts,probably because of the activities of certain enzymes during the course of processing(P<0.01).In a published article,the authors[17]reported that the application of proteomic study to proteolysis occurring in dry-cured ham production has allowed an evaluation of the changes in muscular proteins during ripening[23].The same group reported that they could extract in pure water the following enzymes creatine kinase,enolase B,glyceraldehyde 3-phosphate,dehydrogenase,triosephosphate isomerase.In the 2-DGE map of dry-cured ham,there can be seen:(i) a diminution of spot intensities of proteins with estimated MW between 66.2 and 28 kDa;(ii) the disappearance of creatine kinase;(iii)a partial disappearance of protein with estimated MW ranging between 28 and 14.4 kDa;(iv) the appearance of spots between 150 and 66.2 kDa,two more acidic spots with estimated MW around 38 kDa[23].In the pastirma samples,however,we have discovered similar results that a tremendous number of peptides and small proteins were extracted(77-18 kDa)(Figs.5 and 6).
Because the process lasts about 1 month,degradation may have occurred through the activation of some unidentifie enzymes.One possible analysis on the difference in extracted protein concentrations between the two types of muscles is the initiation of a reaction activated by certain proteases,such as aminopeptidase or by the cemen addition.It is well known that cemen contains fenugreek,which might involve in the protein degradation.In addition to the fenugreek antimicrobial activities,possibly cemen ingredients had an impact on the proteins during storage of pastirma.Based on the results of a study carried out by Yetim et al.[24]cemen paste and garlic significantl inhibited the growth of three pathogenic bacteria.The protein concentration change is likely to be the result of endogenous enzyme activity since low numbers of microorganisms are found inside dry-cured ham[25].In general,the inhibitory effect of cemen paste was higher than that of garlic.This might have resulted from the synergistic effect of cemen ingredients.In general,the pastirma-making process increased the amounts of proteins extracted by all three solutions,implying that the salting and curing process had a beneficia effect(Fig.1).Enzymatic production of bioactive and antioxidative protein hydrolysates and peptides most likely occurred during pastirma-making process.This process might lead to the generation of new biologically active peptides that could provide multifunctional remedies for some cultural diet-related diseases.
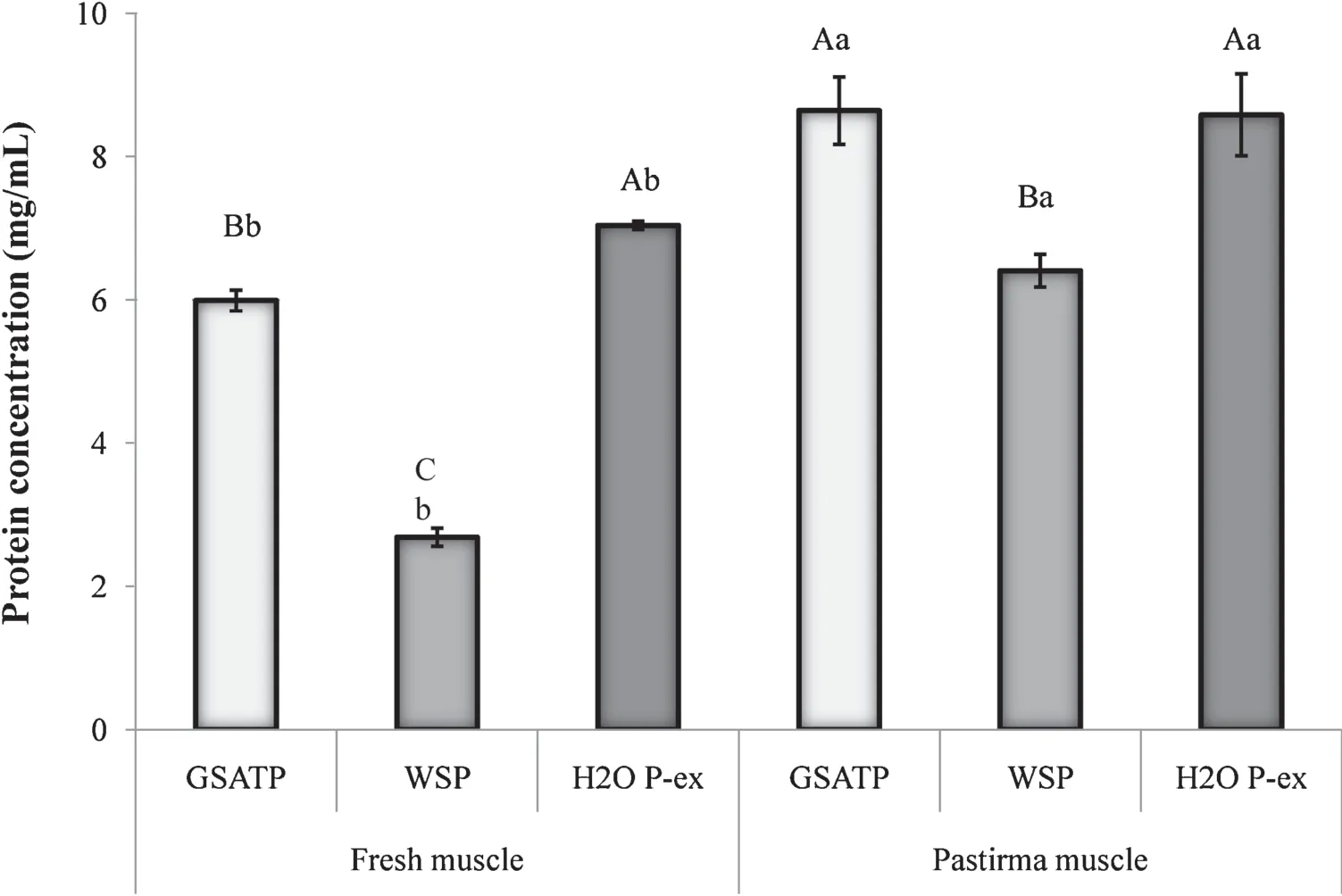
Fig.1.Extractabilities of proteins from fresh SEM muscle in GS-ATP,WSP buffer,and distilled water(mg/mL).Different capital letters in each muscle group show the statistical significanc (P <0.01);different small letters in each extract type show the statistical significanc between the muscle types(P <0.01).
3.2.pH
The curing of beef meat must be done very cautiously under strict conditions,to avoid unfavorable reactions such as lipid auto-oxidation,rancidity,color deterioration,and pH changes.Table 1 shows the pH values of proteins extracted in different buffers.The pH values of fresh samples showed normal readings,except that those extracted in GS-ATP solution were higher than those extracted in WSP and H2O P-ex,which might be due to the fina pH of the buffer.In general,the pastirma-making process had no negative effect on the pH values,with only a slight increase among all samples compared with fresh samples.The increase might have been associated with the degradation of some proteins and enzymes,as indicated by SDS-PAGE.Table 1 also shows the pH values for raw meat and pastirma,measured by inserting a pH meter probe directly into the meat cuts.Surprisingly,the measurements were similar,indicating that the acidity of the pastirma was the same as that of the original fresh-cut meat.Since the pH of pastirma samples ranged from 5.4 to 6.2.We suggest that the following enzymes were coexisted with myofibrilla cells in pastirma muscles:cysteine peptidases (cathepsin B,H,C and L and dipeptidyl-peptidase I) and serine peptidase (dipeptidyl-peptidase II) as they are active within a range of pH 5.5-6.5 and 5.0-6.0,respectively.Cathepsin B and L may play key roles in hydrolyzing insoluble proteins to soluble proteins,and soluble proteins to peptides as a result ofin vitroconditions and the long duration of Jinhua ham processing[26].Furthermore,the same authors reported that the activity of muscle dipeptidyl-peptidase I decreased gradually before the beginning of ripening and then increased progressively until the end of post-ripening.As we believe that there is a relative similarity in processing method between pastirma and Jinhua hams,the results of this study thus in accordance with the results demonstrated by a study conducted by Zhou and Zhao[27].

Table 1 pH values of protein extractions from fresh-meat cuts and pastirma made from the SEM muscle of cattle,also pH values of proteins extracts(dissolved in GS-ATP,WSP buffer,and distilled water).
3.3.Fluorescence intensity
Since Sano et al.[28]and Yongsawatdigul and Park[29]reported that solubilization of myosin or actomyosin in 0.6 mol/L KCl following by fluorimetri determination of hydrophobicity with 1,8-anilino-naphtalene-sulfonate (ANS)probe has been described in molluscs [30]and fish The polar and hydrophobic amino acid residues were examined in proteins extracted from unprocessed and pastirma cuts ofM.semimembranosususing all three solutions.Fluorescence intensity measurements indicated that the surface hydrophobicity and the relative proportions of amino acids in the proteins extracted from fresh muscle raised with increasing guanidine hydrochloride(Gu-HCl)concentration(Fig.2).In general,the hydrophobicity of the control-sample proteins extracted in H2O P-ex was higher than those of proteins extracted in GS-ATP and WSP.The lowest fluorescenc intensity values were associated with proteins extracted in WSP,which might reflec the myoglobin concentration or refers to the EDTA addition,as this reagent inhibits enzyme activity during preparation of samples for tests.The high fluorescenc intensity values of proteins extracted in distilled water may be contributable to the fact that fresh distilled water is chemical free,as well as to their extractability.
In relation with the variations of physico-chemical properties,we suggest the increase in disassociated proteins was associated with an increase in protein hydrophobicity in pastirma cuts.The fluorescenc intensities of the processed samples were higher than those of the control samples at all Gu-HCl concentrations(Fig.3).An increase in the surface hydrophobicity of the treated samples indicates an increase in the polarity of the amino acids.The fluorescenc intensity values of the samples extracted in distilled water were significantl lower than those in samples extracted in GS-ATP and WSP(P<0.01),which might be attributable to oxidation occurring during the processing time.Oxidation of myoglobin would convert it to oxymyoglobin,while further oxygenation would also convert it to metmyoglobin.This could explain why the hydrophobicity of the distilled water samples was lower than that of the other extractions,and also suggests that a considerable number of amino acids split from each other as a result of the salting and curing process.The creation of new small compounds such as peptides and free amino acids by the traditional pastirma-making process is associated with an increase in hydrophobicity and changes of the physical properties.As expected,protein denaturation by Gu-HCl increased the surface exposure of hydrophobic residues,as measured by ANS fluorescenc [13,16,22,31],in a concentration-dependent manner.Free amino acids are generated by proteolysis during the processing of dry-cured meat products [32].The most single free amino acids increased during aging with particular reference to the lipophilic;a rise in lyophilic valine,phenylalanine and tryptophan was also found in Iberian ham processed following a traditional prolonged way [33,34].Such finding led us to suggest that the differences in fluorescenc intensity between the control and pastirma samples were probably associated with the protein degradation and creation of free amino acids.The surface hydrophobicity of the degraded proteins during processing was an indicator of the polarity of the muscle proteins,and the results indicate that the hydrophobicity of the pastirma samples increased as the extracted protein content increased.The differences in the fluorescen intensity indicate that changes within protein composition in the meat cuts resulted in increased protein content and alterations in surface hydrophobicity.
3.4.Metmyoglobin
Changes of myoglobin in meat cuts are one of the fastest chemical reactions occurring during food processing.The mechanism is complex,as myoglobin is converted to oxymyoglobin,which is subsequently converted to metmyoglobin within a few hours.Scientists also consider that metmyoglobin may revert to oxymyoglobin under certain circumstances.The discoloration of fresh meat is an important process,which is determined by the relative concentration of the three redox forms of myoglobin (deoxymyoglobin,oxymyoglobin,and metmyoglobin)[35].Food quality is the sum of three principal components,nutritional value,safety and consumer acceptability[36].Consumer acceptability includes a large array of attributes such as visual appeal,aroma,flvor,texture,mouth feel,convenience and cultural relevance[37].Consumers are more likely to purchase beef products that have an acceptable color,such as bright red or pink,as this is regarded to be an indication of freshness[38].Turkish consumers prefer pastirma with a bright-red color,because they consider that the darker the color,the inferior the pastirma.Over time,the pastirma turns browner and darker,and becomes firme in texture.
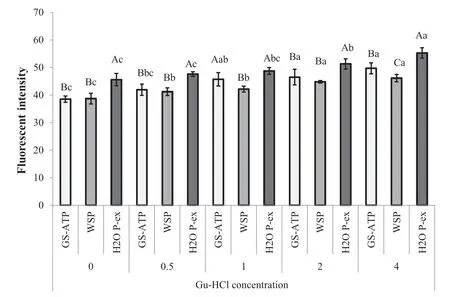
Fig.2.Effects of Gu-HCl(0,0.5,1,2 and 4)on fluorescenc intensity(surface hydrophobicity)of native proteins(extracted in GS-ATP,WSP and H2O P-ex solutions)from fresh SEM muscle.Different capital letters in each extract type show the statistical significanc (P <0.01);different small letters in each extract type show the statistical significanc between the Gu-HCL concentrations molarity(M)(P <0.01).
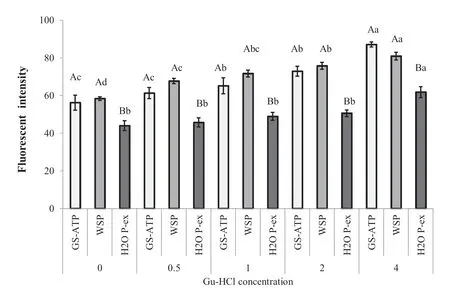
Fig.3.Effects of Gu-HCl (0,0.5,1,2 and 4) on fluorescenc intensity (surface hydrophobicity) of degraded proteins (extracted in GS-ATP,WSP and H2O P-ex solutions)from pastirma of SEM muscle.Different capital letters in each muscle group show the statistically significanc (P <0.01);different small letters in each extract type show the statistically significanc between the Gu-HCl concentrations molarity(M)(P <0.01).
The results of the current study indicated that the metmyoglobin percentage in pastirma samples was significantl increased (P<0.01),by as much as 89% compared with fresh samples (Fig.4).The pastirma-making process lasts about 30 d,which is long enough to allow most of the myoglobin to be converted to oxymetmyoglobin and then to metmyoglobin.However,we suggest that the metmyoglobin reverted to oxymetmyoglobin as a result of wrapping the pastirma in a spice paste(cemen).This process provided an anaerobic environment that isolated the meat cut from further contact with oxygen.The characteristic flvor and aroma of pastirma can be attributed to the ingredients used in its processing,namely fresh mashed garlic,red pepper,and ground fenugreek (Trigonella foenum graecum) seeds [39].The paste increases the aromatic properties of the meat and protects it from drying and spoiling through contact with the open air,which would otherwise cause the fat in the pastirma to oxidize and give a rancid flvor.Visually,pastirma is esthetically unique but darker than the equivalent fresh cuts of meat.That can be explained by the hypothesis of Swatland[40]as reported that after prolonged exposure to the atmosphere,however,the iron atoms in Mb may be converted to the ferric form,resulting in the formation of brown Met-Mb.The Met-Mb%is not only an indicator of oxidation but plays an important role in the coloration of the meat[41]and that can be avoided by using natural wrapping materials.Cemen is considered one of those excellent natural wrapping materials,which composed of ground classical fenugreek seeds,local garlic,and chili pepper powder,mixed into a paste with a little tomato and plenty of water.
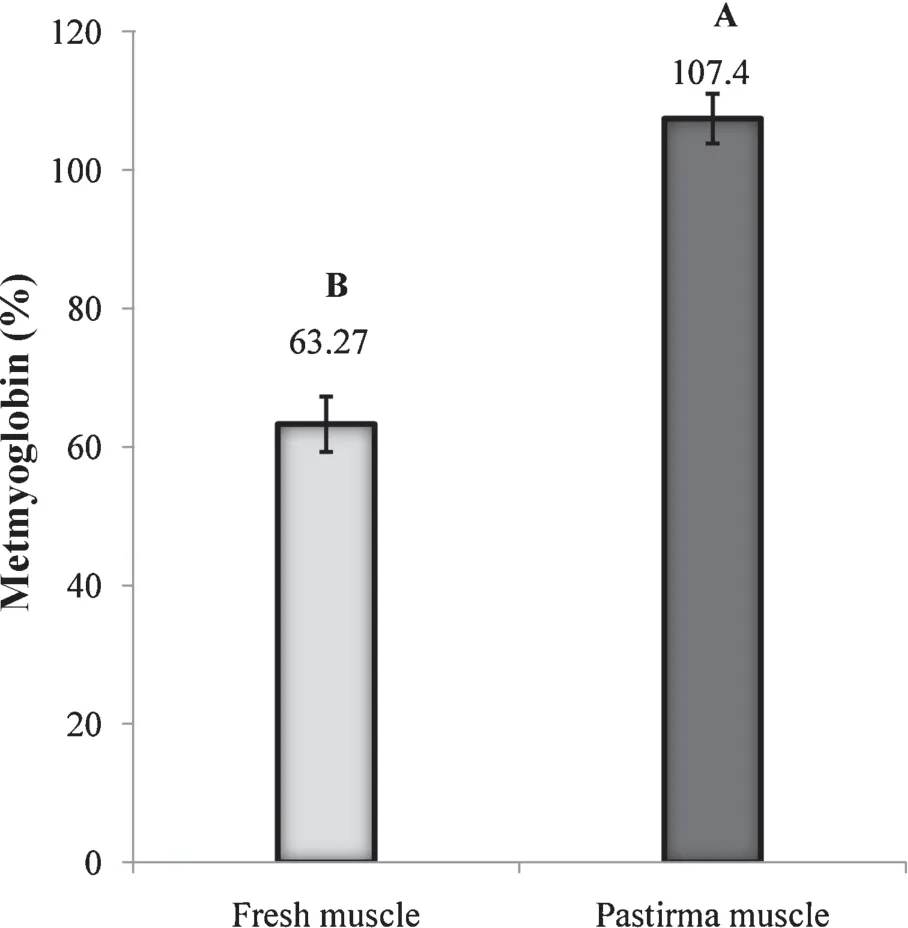
Fig.4.Metmyoglobin percentage in fresh beef cuts and pastirma,both from the SEM muscle of cattle.Different letters show statistically significanc between cut types(P <0.01).
3.5.Electrophoresis
The extracted proteins (GS-ATP,WSP,and distilled water)from the fresh and pastirma cuts were separated by SDS-PAGE to evaluate their molecular weights and to detect changes in the native proteins(Fig.5A and B).In general,the SDS-PAGE pattern indicated that most muscle proteins were metabolized to new,smaller molecules,including peptides(Fig.5B).
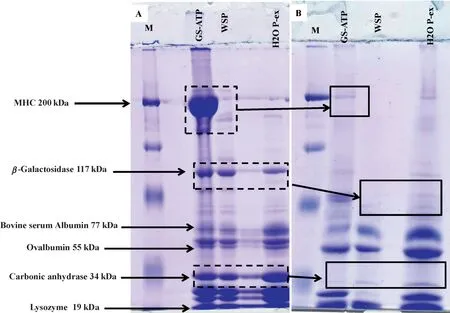
Fig.5.SDS-PAGE showing effects of traditional pastirma-making process on MHC and other low molecular mass proteins in the SEM muscle of cattle.Samples were extracted in GS-ATP solution.(A)Samples of proteins extracted from fresh muscle and(B)samples of proteins extracted from pastirma.Gradient slab gel was 7.5%.Dotted boxes indicate the original bands in(A)that are undetectable in(B).
In the fresh meat cuts,the MHC protein band in the GS-ATP sample was clear,with another band representing a subunit of MHC.There are likely to be several bands of different molecular weights.In contrast,the MHC bands were absent in pastirma samples,suggesting degradation of the muscle proteins during the pastirma-making process.Other bands,possibly representingβ-galactosidase with a molecular weight of 117-kDa and carbonic anhydrase with a molecular weight of 34 kDa,were present in the GS-ATP,WSP,and distilled-water extracts of fresh meat,but absent from the pastirma samples,probably as a result of degradation into smaller peptides and possibly functional compounds.
Furthermore,separation of the same samples on different gradients (7.5%-17.5%) showed less degradation of lowmolecular-weight proteins(Fig.6A).Most major small proteins retained their native structure,but some changes occurred:the WSP 36-kDa Glyceroaldehyde3-Phosphatedehydogenase band disappeared in the pastirma samples,as did carbonic anhydrase,with a molecular weight 34-kDa bands in the three extracts(GS-ATP,WSP and H2O P-ex),all of which were apparently degraded into smaller peptides.Additionally,many small peptides are absent from Fig.6B such as the 29-kDa trypsin inhibitor band in GS-ATP samples.A substantial increase in peptide concentration in the 3-17 kDa MW range with time post-mortem in both fresh pork and beef samples that aged for 14,22 and 29 d was observed [42].Similarly,might be happened,since the pastirma making procedure takes about 4 weeks.These results thus demonstrate that certain proteins were degraded by enzymes activated during or after the processing.This suggestion of enzyme action is supported by the increases in protein content and hydrophobicity of the extracts,as well as the increased concentration of metmyoglobin.Thus,the traditional pastirma-making process results in the degradation of many proteins into peptides,which might then be obtainable for treating some diet-related diseases.However,further biochemical and physiological studies are needed to confir this hypothesis.It is important to study techno-functional properties of active peptide fractions and how these peptides can retain their antioxidant activities in different targeted food matrices[43].
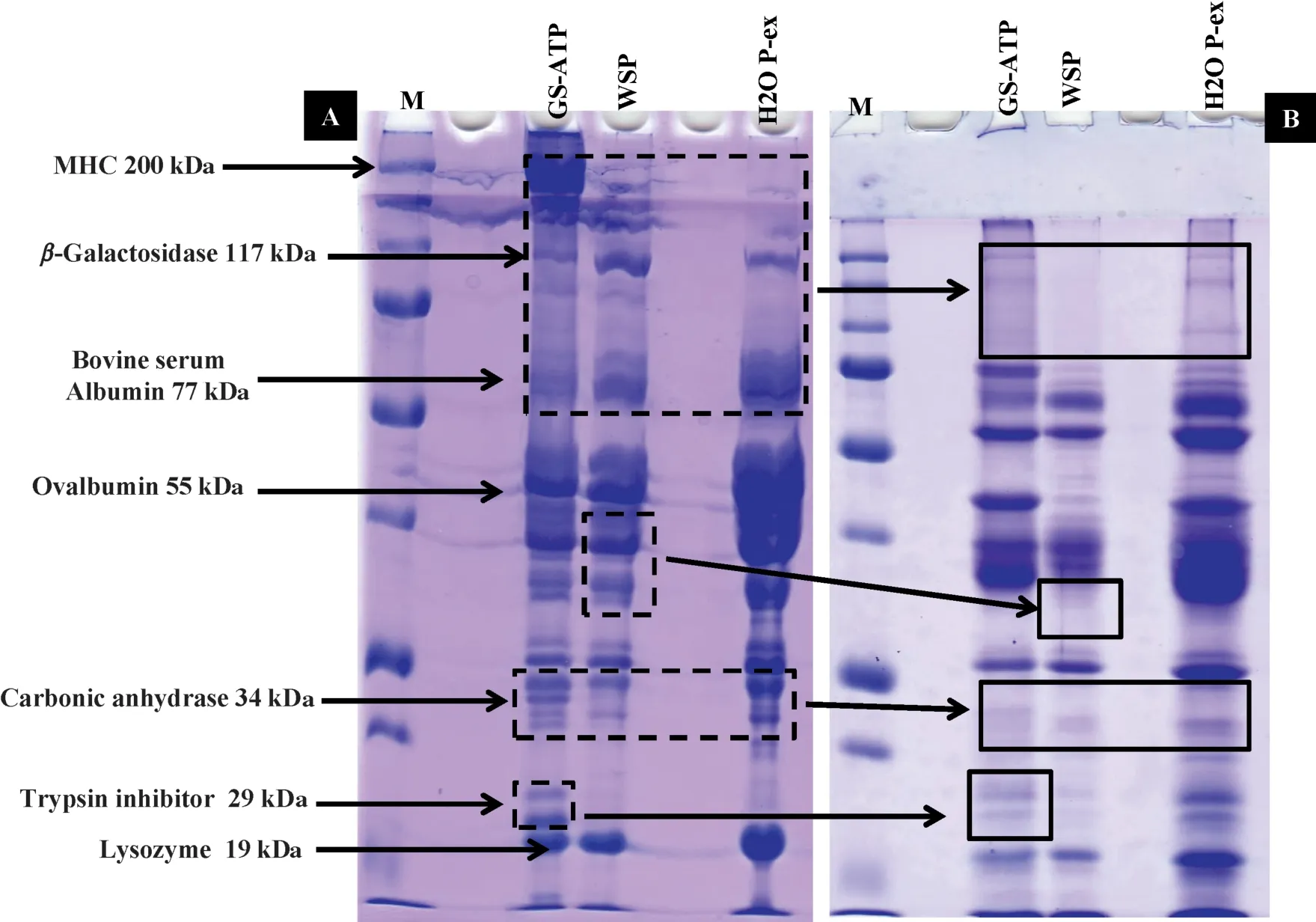
Fig.6.SDS-PAGE showing effects of traditional pastirma-making process on MHC and other low molecular mass proteins in the SEM muscle of cattle.(A)Samples of proteins extracted from fresh muscle and (B) samples of proteins extracted from pastirma.Samples were extracted in GS-ATP solution.Gradient slab gel was 7.5%-17.5%.Dotted boxes indicate the original bands in(A)that are undetectable in(B).
3.6.Histology
Histological techniques are often used in combination to obtain a maximum ofinformation from a given tissue[44].Histological study was used in this work to examine the changes in myofibrilla proteins structures.Fig.7A shows the structure of the muscle protein filaments as well as the nucleus,intracellular structure,and tubular arteries.The structure of the muscle protein filament in the pastirma samples had changed after the course of processing.The most recognizable change was occurred within the intracellular and tubular gaps were smaller than in fresh muscles.Fig.7B shows that the muscle protein filament (arrows)were attracted to each other and were closer than in unprocessed samples.The filament and myofibrilla proteins are magnetized due to the hydration process.Minimizing the gaps in muscles maximizes the firmnes of cured-meat cuts.From the preference point of view,however,the traditional pastirma making process had no negative impact on the structure of the muscle.This indicates that this method of processing meat products is not detrimental to the texture,and results in a firme texture of the processed meat products.Conversely,Huff-Lonergan and Lonergan[45]have reported that fresh meat that retains its moisture,and tenderness is arguably one of the most important quality characteristics of raw products.It is not the case for cured meat products such as pastirma,this sort of product unrestrained most of the moisture that later it becomes slightly harder in texture and had a drastic change in the color as it turned to dark brown.The background of the fiber and some intramuscular fat tissue appeared in a similar color that refers to one possible reason in which we did not wash the samples thoroughly (distaining process) or the pigmentation process was a bit longer than usual.
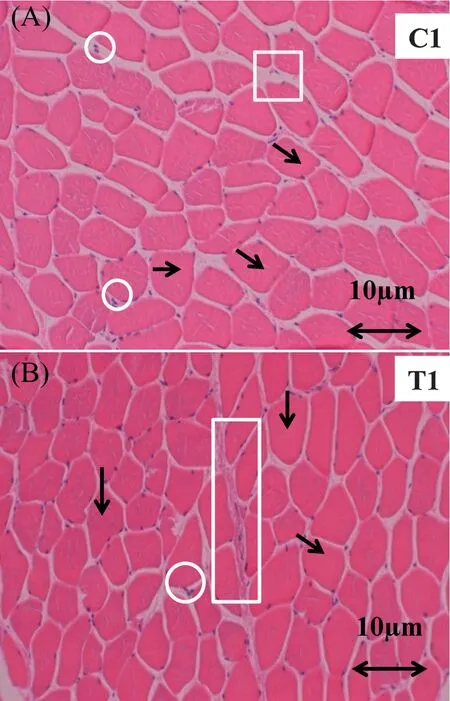
Fig.7.Histological images showing the effects of the traditional pastirmamaking process on the structure of the SEM muscle of cattle.C:fresh cut sample and T:treated cut sample (pastirma).Box:arteries;circles:nucleus;arrows:fiber or filaments and rectangle:endomysin or connective tissue.Images were obtained at the magnification indicated.
4.Conclusion
Changes of physicochemical properties between fresh samples and pastirma cuts(dry-cured meat)sourced from the same carcass,shoulder rose muscleM.semimembranosus(SEM) of cattle were significantl different.The salting and curing process had no negative impact on the firmnes of pastirma cuts,only the filament and myofibrilla proteins are magnetized due to the hydration process.These results demonstrate that the protein degradation capacity in pastirma cuts was considerable,which refers to certain enzymes that activated during or after the curing process.The traditional pastirma-manufacturing process does not retain many of the physicochemical parameters of fresh muscle.Thus,the pastirma-making process results in the degradation of many muscle proteins into peptides,which might then be obtainable to treat some diet-related diseases.The results suggest that pastirma may contain newly generated peptides that can serve as nutraceuticals suitable for reducing some chronic diseases.However,further biochemical biological assays,and bioavailability and metabolism studies are needed to confir this hypothesis.
- 食品科学与人类健康(英文)的其它文章
- Black tea in chemo-prevention of cancer and other human diseases
- Polyphenolic extract of Sorghum bicolor grains enhances reactive oxygen species detoxificatio in N-nitrosodiethylamine-treated rats
- Protective role of concomitant administration of fla lignan concentrate and omega-3-fatty acid on myocardial damage in doxorubicin-induced cardiotoxicity
- Epigenetic origins of metabolic disease:The impact of the maternal condition to the offspring epigenome and later health consequences
- GUIDE FOR AUTHORS
- Natural products for cancer prevention associated with Nrf2-ARE pathway

