Protective role of concomitant administration of fla lignan concentrate and omega-3-fatty acid on myocardial damage in doxorubicin-induced cardiotoxicity
Annd A.Znwr Mhleshwr V.Hegde Suhsh L.Bodhnkr
a Department of Pharmacology,Poona College of Pharmacy,Bharati Vidyapeeth University,Paud Road,Erandwane,Pune 411038,Maharashtra,India
b Interactive Research School for Health Affairs,Medical College Campus,Bharati Vidyapeeth University,Dhankawadi,Pune 411043,India
Abstract The severe cardiotoxicity incurred due to doxorubicin limits the use of their therapeutic potential.The current study aims to investigate the cardioprotective effect of concomitant administration of fla lignan concentrate(FLC)and omega-3-fatty acid(ω-3-FA)on myocardial damage in doxorubicin-induced cardiotoxicity in Wistar rats.Cardiotoxicity was induced by intraperitoneal injection of doxorubicin (4 mg/kg) on day 7th,14th,21st and 28th day in normal saline.Concomitant administration of FLC (500 mg/kg) and ω-3-FA (1 mL/kg) lowered TNF-α level,normalized ST,QT and mean arterial blood pressure,elevation in endogenous enzymes levels such as glutathione and lowering in malondialdehyde,super oxide dismutase followed by normalized lipid profil and reduced the mortality rate.The treatment had antiapoptotic potential at cellular level also histopathology of heart tissue (light and electron microscopical).Thus concomitant action of FLC and ω-3-FA may be antioxidant,antihyperlipidemic,anti-inflammator and anti-apoptotic actions seem to the probable mechanisms in doxorubicin induced cardiotoxicity.It can be concluded that FLC and ω-3-FA both have distinct mechanism for cardioprotection and hence the additive effect was observed in the present study due to concomitant administration of FLC and ω-3-FA.
Keywords:Apoptosis;Cardiotoxicity;Doxorubicin;Flax lignan concentrate;Poly unsaturated fatty acid;TNF-α
1.Introduction
Flaxseed has been used as human food for thousands of years;industrial uses of fla oil have predominated since the industrial revolution.There has been considerable interest in the inclusion of flaxsee in western diet,medicinal food or in the development ofisolated and purifie compounds for food,animal feed,and the improvement of human health.Flaxseed seed with its high level of omega-3-fatty acid (ω-3-FA),assumes super food status.But due to the presence of several antinutrients such as cynogenic glycosides,linatine anti-vitamin B6,flaxsee is not generally regarded as commonly edible food [1].An array of therapeutic activities of flaxsee is well proven one of them is antiatherosclerotic activity which is mainly attributed to secoisolariciresinol diglucoside(SDG)[2].In our previous study,we have reported the cardioprotective activities in isoprenaline induced cardiotoxicity of fla lignan concentrate (FLC) extracted from flaxsee [3]andin vitroantioxidant activity of SDG lignan is previously reported[4].
For many years,scientists were puzzled by the fact that heart disease among Greenland Eskimos was extremely rare despite their consumption of a high fat,high-cholesterol diet.Later research revealed that the Eskimos were protected by diets largely based on seals,whales and fish all of which provide high intakes of omega-3 polyunsaturated fatty acids (ω-3-PUFA),especially eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) resourced from marine origin [5].This observation established “Eskimo paradox” wherein little evidence of heart disease and low blood cholesterol levels was reported [6].Now it is well established that there is an inverse relationship between high fis oil consumption and mortality following cardiovascular disease (CVD).The mechanism by whichω-3-FA may reduce risk for cardiovascular disease includes reduction of susceptibility of the heart to ventricular arrhythmia,antithrombogenic,hypotriglyceridemic,retardation of growth of atherosclerotic plaque,reduction of adhesion molecule expression,reduction of platelet-derived growth factor,anti-inflammator,promotion of nitric oxide-induced endothelial relaxation and mild hypotension[7].
Despite of controversy about the mechanism of doxorubicin(Dox) on tumor cells,doxorubicin induced cardiotoxicity is a well established model to study the cardiovascular effects of different natural and synthetic drugs in rodents.Various mechanism of doxorubicin toxicity in rodents has been suggested.Doxorubicin administration increased oxidative stress due to production free radicals and caused cardiac defici of antioxidants.At the nuclear level,intercalation into DNA,leading to inhibition of synthesis of macromolecules has been suggested,which precipitate DNA abnormalities like alkylation,crosslinking,strand separation and helicase activity.DNA damage may be initiatedviainhibition of topoisomerase II and induction of apoptosis in response to topoisomerase II inhibition[8].There are several reports of reversal of doxorubicin cardiotoxicity by natural products[9]including fis oil containingω-3-FA[10],apart from this model has been successfully used in evaluation of cardioprotective action of number of extracts from medicinal plants such asZingiber officinale[11],garlic[12].
However,different types of events,such as anti-inflammator,anti-atherosclerotic and anti-immunomodulatory effects,have not yet been well explained and further focused studies are required to explore these properties to establish an optimized conventional drug therapy for cardioprotection [13].Several reports have been published,emphasizing that statins alone are not sufficien and the use of combination therapy withω-3-FA and statin is recommended for cardiovascular prognosis [14].Nadtochiy and Redman [15]reported a very systematic approach for cardioprotective effects of the Mediterranean diet explained,which is due to PUFA and different types of polyphenol,they possibly act through different,distinct and convergent mechanisms.These finding explain the need for a novel design for the treatment of cardiovascular disorder.Therefore,the objective of the present study was to investigate the protective effect of concomitant administration of fla lignan concentrate andω-3-FAon myocardial damage in doxorubicin-induced cardiotoxicity in Wistar rats.
2.Materials and methods
2.1.Collection and authentication of plant
Authenticated seeds ofLinum usitatissimum(Linn.) were obtained from Dr.P.B.Ghorpade,Principal,Scientist,Punjabrao Deshmukh Krushi Vidyapeeth,College of Agriculture,Nagpur,India and voucher specimen was deposited at the institute.Flaxseeds were stored in cold room before processing.Further processing for oil extraction was carried out at our Omega-3-oil unit,Sangamner,Maharashtra,India.
2.2.Drugs and chemicals
ω-3-FA mainly containing docosahexaenoic acid derived from algal sources was obtained from Martek Biosciences Corporation,Columbia,USA.Doxorubicin(Dox)was received as a gift sample from Serum Institute of India,Pune,India.Absolute alcohol(Changshu Yangyuan Chemicals,China)was purchased from respective vendors.n-Hexane,hydrochloric acid,sodium hydroxide and sodium chloride of analytical grade were purchased from Qualigene fine-chem Ltd.,Mumbai,India.Rat tumor necrotic factor-α kit(Thermo Scientific Rat TNF-α kit,Pierce Biotech Int.,Rockford,IL,USA),was purchased from respective vendors.Triglycerides (TG),total cholesterol (TC)and high density lipoprotein(HDL-C)kits ware purchased from Accurex Biomedical Pvt.Ltd.,India.Epinephrine hydrochloride,super oxide dismutase(SOD)and malondialdehyde(MDA)were purchased from Sigma Chemical Co.,USA.Reduced glutathione (GSH),5,5′-dithiobis (2-nitro benzoic acid) (DTNB)and thiobarbituric acid (TBA) were obtained from Himedia,India.
2.3.Preparation of flax lignan concentrate(FLC)
Preparation of fla lignan concentrate was carried out as described previously [3].Briefl,the flaxsee were subjected for oil extraction by double cold pressed technique which was carried out at Indian Council of Agricultural Research under National Agriculture Innovation Project,Omega-3-oil unit,Sangamner,Maharashtra,India.In order to remove more residual lipids from the matrix use of non-polar organic solvent such as hexane in a soxhlet apparatus was used,as they might interfere with the analysis of lignans.In order to liberate SDG from its polymeric lignan precursor by breaking the esterlinkages present in the complex,direct alkaline hydrolysis was preferred,hence defatted flaxsee cake was then hydrolyzed with 1 M aqueous sodium hydroxide for 1 h at room temperature with intermittent shaking,followed by extraction with 50%ethanol.In order to prevent the ionization of any functional groups in the aliphatic and aromatic part of the SDG molecule,the filtrat was acidifie to pH 3 using 1 N hydrochloric acid.The filtrat was concentrated in rotary evaporator.An additional benefi of this process is the destruction of the cyanogenic glycosides yielding an extract free of cyanogenic glycosides.The yield of FLC was 14% (w/w).The powdered lignan rich extract was dissolved in distilled water to prepare the different concentrations of FLC and used for pharmacological studies.Fresh drug solution was prepared from each day.
2.4.Characterization and identification of active compound in FLC
Identificatio and quantificatio of SDG lignan content in FLC was carried out by using high pressure thin layer chromatography.SDG lignan content in FLC was found to be 40 mg/g[3].Preliminary phytochemical analysis of FLCshowed the presence of flvonoids and phenolics[16].In acute oral toxicity studies,no mortality was observed in mice up to the dose of 5000 mg/kg[3].
2.5.Research protocol approval
The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) constituted in accordance with the rules and guidelines of the Committee for the Purpose of Control and Supervision on Experimental Animals(CPCSEA),India (Approved protocol no-CPCSEA/2011/53;institutional approval no-CPCSEA/1999/100).
2.6.Experimental animals
Male Wistar rats weighing between 200 and 220 g were purchased from National Institute of Bioscience,Pune,India.The animals were housed at an ambient temperature (25±2)°C and relative humidity(50±2)%and light and dark cycle(12 h light/dark).The animals had access to pellet diet (Chakan oil mills,Pune)and waterad libitum.
2.7.Selection of dose of flax lignan concentrate
Dose of FLC in present study was selected as reported previously[3,16].
2.8.Selection of dose of ω-3-FA
The study was carried out using three doses ofω-3-FAi.e.0.5 L,1 mL/kg and 2 mL/kg.As judged by the hemodynamic parameters,0.5 mL/kg had no significan action and 1 L,2 mL/kg gave same results,therefore 1 mL/kg was selected for present the study.
2.9.Experimental design and protocol
After 2 weeks of an acclimatization period,animals were randomly divided into fie groups.Taking into account the high toxicity of doxorubicin and possible higher mortality,more number of animals were assigned to these groups.Each group contained a different number of animals.Animals received FLC orω-3-FA orally and doxorubicin by the intrapritoneally(i.p.)route as indicated below:
Group I:Control group,animals received distilled water orally as a vehicle for 7 weeks and normal saline(i.p.)on the 7th,14th,21st and 28th day(n=6).
Group II:Dox alone group,animals received distilled water orally for 7 weeks and Dox (4 mg/kg,i.p.) on the 7th,14th,21st and 28th day in normal saline[17](n=12).
Group III:Dox+FLC group,animals received FLC(500 mg/kg,p.o.) dissolved in distilled water for 7 weeks and Dox (4 mg/kg,i.p.) on the 7th,14th,21st and 28th day in normal saline(n=9).
Group IV:Dox+ω-3-FA group,animals receivedω-3-FA(1 mL/kg,p.o.)for 7 weeks and Dox(4 mg/kg,i.p.)on the 7th,14th,21st and 28th day in normal saline(n=9).
Group V:Dox+FLC+ω-3-FA group,animals receivedω-3-FA(1 mL/kg,p.o.)+FLC(500 mg/kg)for 7 weeks and Dox(4 mg/kg,i.p.)on 7th,14th,21st and 28th day in normal saline(n=9).
2.10.Survival study
Mortality and general conditions of the animals were observed daily throughout the experimental period.
2.11.Tumor necrotic factor-α level(TNF-α)by enzyme-linked immunosorbent assay(ELISA)
Myocardial tissue homogenate levels of TNF-α were determined by using an enzyme-linked immunosorbent assay according to the manufacturer’s instructions.TNF-α level was determined from a standard curve.The concentrations were expressed as pg/mL.
2.12.Hemodynamic parameters and left ventricular contractile function
At the end of the study period,animals were anaesthetized by urethane(1.25 g/kg,i.p.).The right carotid artery of rat was cannulated for the measurement of systolic blood pressure(SBP),diastolic blood pressure (DBP) and mean aterial blood pressure (MABP).The polyethylene cannula (PE 50) was fille with heparinized saline and connected to pressure transducer.The cannula was connected to a transducer and the signal was amplifie by means of a bioamplifie.The hemodynamic parameters were recorded by eight channel power lab(AD Instruments Pty.Ltd.,Australia),having LABCHART-6 pro software.Left ventricular systolic pressure was measured by means of a Millar mikro-tip transducer catheter (Model SRP-320,Millar instrument,Texas)inserted into the left ventricleviathe right carotid artery and connected to a bioamplifie.dP/dtmaxand left ventricular end diastolic pressure signals were obtained from primary signals (left ventricular systolic pressure and blood pressure)by means of an acquisition data system (AD Instruments Pty.Ltd.with LABCHART 6 Pro software) as standardized in our laboratory[18].
2.13.Assessment of electrocardiographic(ECG)abnormalities
For the measurement of electrocardiogram the leads were placed on the right foreleg (negative electrode),left foreleg(positive electrode),and right hind leg(neutral electrode).Electrocardiographic changes and heart rate (HR) were recorded using 8 channels Power Lab System (AD Instruments Pty.Ltd.,Australia) with LABCHART 6 Pro software.To study doxorubicin-induced cardiotoxicity ST and QT intervals were determined as standardized in our laboratory[18].
2.14.Myocardial endogenous antioxidant enzymes
A portion of myocardial tissue was individually homogenized in 10% ice cold Tris-hydrochloride buffer (10 mmol/L,pH 7.4) in tissue homogenizer (Remi,India) and centrifuged at 7500 r/min for 15 min at 0°C.The clear supernatant was collected after centrifugation and used for the assays of endogenous antioxidant enzymes.Superoxide dismutase (SOD) was determined by the method of Misra and Fridovich[19].Glutathione(GSH)was determined by the method of Moron et al.[20].Lipid peroxidation(MDA)formation was estimated by the method of Slater and Sawyer[21].Total proteins were determined by the method of Lowry et al.[22].
2.15.Lipid profile
The serum total cholesterol,triglycerides and HDLcholesterol were quantifie using enzymatic kits (Accurex Biomedical Pvt.Ltd.,India) as per manufacturer’s instruction manual.Very low density lipoprotein (VLDL) was calculated using Frieldwann’s equation[23].
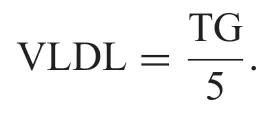
2.16.DNA extraction and gel electrophoresis
DNA was extracted from heart tissue using the phenolchloroform C-TAB method by using DNA isolation kit(Bangalore genei,India).10 μg of DNA was loaded onto 1%agarose gel and DNA electrophoresis carried out at 50-100 V for 1-2 h.The gel was stained with ethidium bromide 0.5 mg/mL and examined under an ultraviolet transilluminator.The image of the gel was captured using a gel documentation instrument.
2.17.Histopathology of heart
After recording of hemodynamic parameters the animals were euthanized,heart was removed and placed in 10%formalin solution.The organ specimens were subjected to dehydration with xylene (1 h each) and alcohol of strength 70%,90% and 100%respectively,each for 1 h.The infiltratio and impregnation was carried out by treatment with paraffi wax twice for each time for 1 h.Parrafi wax was used to prepare paraffi L molds.Specimens were cut into sections of 3-5 μm thickness and stained with hematoxylin and eosin(H and E).The sections were mounted by diestrene phthalate xylene.The parameters of histopathology assessment of heart sections were myocardial atrophy,nuclear pyknosis,cytoplasmic eosinophilia and inflam mation.The grading system used for assessment of parameter was[--:absence of change,+:0%-30%area shows changes,++:30%-60%area shows changes,and+++:60%-100%area shows changes]as described previously[3].
2.18.Ultrastructural changes in rat heart in doxorubicin induce cardiotoxicity by electron microscopy
Myocardial tissue samples were fi ed in 2.5%-3% gluteraldehyde in 0.1 mol/L phosphate buffer(pH 7.2)for 24 h at 4°C and post fi ed in 2% aqueous osmium tetraoxidein the same buffer for 2 h.Dehydrated in series of graded alcohols,infiltrate and embedded in araldite 6005 resin or spur resin [24].Ultra thin(50-70 nm)sections were made with a glass knife of ultra microtome (Leica Ultra cut UCT-GA-D/E-1/00),mounted on copper grids and stained with saturated aqueous uranyl acetate and counter stained with Reynolds lead citrate.Viewed under electron microscope(Hitachi,H-7500,Japan)at required magnificatio (12,000×) as per the standard procedures at Ruska labs,College of Veterinary Sciences,SVVU,Hyderabad,India[25].
2.19.Statistical analysis
Data were expressed as the mean±S.E.M.Statistical analysis was carried out by one-way ANOVA followed bypost hocBonferroni test using graphpad prism 5.00 for Windows 7,GraphPad Software,San Diego,CA,USA,www.graphpad.com.ThePvalue was considered significan when <0.05.
3.Results
3.1.General observations and mortality
Within 3 weeks after the Dox administration,animals in the Dox and Dox+FLC groups developed scruffy,yellowish fur around the eyes.Animals in the Dox group appeared sicker and more lethargic than the Dox+FLC group.However these symptoms were less prominent in Dox+ω-3-FA and concomitant treatment with FLC andω-3-FA group did not show any of these changes.After 3 weeks of treatment,the Dox and Dox+FLC treated groups of animals had enlarged abdomens.In case of Dox+ω-3-FA and concomitant administration ofω-3-FA and FLC groups only slightly distended abdomen were observed.The mortality rate was highest in the Dox alone group (50%).There was 22%mortality inω-3-FA treated and 33%mortality in FLC treated group(Fig.1).All animals in the control group and concomitant administration ofω-3-FA and FLC groups were survived.Heart weight/body weight ratio was decreased in Dox treated group.Table 1 illustrates body weight and heart weight in control and DOX-treated rats.After 4 weeks ofinjections,Dox-treated rats failed to maintain weight gains as observed in control rats(data not shown).
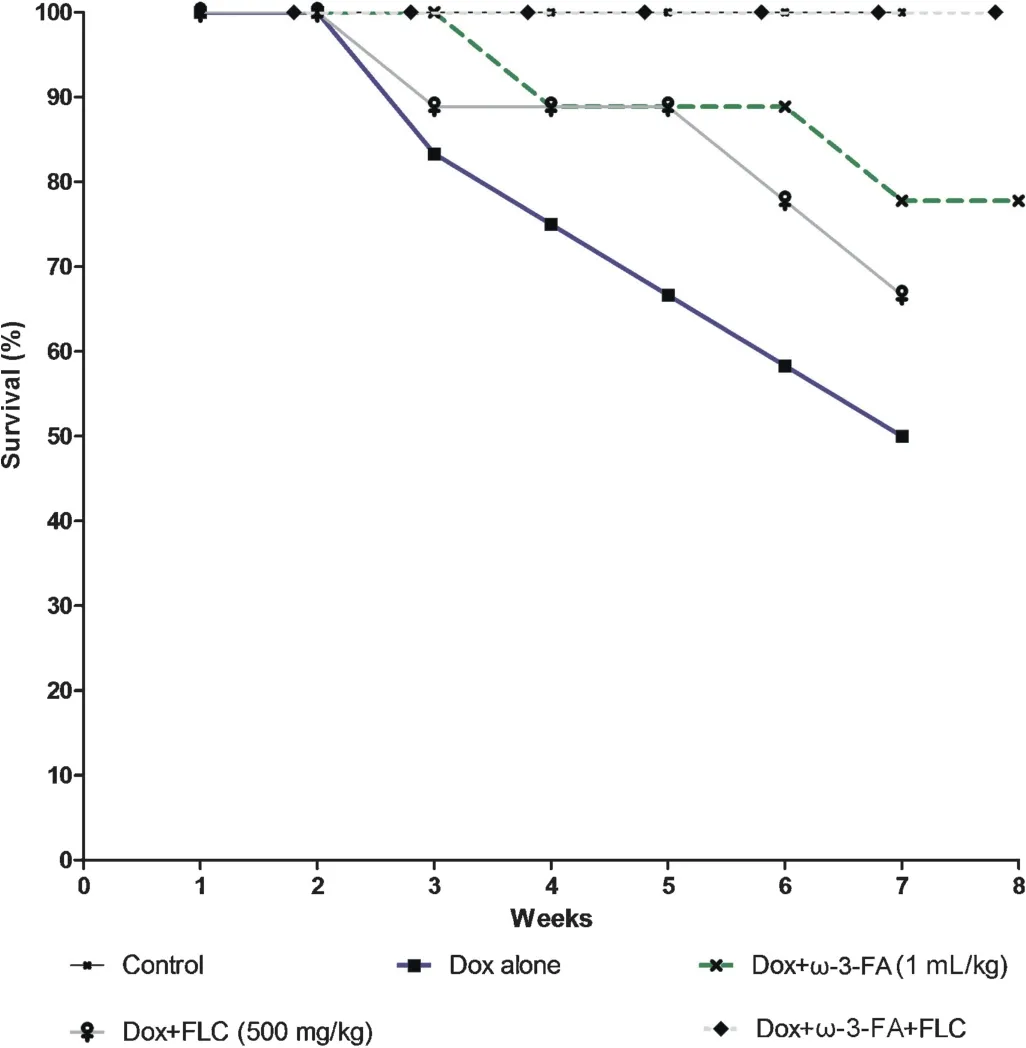
Fig.1.Effect of doxorubicin on survival rate after pretreatment with FLC,ω-3-FA and FLC+ω-3-FA.
3.2.Tumor necrotic factor-α level(TNF-α)by ELISA
TNF-α level in myocardial tissue homogenate level was significantl increased (P<0.001) in Dox alone group as compared to control group,whereas in all pretreated groups TNF-α was significantl decreased as compared to Dox alone group(P<0.001),however maximum significan decrease was observed in case of Dox+FLC+ω-3-FA group(Table 1).
3.3.Hemodynamic parameters and left ventricular contractile function
There was significan (P<0.001) alterations of the basal hemodynamic parameters such as SBP,DBP,MABP,LVSP anddP/dtmaxin animals receiving doxorubicin alone.SBP was significantl lowered (P<0.01) in Dox+ω-3-FA and Dox+FLC+ω-3-FA treated groups compared to Dox alone group,DBP was significantl (P<0.001)lowered in Dox+FLC treated group and also (P<0.01) in Dox+FLC+ω-3-FA and Dox+ω-3-FA treated groups.In case of MABP,significan lowering was observed only in Dox+FLC+ω-3-FA treated group compared to Dox alone group (P<0.05).Similar results were observed in case ofdP/dtmax.LVSP was significantl lowered in all treated group compared to Dox alone group (P<0.001).Considering hemodynamic parameters and left ventricular contractile function maximum significan decrease was observed in case of Dox+FLC+ω-3-FA treated group than that FLC orω-3-FA alone group.Non-significan changes were observed in case of LVEDP in all groups(Fig.2A-F).
3.4.Electrocardiographic evaluations
Dox alone treated group showed significan (P<0.001)prolongation of ST interval and QT interval of the ECG compared to control group.On other hand,Dox+ω-3-FA and Dox+FLC treated significantl (P<0.01) prevented changes in QT and Dox+ω-3-FA and Dox+FLC treated significantl (P<0.05)prevented changes in ST interval induced by doxorubicin,whereas in animals which received concomitant administration ofω-3-FA+FLC showed maximum significan (P<0.001)effect in doxorubicin-induced prolongation of the QT and ST interval although not to normal.There were no significan changes in heart rate in all groups except in concomitant administration ofω-3-FA and FLC compared to Dox alone group(Fig.2G and H).
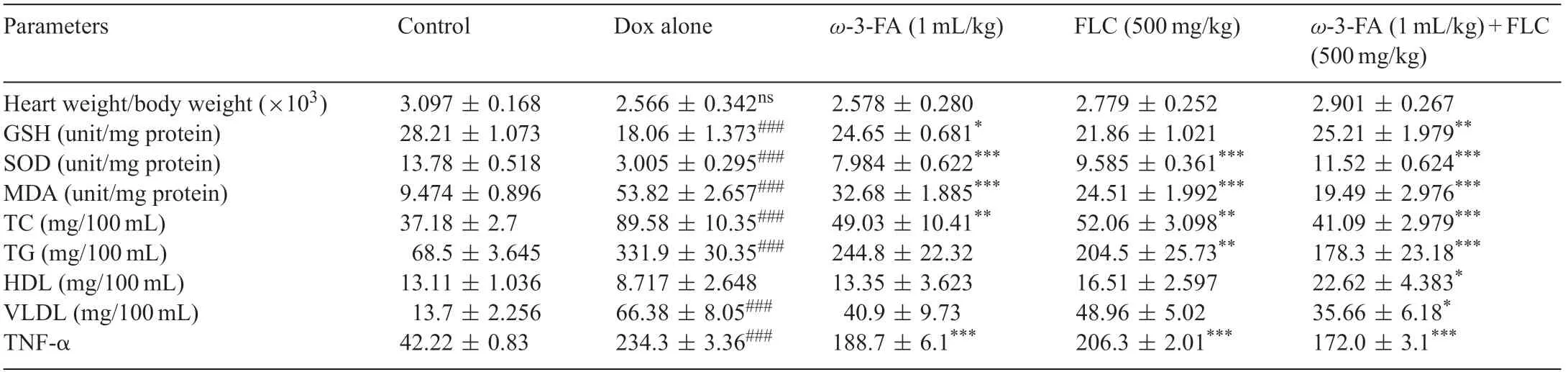
Table 1 Effect of FLC,ω-3-FA and FLC+ω-3-FA in doxorubicin induced cardiotoxicity on heart weight/body weight ratio,mortality rate,endogenous antioxidant enzymes,lipid profil and TNF-α levels.
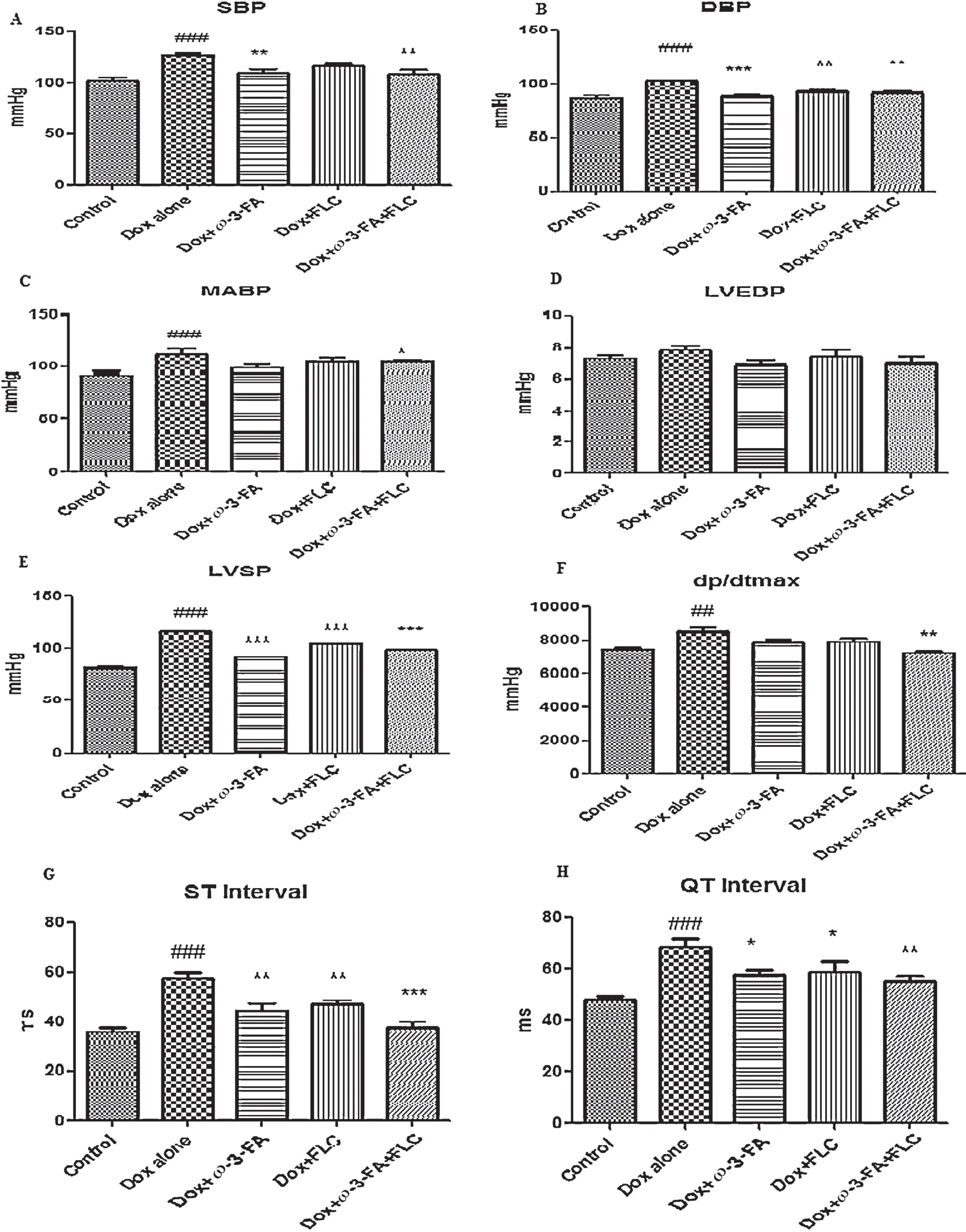
Fig.2.Effect of doxorubicin on electrocardiographic abnormalities,hemodynamic changes and LV contractile function after pretreatment with FLC,ω-3-FA and FLC+ω-3-FA.
3.5.Myocardial endogenous antioxidant enzymes
Cardiac content of GSHwas reduced significantl (P<0.001)in the Dox alone group as compared to control group.Animals receiving concomitant pretreatment of FLC+ω-3-FA showed significantl (P<0.001) higher levels of GSH and Dox+ω-3-FA also showed significantl higher(P<0.05)levels of GSH as compared to Dox alone treated animals.However,GSH level in the Dox+FLC group was non-significantl lowered than that of control group.Cardiac levels of MDA contents were increased significantl (P<0.001) in the Dox alone treated group compared with control group.It was observed that pretreatment with FLC,ω-3-FA and concomitant administration of FLC andω-3-FA significantl reduced the levels.Similarly in case SOD Dox alone group showed significan decrease(P<0.001)as compared to control group and concomitant administration of FLC+ω-3-FA showed maximum significan increase(P<0.001)than that of alone treatment(Table 1).
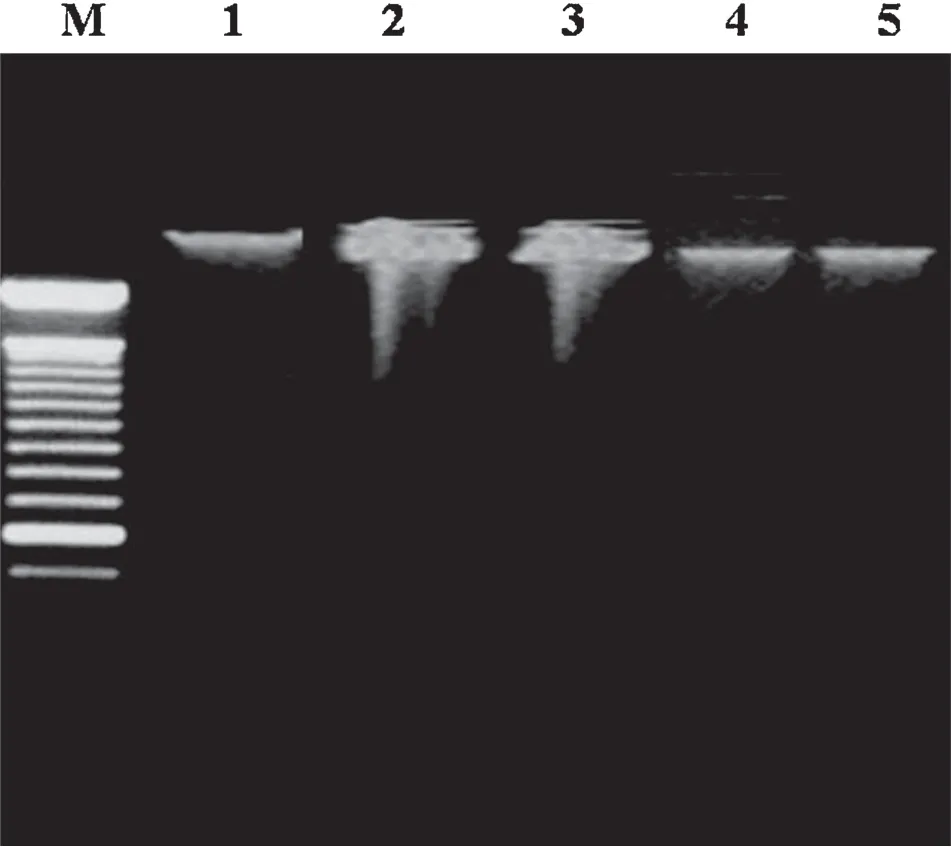
Fig.3.Effect of doxorubicin on DNA fragmentation after pretreatment with FLC, ω-3-FA and FLC+ω-3-FA.M:marker,Lane 1:Control group,Lane 2:Dox alone group,Lane 3:Dox+FLC group,Lane 4:Dox+ω-3-FA group,and Lane 5:Dox+FLC+ω-3-FA group.
3.6.Lipid profile
Serum triglycerides,total cholesterol,high-density lipoproteins and very low-density lipoproteins levels were analyzed in all groups.Dox alone treatment caused significan (P<0.001)increase in the serum triglycerides,total cholesterol,low density lipoproteins and decrease in high-density lipoproteins nonsignificantl.Although there was a trend toward a decrease in all lipids in the all groups,the prominent change was signifi cant only in the case of the concomitant administration of FLC andω-3-FA in which total cholesterol was decreased significantly (P<0.001) and TG and VLDL levels also decreased significantl (P<0.05).Total cholesterol levels were signifi cantly decreased (P<0.01) inω-3-FA and FLC treated group.Total triglyceride levels were significantl decreased(P<0.01)in FLC treated group.VLDL levels were significantl decreased(P<0.05)only in case of concomitant administration of FLCandω-3-FA group(Table 1).
3.7.DNA extraction by gel electrophoresis
DNA fragmentation in heart tissue was analyzed with agarose-gel electrophoresis.Dox alone treated heart showed large amount degradation of DNA (Fig.3,lane 2).However,Dox+FLC and Dox+ω-3-FA treated hearts had reduced amount of DNA degradation(Fig.3,lanes 3 and 4,respectively),whereas concomitant administration of FLC andω-3-FA+Dox heart samples showed very little of DNA degradation (Fig.3,lane 5).
3.8.Histopathology of heart tissue
Fig.4 illustrates the section of heart tissues in control rat(A),in rat that received Dox alone (B),in rat that received Dox+ω-3-FA (C),in rat that received Dox+FLC (D),in rat that received Dox+FLC+ω-3-FA (E).The histopathology of the heart of control group showed absence of myocardial atrophy,nuclear pyknosis and inflammatio (graded as-:absence of changes).Doxorubicin administration in the control group showed myocardial atrophy(+++),nuclear pyknosis(++),cytoplasmic eosinophilia(++)and inflammatio (++)which means that 30%-60% area of the rat heart shown inflammatio and pyknosis and myocardial atrophy in large areas of heart in rats.On the other hand,myocardial atrophy (++),nuclear pyknosis(+),cytoplasmic eosinophilia (+) and inflammatio (+) which means that 30%-60% area of the rat heart was inflammed pyknosis and myocardial atrophy occurred in 30%-60% area of heart in case of Dox+ω-3-FA treated group,similar results were observed in case of Dox+FLC treated group.Whereas,myocardial atrophy(+)was observed in 30%area of heart and nuclear pyknosis(-),cytoplasmic eosinophilia(-)and inflam mation (-) were absent in Dox+FLC++ω-3-FA.The results of histopathological analysis thus indicated that the rat heart did get partially protected by pre-treatment of concomitant administration withω-3-FA and FLC against the doxorubicin induced cardiotoxicity.
3.9.Ultrastructural changes in rat heart in doxorubicin induced cardiotoxicity by electron microscopy
Electron microscopy showed no cardiac injury in control group animals,as no mitochondrial damage was observed;dense chromatin,prominent nucleus and nucleolus were observed(Fig.4A1).The heart muscle samples from Dox alone group showed characteristic features of cardiotoxicity which manifested in the form ofintracellular vacuolization,degeneration of mitochondria,margnisation of chromatin and perinuclear membrane dilation (Fig.4B1).Dox+ω-3-FA and Dox+FLC group showed moderate changes as streaky vacuolization,partial loss of architecture,moderate perinuclear dilatation and moderate disruption of chromatin material was observed(Figs.3D1 and 4C1),whereas in case of Dox+FLC+ω-3-FA group,showed chromatin,preserved intramuscular bundle and moderately less perinuclear space was observed(Fig.4E1).
4.Discussion
In the present study,with 16 mg/kg cumulative dose ofi.p.administration of the Dox,the sign of systemic and severe cardiac toxicity could be observed.Fifty percent of the animals died before termination of the experiment in the doxorubicin alone group possibly due to lethal toxicity,Jensen et al.[26]has stated that after administration of doxorubicin,deaths may be due to some degree of bone marrow depression induced even at a small total dose of doxorubicin[26,27].In heart weight to body weight ratio,linear weight reduction was observed after 3rd week which may be attributed to decrease in food intake,resulting in both heart weight and body weight loss.Similar results have been reported by other researchers in doxorubicin induced cardiotoxicity[28].
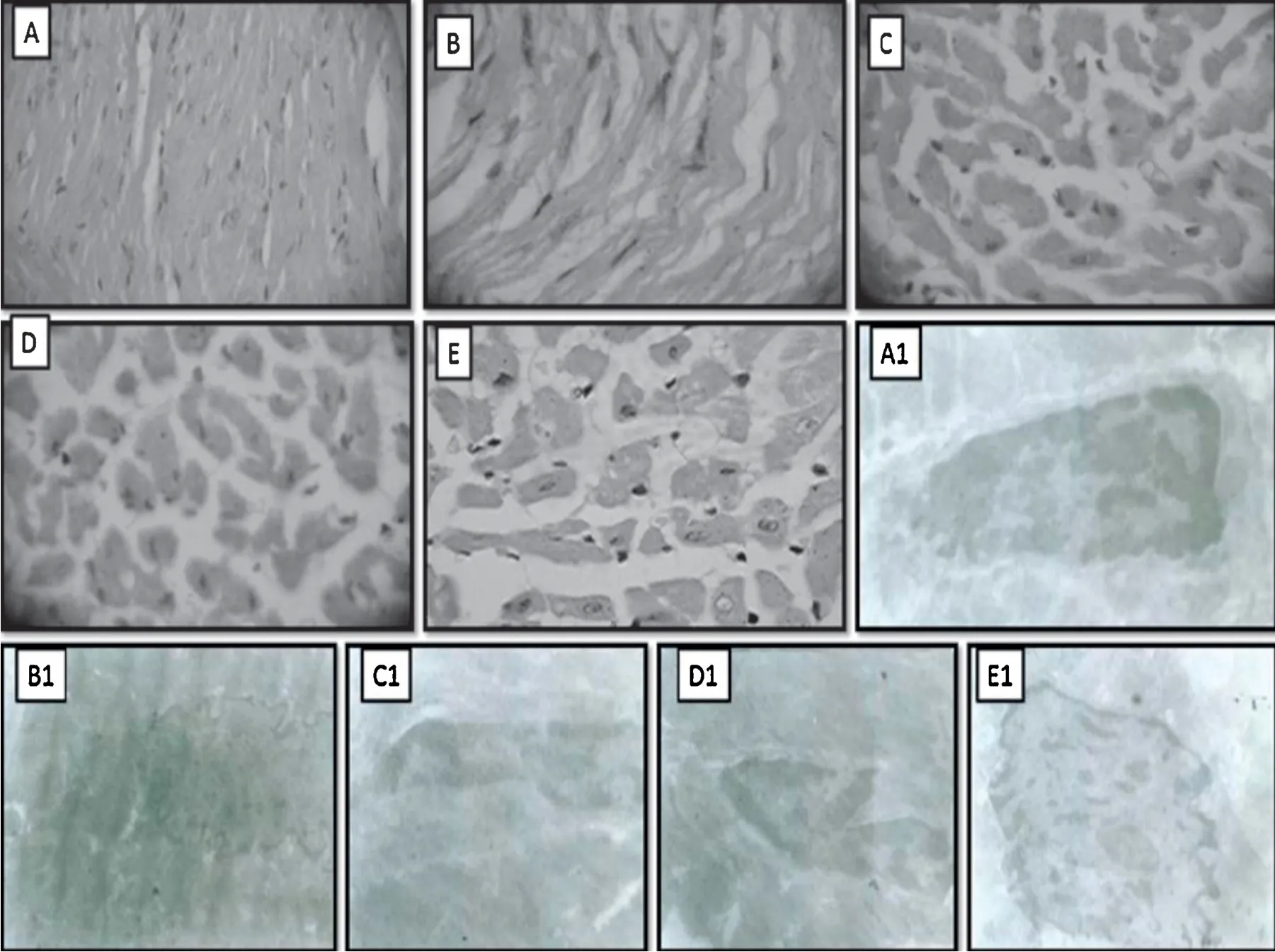
Fig.4.Effect of doxorubicin on histological changes of rat heart at 40×after pretreatment with FLC,ω-3-FA and FLC+ω-3-FA.Light electron microscopy:(A)Control group,(B)Dox alone group,(C)Dox+FLC group,(D)Dox+ω-3-FA group,and(E)Dox+FLC+ω-3-FA group;Transmission electron microscopy:(A1)Control group,(B1)Dox alone group,(C1)Dox+FLC group,(D1)Dox+ω-3-FA group,and(E1)Dox+FLC+ω-3-FA group.
TNF-α is a sensitive anti-inflammator biomarker for detecting myocardial damages in doxorubicin induced cardiotoxicity[29].Our finding showed restoration of TNF-α level in FLC alone andω-3-FA alone and concomitant administration of FLC andω-3-FA against Dox-induced cardiotoxicity was observed.
Cardiac function protective effects of Dox+FLC alone and Dox+ω-3-FA alone and concomitant administration of FLC andω-3-FA against Dox-induced cardiotoxicity were observed in our study.The values of LVSP,+dP/dtmax,SBP,DBP,and MABP increased in the Dox group which was partially restored in these treated groups.Dox+ω-3-FA alone was more effective than Dox+FLC alone in Dox induced cardiotoxicity.Concomitant administration of FLC andω-3-FA had slight additive effect on cardiac function damage induced by Dox and can improve cardiac function significantl.
The lengthening of the QT interval in ECG is regarded as an appropriate sign of myocardial injury induced by chronic treatment of doxorubicin in rats[26].Similar results were observed in animal treated with doxorubicin alone when compared to control group.This indicates a protective effect of concomitant administration of FLC andω-3-FA in doxorubicin-induced cardiotoxicity.Non-significan changes were observed with respect to heart rate.
Oxidative stress is a major risk factor in Dox-induced cardiotoxicity[8],hence it was essential to investigate the oxidant/antioxidant status of the rats.In the present investigation MDA was significantl elevated while GSH and SOD were significantl reduced in Dox alone treated group compared to control group indicating an overt oxidative stress.These data are in accordance with previous investigators[30].In proceeding work,we reported cardioprotective activity of FLC against isoprenalin induced cardiotoxicity due to antioxidant activity of FLC.The major chemical constituents present in the FLC(SDG and flvonoids alone or synergistically) may contribute to the antioxidant activity[3,4].Pretreatment of rats with FLC alone,ω-3-FA alone and concomitant administration of FLC andω-3-FA reduced the oxidative stress produced by Dox.
Increased serum levels of triglycerides,cholesterol,high density lipoproteins and very low density lipoprotein were observed in Dox alone group which indicated that doxorubicin may interfere with metabolism or biosynthesis of lipid.Similar observations were also reported by[9,31].Pretreatment of rats with FLC alone,ω-3-FA alone and concomitant administration of FLC andω-3-FA significantl prevented the myocardial alterations and normalized the serum lipid profile The protective effect exerted by FLC andω-3-FA can be attributed to the combination of antioxidant and lipid lowering actions of these agents independently.The antiatherosclerotic[2]and antioxidant[3,4]activities of FLC containing SDG have been reported.Holub[32]reported cholesterol and triglyceride lowering effect ofω-3-FA containing algal DHA.
Although the mechanism for Dox-induced apoptosis is unclear.It is observed that Dox strongly increased reactive oxygen species (ROS),a known trigger of apoptosis [33].We observed Dox-treated heart samples higher degree of DNA degradation which is an indication of oxidative stress and fragmentation of chromosome possibly leading to apoptosis.However,Dox+FLC,Dox+ω-3-FA treated hearts had reduced amount of DNA degradation,whereas concomitant administration of FLC andω-3-FA+Dox-treated heart samples showed less degree of DNA degradation.
Administration of Dox produced severe changes in myocardium with respect to myocardial atrophy,nuclear pyknosis,cytoplasmic eosinophilia and inflammatio which confirm cardiotoxicity in rats.These changes are previously reported by other researchers[34].Pretreatment of rats with FLC alone andω-3-FA alone exhibited comparatively less degree of damage compared to Dox alone group whereas in case of concomitant administration of FLC andω-3-FA showed partial protection.Further transmission electron microscopic studies provided credence to the cardioprotective effect.Restoration of vacuolization and reduction ofintracellular damage in case of concomitant administration of FLC andω-3-FA indicated protection in terms ofintegrity of the subcellular structure in doxorubicin-treated rat hearts.
Micallef and Garg [35],showed cardioprotective benefit of combinedω-3-PUFA and plant sterol supplementation in hyperlipidemic individuals,whereinω-3-PUFA and plant sterols as lipid-lowering agents provided greater risk reduction compared to either of the supplements alone.They used non-pharmacological approach which showed lipid-lowering benefits down regulation of markers of systemic inflammatio and reduction in overall cardiovascular risk.In present study,pharmacological approach was used by oral administration of test substances(ω-3-FA and FLC).
5.Conclusion
In conclusion,the present finding demonstrated that,concomitant administration of FLC (500 mg/kg) andω-3-FA(1 mL/kg)lowered TNF-α level,normalized ST,QT and MABP,elevation in GSH and MDA,SOD lowered and normalized lipid profile The treatment provided protection from DNA degradation at cellular level.Histopathology of heart tissue (both light and electron microscopical)confirme the cardioprotective effect.The concomitant treatment reduced the mortality due to Dox administration.Thus antioxidant,antihyperlipidemic,antiinflammator and anti-apoptotic actions seem to be the probable mechanisms in doxorubicin induced cardiotoxicity.Hence,it can be concluded that FLC andω-3-FA both have distinct mechanism for cardioprotection and the additive effect was observed in the present study due to concomitant administration of FLC andω-3-FA.These data suggest a possible usefulness combination of FLC andω-3-FA as a cardioprotective agent.The study reveals the role of fla lignan concentrate andω-3-FA in preventing doxorubicin-induced cardiotoxicity.Additional clinical trials using this combination to further extrapolate these experimental results are necessary,also more molecular studies to reveal the underlying mechanisms in this direction is warranted.
Acknowledgements
The authors would like to acknowledge Dr.S.S.Kadam,Vice-Chancellor and Dr.K.R.Mahadik,Principal,Poona College of Pharmacy,Bharati Vidyapeeth University,Pune,India,for providing necessary facilities to carry out the study.The research work was carried out as a part of Indian Council for Agriculture Research sponsored project under National Agriculture Innovation Project entitled“A value chain on Linseed-processing and value addition for profitabilit (grant no-F.No.NAIP(SRLS-S)II)”.Authors are thankful to Serum Institute of India,Pune,India for providing Doxorubicin as a gift sample and also thanks to Dr.Laxman,Ruska Labs,College of Veterinary Sciences,SVVU,Hyderabad,India for analyzing and interpretation of electron microscopy results.
- 食品科学与人类健康(英文)的其它文章
- Black tea in chemo-prevention of cancer and other human diseases
- Changes in physicochemical properties of proteins in Kayserian Pastirma made from the M.semimembranosus muscle of cows during traditional processing
- Polyphenolic extract of Sorghum bicolor grains enhances reactive oxygen species detoxificatio in N-nitrosodiethylamine-treated rats
- Epigenetic origins of metabolic disease:The impact of the maternal condition to the offspring epigenome and later health consequences
- GUIDE FOR AUTHORS
- Natural products for cancer prevention associated with Nrf2-ARE pathway

