Anatomical and chemical characteristics associated with lodging resistance in wheat
Eryn Kong,Dongheng Liu,Xioli Guo,Wenlong Yng,Jizhu Sun,Xin Li, Kehui Zhn,Dngqun Cui,Jinxing Lin,Aimin Zhng,,*
aThe State Key laboratory of Plant Cell and Chromosome Engineering,Institute of Genetics and Developmental Biology, Chinese Academy of Sciences(CAS),Beijing 100101,China
bThe Collaborative Innovation Center for Grain Crops in Henan,College of Agronomy,Henan Agricultural University, Zhengzhou 450002,Henan,China
cCollege of Biology,China Agricultural University,Beijing 100193,China
dKey Laboratory of Plant Photosynthesis and Environmental Molecular Physiology,Institute of Botany,CAS,Beijing 100093,China
Anatomical and chemical characteristics associated with lodging resistance in wheat
Eryan Konga,Dongcheng Liua,Xiaoli Guoc,Wenlong Yanga,Jiazhu Suna,Xin Lia, Kehui Zhanb,Dangqun Cuib,Jinxing Lind,Aimin Zhanga,b,*
aThe State Key laboratory of Plant Cell and Chromosome Engineering,Institute of Genetics and Developmental Biology, Chinese Academy of Sciences(CAS),Beijing 100101,China
bThe Collaborative Innovation Center for Grain Crops in Henan,College of Agronomy,Henan Agricultural University, Zhengzhou 450002,Henan,China
cCollege of Biology,China Agricultural University,Beijing 100193,China
dKey Laboratory of Plant Photosynthesis and Environmental Molecular Physiology,Institute of Botany,CAS,Beijing 100093,China
A R T I C L E I N F O
Article history:
Received 4 April 2013
Received in revised form 7 June 2013
Accepted 18 July 2013
Available online 26 July 2013
Molecular marker
Anatomical and chemical characteristics of stems affect lodging in wheat(Triticum aestivum L.)cultivars.Traits associated with lodging resistance,such as plant height,stem strength,culm wall thickness,pith diameter,and stem diameter,were extensively investigated in earlier studies.However,the solid stem trait was rarely considered.In this study,we measured a range of anatomical and chemical characteristics on solid and hollow stemmed wheat cultivars.Significant correlations were detected between resistance to lodging and several anatomical features,including width of mechanical tissue,weight of low internodes,and width of stem walls.Morphological features that gave the best indication of improved lodging resistance were increased stem width,width of mechanical tissue layer,and stem density.Multiple linear regression analysis showed that 99%of the variation in lodging resistance could be explained by the width of the mechanical tissue layer,suggesting that solid stemmed wheat has several anatomical features for increasing resistance to lodging.In addition,microsatellite markers GWM247 and GWM340 were linked to a single solid stem QTL on chromosome 3BL in a population derived from the cross Xinongshixin(solid stem)/Line 3159(hollow stem).These markers should be valuable in breeding wheat for solid stem.
©2013 Production and hosting by Elsevier B.V.on behalf of Crop Science Society of China and Institute of Crop Science,CAAS
1.Introduction
Lodging in cereal crops causes significant economic losses associated with reduced yields,quality,and harvesting efficiency.Previous studies showed that lodging resistance was significantly correlated with some morphological and chemical characteristics[1–5].
Solid stemmed wheat(Triticum aestivum L.)has thin but very hard stems,in which the stem pith is filled with solid materials. The morphological features ofsolid stemmed wheat suggest that it could be highly resistant to lodging.It is known that solid stemmed crop plants have increased resistance to damage from sawfly larvae,as the presence of solid pith impedes larval growth and migration[6].Some wheat cultivars with high yield potential, such as Genou,Rampart,Choteau,Bynum,and Duclair,developed by Montana Agricultural Experimental Station,USA,have solid stems[7–10].
The hereditary characteristics of solid stem in durum wheat(Triticum durum Desf.,2n=4x=28)were simple,dominant,recessive or complex,depending on the manner in which studies were carried out and/or the genetic characteristics of the parental plants[11].Cook et al.[12]reported four microsatellite markers linked to Qss.msub-3BL for stem characteristics in a double haploid winter wheat population derived from a cross between‘Rampart'(solid stem)and“Jerry”(hollow stem).However,few studies have investigated the anatomical features and chemical composition of solid stemmed wheatvarieties.Suchcharacteristics are potentially importantfor stem strength at physiological and anatomical levels.
The aim of this study was to improve our understanding of the relationship between the anatomical features and chemical composition of stems in different wheat cultivars and their influence on resistance to lodging.In addition,the gene(s) controlling stem solidness was mapped based on an F2population derived from a cross between a solid stemmed variety and a hollow stemmed one.The result will be helpful for molecular marker assisted selection(MAS)for solid stemin wheat breeding.
2.Materials and methods
2.1.Plant materials
Solid stemmed wheat line Xinongshixin(XNSX),hollow stemmed Line 3159,the F1and F2populations from cross XNSX/Line 3159 and Chinese Spring(CS)were planted at Changping Experimental Station,CAS,Beijing,China.Plant samples were collected fromearly April(three-leafstage)to late June(mature stage).To evaluate stem solidness,more than 10 stems were randomly selected at post-anthesis and were cross-sectionally cut at the center of each internode.The level of pith solidness was rated on a previously established score system[12]ranging from 1 to 5(1 for hollow and 5 for solid).All samples were collected from main tillers.
2.2.Anatomical and chemical evaluation
The internodes on samples were numbered consecutively from the base to the top of the stem.Sections were cut at the center of each internode and stained with either phloroglucine-HCl or Calcoflour(Sigma)according to the procedure described in our previous study[13].The following morphological characteristics were measured and analyzed using a statistical software package attached to fluorescence microscope(Axioskop 40 with UV excitation,ZEISS),i.e.,outer and inner stem diameters, area of stem wall,radius of stem wall(RSW),width of stem wall (WOSW),area of vascular bundles(AOVB),area of transverse section(AOT),width of the mechanical tissue layer(WOMT), number of vascular bundles(NOVB),number of large and small vascular bundles(NLVB and NSVB),weight of the three lower internodes(WOL),and stem length.
Carbohydrate contents(lignin and cellulose)were assayed according to the methods described previously[13–15].Three internodes from the bottom upwards collected from stems were ground to fine powder in liquid nitrogen using a mortar and a pestle.Lignin content was assayed using the methods described by Kirk and Obst[16]and histochemical detection (the Wiesner reaction)following established protocols[17].For cellulose staining,polyethylene glycol(PEG)-embedded sections (10 μm)were treated with a 0.005%aqueous solution of Calcoflour (fluorescent brightener 28,Sigma)for 2 min and then observed with a fluorescence microscope(Axioskop 40,ZEISS).Lodging resistance was ranked according to the measured resistance of stems to pushing,which was carried out on the bottom part of the stem following Kashiwagi and Ishimaru[18].
2.3.Statistical analysis
The data were analyzed by multiple ANOVAwith 95%confidence limits using mean values measured for each genotype.The relationships between morphological characteristics and lodging resistance were revealed by linear regression analysis following the procedure for stepwise forward regression analysis described in the SPSS 11.0 software package for Windows.Lodging resistance was used as the dependent variable,while lignin, cellulose,AOVB,NOVB,AOT and WOMT were used as independent variables.
2.4.Microsatellite markers linked to gene(s)conferring stem solidness
Potential microsatellite markers linked to stem solidness genes were identified by screening the F2population using bulked segregant analysis.DNA was extracted from young leaf tissues using the CTAB method.The solid and hollow stem DNA pools were composed of 5 solid and 5 hollow stemmed F2plants,respectively.Along with the parental DNA,the bulked DNA samples were used to screen 607 SSR markers(210 GWM[19]and 397 BARC[20]).The PCR mixture (20 μL)consisted of 2.0 μL of 10×buffer,1.6 μL of Mg2+(25 mmol L−1),2.0 μL of dNTP(2 mmol L−1),2.0 μL of DNA (10–20 ng μL−1),2.0 μL of primer(2 μmol L−1),0.2 μL of Taq DNA polymerase(5 U μL−1),and 10.2 μL of ddH2O and was subjected to a thermocycler program of 94°C for 5 min; followed by 30 cycles at 94°C for 1 min,60,55,or 50°C for 1 min(depending on each primer set),and 72°C for 1 min; with a final extension at 72°C for 5 min.The PCR products were electrophoresed in 4%polyacrylamide gels and detected by silver staining[21].Marker-trait associations were identified bysingle factor ANOVA and the proportion of phenotypic variation explained by single marker loci was determined as the ratio of sum of squares for marker class divided by sum of squares of entries[12].
3.Results
3.1.Morphological characteristics and anatomical features
The characteristics of stem pith varied significantly among the four genotypes examined(Fig.1).Solid stemmed XNSX showed the greatest amount of pith material(Fig.1B),whereas CS and Line 3159 had hollow stems(Fig.1A and C),and the characteristics of F1plants were similar to the solid-stemmed parent except for the third and fourth internodes(Fig.1D).Significant differences were also detected in the anatomical characteristics of the four genotypes,especially the transverse sections of solid stemmed wheat XNSX,which had more mechanical and parenchyma tissues(Fig.2C and D)than the other three genotypes(Fig.2A,B,E and F);F1plants were almostintermediate between theirparents in the corresponding values(Fig.2Gand H).
The morphological data for the four wheat genotypes are shown in Table 1.AOT in the solid stemmed and F1plants were significantly larger than that of CS.In contrast,there were only minor differences in AOVB among the four genotypes(Table 1).The widths of stem walls in XNSX and F1were 2.7-and 2.6-fold that in CS,and WOMT values were 2.1-and 1.7-fold that in CS.Only slight differences were observed in TNVB among the four genotypes,but the WOL of XNSX and F1plants were significantly higher than those of Line 3159 and CS(Table 1).
3.2.Chemical composition and histochemical staining
The contents of cellulose and lignin showed slight differences among the four genotypes.In regard to WOSW the lignin and cellulose compositions per unit length of stem in XNSX and the F1were significantly higher than in CS and Line 3159 (Figs.3 and 4).Correspondingly,no significant differences were observed in histochemical staining among the four wheat genotypes;however,the area of the mechanical tissue in the solid stemmed variety was obviously larger than any of the other three genotypes(Fig.2A to L).
3.3.Correlation of the anatomical features and chemical composition of stems with lodging resistance
Lodging resistance of XNSX was 3.0-and 4.1-fold that of Line 3159 and CS,respectively.F1plants had lower lodging resistance than XNSX,but had 2.27-fold higher values than Line 3159(Table 1).Lodging resistance was positively correlated with WOMT(r=1.000,P<0.01),WOSW(r=0.972, P<0.05),and WOL(r=0.986,P<0.05)(Table 2).In addition, significantly positive correlations were found between WOMT and WOSW(r=0.968,P<0.05),WOMT and WOL(r=0.988, P<0.05),AOT and NOVB(r=0.955,P<0.05),AOT and NSVB (r=0.982,P<0.05),cellulose and lignin content(r=0.993,P<0.01),whereas no significant correlations were found between lodging resistance and the chemical compositions, RSW,AOVB,or AOT(Table 2).
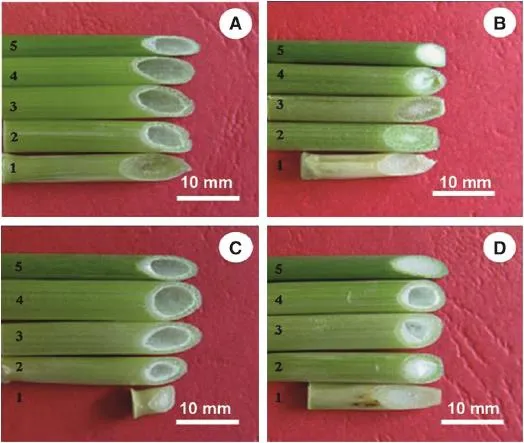
Fig.1–Morphological characteristics of different wheat genotypes.A and C:empty stems internodes of(A)CS and Line 3159(C). B and D:solid stems XNSX(B)and the F1(D).Numbers 1,2,3,4 and 5 in the figures mean the first,second,third,fourth,and fifth stem internodes(from the base to the top),respectively.
The relationships between lodging resistance and the chemical and anatomical characteristics of the four genotypes were tested using a stepwise forward regression,where lignin, cellulose,AOT,AOVB,WOMT,WOSW,RSW and WOL were used as independent variables.Each variable was added in the order of statistical significance(P<0.05).The best predictor of lodging resistance was obtained from a model incorporating WOMT,AOVB and WOSW as follows:
LR=−20.251+0.397 WOMT+5.287E−06 AOVB+0.009 WOSW

Fig.2–Anatomical and chemical characteristics of different wheat genotypes.Wiesner staining of transverse stem sections of hollow stemmed CS(A and B),solid stemmed XNSX(C and D),hollow Line 3159(E and F),and the F1(G and H),showing slight differences in lignin composition in the walls of sclerenchyma cells and vascular bundles(sections were taken from the middle of the third internodes,respectively).C and D(magnified section of C)have the highest amounts of mechanical and parenchyma tissues.Calcofluor staining of the transverse stem sections of CS(I),XNSX(J),Line 3159(K),and the F1(L),showing similar amounts of cellulose in the cell walls of sclerenchyma cells and vascular bundles.LV:large vascular bundle;SV:small vascular bundle;P:parenchyma;MT:mechanical tissue.
3.4.Marker identification
For 607 microsatellite markers,120 showed polymorphisms between XNSX and Line 3159.Among these,Xgwm340 and Xgwm247 on chromosome 3BL exhibited amplification profiles that distinguished between the solid and hollow stemmed parents in corresponding bulks,suggesting a possible association between stemsolidness and these markers(Fig.5).Subsequently, the entire F2population was genotyped for these markers. Both markers were located proximally to the solid stem locus (Xgwm340–4.0 cM–Xgwm247–12.1 cM–Solid stem QTL peak)and results from ANOVA showed that the solid stem phenotypic variance explained by the Xgwm-247 locus was about 77%,and that explained by Xgwm-340 was 62%.
4.Discussion
Lodging resistance is of importance in cereal crop breeding.It is well known that morphological characteristics significantly affect lodging resistance.As a result,morphological differences among cultivars have been studied to identify morphological and anatomical traits associated with lodging response so that they could be used to breed for lodging resistance [22,23].Previous studies showed that lodging resistance is negatively correlated with stem diameter[3,24],whereas we found that lodging resistance in the four wheat genotypes examined was positively correlated with stem wall thickness of(r=0.972,P<0.05).Other workers have also suggested that thicker stem walls increase lodging resistance in wheat[3,25]. In addition,we investigated the significance of WOMT in lodging resistance.The lignin and cellulose contents were higher in the mechanical tissue layer,where the cells around the vascular bundles are rich in lignin and cellulose[26].In our study,a strong relationship was observed between lodging resistance and WOMT(r=1.000,P<0.01),indicating thatmechanicaltissues play an importantrole in lodging resistance of wheat.Compared with hollow stemmed wheat,the solid stemmed genotype was more resistant to lodging as a result of its comparatively wider stem wall and greater amount of mechanical support tissues.
Zuber et al.[22]reported that 49.7%of the variation in lodging in wheat was explained by variation in stem weight.It is suggested that,along with plant height,stem weight and stem diameter might be helpful in developing new lodging-resistant wheat cultivars.In this study,the high correlation between WOL and lodging resistance(r=0.986,P<0.05)suggested that WOL was also an importantfactor affecting the rigidity ofwheatstems. However,WOL was not included in the model of predicting lodging resistance.This probably results from the strong correlation between WOL and WOMT(r=1.000,P<0.01).
Khanna[27]and Hamilton[28]found that the stem lodging of wheat,triticale(×Triticosecale Wittmack),rye(Secale cereale L.) and oat(Avena sativa L.)decreased in proportion to the number of vascular bundles.In contrast,Dunn and Briggs[3]found no relationship between the number of vascular bundles and lodging response in barley(Hordeum vulgare L.).Among the four wheat genotypes investigated in this study,few differences were found with respect to the number of vascular bundles,and there were no significant correlations between the presence of large or small vascular bundles and lodging response.These inconsistent results might be due to the inherent genetic differences between the genotypes used in different studies.
A layer of thick-walled,lignified sclerenchyma near the periphery of the stem and around the vascular bundles significantly increases lodging resistance[25,27,29].In our study,the correlation between lodging resistance and AOVB was not significant.In a one-variable model with WOMT,the coefficient of determination(R2)was 0.999(P<0.01).The value increased to 1.000(P<0.01)in a two-variable model with the addition of AOVB(data not shown),suggesting that AOVB might also play an important role in lodging resistance.
Wiesner staining involves the cinnamaldehyde residue of lignin,and the color intensity reflects the total lignin content. However,there was no difference in the color ofthe mechanicaltissue layer among the four wheat genotypes examined, indicating similar lignin contents.Li[30]reported that maize (Zea mays L.)hybrids with higher amounts of lignin were more prone to stalk breakage.In contrast,Hondroyianni et al.[31] found no significant correlation between lodging and stalk lignin content.The results of this study showed no significant correlation between lodging and lignin or cellulose contents. However,the solid stemmed genotype had the highest lignin content and correlations between the degree of lodging resistance and lignin(R2=0.978,P<0.01)and cellulose(R2= 0.944,P<0.05)contents were both significant.Considering the results obtained from histochemical staining,we propose that lignin and cellulose play an important role in lodging resistance. All four genotypes analyzed in this study contained high levels of both lignin and cellulose.Further research is needed to increase our understanding of the role of chemical composition in lodging resistance in a wider range of wheat cultivars.

Table 1–Morphological traits and compositions of second and third stem internodes of XNSX,Line 3159,the F1(XNSX/3159) and Chinese Spring(CS).

Fig.3–Lignin contents oflowerstemsegments fromfour wheat genotypes.Error bars were obtained from five measurements.

Fig.4–Cellulose contents of lower stem segments from four wheat genotypes.The error bars were obtained from five measurements.
Pith parenchyma plays an important role in stabilizing the stem against ovalisation and reducing the risk of local buckling and collapse[32–34].Stem stability is known to increase with the thickness of the parenchyma layer[3].The effects of wind,rain,and other environmental forces can be absorbed by the parenchyma without heating or mechanical damage[4].The available evidence suggests that pith parenchyma also plays an important role in lodging resistance.In this study,the percentage of pith varied significantly among the four genotypes.Since the solid stemmed wheat genotype had the greatest amount of pith,it was likely to be more resistant to lodging than the hollow ones.
Solid pith parenchyma also inhibits the growth and development of sawfly(Cephus cinctus Norton)larvae in wheat stems, which is important for the control of wheat stem sawfly infestations.Sawfly larvae feed on vascular tissue and parenchyma cells from the hollow regions inside the stem.When the larvae reach maturity,they migrate toward the base of stem, which at the time they eat a ring or girdle around the stem wall weakens the stem base and increases susceptibility of the plant to lodging[35].The results of this study suggest that a solid-stem wheat cultivar is less susceptible to lodging.The thick layer of mechanical tissue in the outer ring,as well as the solid pith parenchyma in the inner ring,of the solid stem increases resistance to lodging.
In a U.S.A.study four microsatellite markers on chromosome 3BL(GWM247,GWM340,GWM547 and BARC77)were linked to a single solid stem QTL,which accounted for 76%of the variation in stem solidness in wheat[12].In our study,only GWM247 and GWM340 were polymorphic and GWM247 was more closely linked to the single solid stem QTL and accounted for 77%of the variation in stem solidness.Our results were thus in agreement with the mapping results for solid stem in wheat cultivar Rampart[12],indicating that the same gene for solid stem may be presentin XNSXand Rampart.In addition,our results showed that the solidness gene is closer to Xgwm247(12.1 cM)than to Xgwm340(16.1 cM),suggesting that Xgwm247 is a better marker for selection of solid stem in developing new wheat cultivars.
In conclusion,solid stemmed wheat cultivars with relatively thin stems and large spikes could be used as parents for crossing in wheat breeding programs.In wheat,stem solidness is controlled by a single chromosome region on chromosome 3BL and two SSR markers,Xgwm247 and Xgwm340,could be used in wheat breeding for selecting solid stemmed individuals with lodging resistance.

Table 2–Correlation coefficients for lodging resistance and stem characteristics of XNSX,Line 3159,F1(XNSX/3159)and Chinese Spring(CS)used as a control.

Fig.5–Banding patterns of GWM247 and GWM340 in solid stemmed XNSX and hollow stemmed Line 3159.
Acknowledgments
We thank Dr.Zhengqing Li and Dr.Hongfei Lue,Institute of Botany,Chinese Academy of Sciences,for technical assistance. This study was supported by the National Basic Research Program of China(2011CB100302)and the Knowledge Innovation Program of CAS(KSCX2-EW-N-02).
R E F E R E N C E S
[1]P.M.Berry,M.Sterling,J.H.Spink,C.J.Baker,R. Sylvester-Bradley,S.J.Mooney,A.R.Tams,A.R.Ennos, Understanding and reducing lodging cereals,Adv.Agron. 84(2004)217–271.
[2]C.R.Duan,B.C.Wang,P.Q.Wang,D.H.Wang,S.X.Cai, Relationship between the minute structure and the lodging resistance of rice stems,Colloids Surf.B:Biointerfaces 35 (2004)155–158.
[3]G.J.Dunn,K.G.Briggs,Variation in stem anatomy among barley cultivars differing in lodging resistance,Can.J.Bot.67 (1989)1838–1843.
[4]A.Kokubo,S.Kuraishi,N.Sakurai,Stem strength of barley, Plant Physiol.91(1989)876–882.
[5]J.B.Yao,H.X.Ma,P.P.Zhang,L.J.Ren,X.M.Yang,G.C.Yao,P. Zhang,M.P.Zhou,Inheritance of stem strength and its correlations with culm morphological traits in wheat,Can.J. Plant Sci.91(2011)1065–1070.
[6]L.E.Wallace,F.H.McNeal,Stem sawflies of economic importance in grain crops in the United States,USDA Tech. Bull,1966.,(No.1350).
[7]P.L.Bruckner,J.E.Berg,G.D.Kushnak,R.N.Stougaard,J.L. Eckhoff,G.R.Carlson,D.M.Wichman,K.D.Kephart,N. Riveland,D.L.Nash,Registration of Genou wheat,Crop Sci.46 (2006)982–983.
[8]G.R.Carlson,J.E.Berg,R.N.Stougaard,K.D.Kephart,N. Riveland,G.D.Kushnak,D.M.Wichman,J.L.Eckhoff,D.L. Nash,E.S.Davis,W.E.Grey,P.L.Bruckner,Registration of‘Bynum'wheat,J.Plant Regist.1(2007)16–17.
[9]S.P.Lanning,G.R.Carlson,D.L.Nash,D.M.Wichman,K.D. Kephart,R.N.Stougaard,G.D.Kushnak,J.L.Eckhoff,W.E. Grey,L.E.Talbert,Registration of Choteau wheat,Crop Sci.44 (2004)2264–2265.
[10]S.P.Lanning,G.R.Carlson,P.F.Lamb,D.L.Nash,D.M.Wichman, K.D.Kephart,R.N.Stougaard,J.Miller,G.D.Kushnak,J.L.Eckhoff, W.E.Grey,N.K.Blake,L.E.Talbert,Registration of Duclair hard red spring wheat,J.Plant Regist.5(2011)349–352.
[11]F.R.Clarke,J.M.Clarke,R.E.Knox,Inheritance of stem solidness in eight durum wheat crosses,Can.J.Plant Sci.82 (2002)661–664.
[12]J.P.Cook,D.M.Wichman,J.M.Martin,P.L.Bruckner,L.E.Talbert, Identification of microsatellite markers associated with a stem solidness locus in wheat,Crop Sci.44(2004)1397–1402.
[13]J.Wang,J.M.Zhu,Q.Q.Lin,X.J.Li,N.J.Teng,Z.S.Li,B.Li,A.M. Zhang,J.X.Lin,Effects of stem structure and cell wall components on bending strength in wheat,Chin.Sci.Bull.51 (2006)815–823.
[14]C.Hoebler,L.D.Barry,J.Delort-Laval,Rapid hydrolysis of plant cell wall polysaccharides by gas–liquid chromatography,J.Agric.Food Chem.37(1989)360–367.
[15]D.M.Updegraff,Semimicro determination of cellulose in biological materials,Anal.Biochem.32(1969)420–424.
[16]T.K.Kirk,J.R.Obst,Lignin determination,Methods Enzymol. 161(1988)87–101.
[17]L.M.Strivastava,Histochemical studies on lignin,Tappi 49 (1966)173–183.
[18]T.Kashiwagi,K.Ishimaru,Identification and functional analysis of a locus for improvement of lodging resistance in rice,Plant Physiol.134(2004)676–683.
[19]M.S.Röder,V.Korzun,K.Wendhake,J.Plaschke,M.H.Tixier, P.Leroy,M.W.Ganal,A microsatellite map of wheat,Genetics 149(1998)2007–2023.
[20]Q.J.Song,E.W.Fickus,P.B.Cregen,Characterization of trinucleotide SSR motifs in wheat,Theor.Appl.Genet.104 (2002)286–293.
[21]J.B.Bassam,G.Caetano-Anollés,P.M.Greshhoff,Fast and sensitive silver staining of DNA in polyarcylamide gels,Anal. Biochem.196(1991)80–83.
[22]U.Zuber,H.Winzeler,M.M.Messmer,M.Keller,B.Keller,J.E. Schmid,P.Stamp,Morphological traits associated with lodging resistance of spring wheat(Triticum aestivum L.), J.Agron.Crop Sci.182(1999)17–24.
[23]M.J.Pinthus,Spread of the root system as an indicator for evaluating lodging resistance of wheat,Crop Sci.7(1967) 107–110.
[24]A.J.Norden,K.J.Frey,Factors associated with lodging resistance in oats,Agron.J.51(1970)561–568.
[25]C.A.Cenci,S.Grando,S.Ceccarelli,Stem anatomy in barley (Hordeum vulgare L.),Can.J.Bot.62(1984)2023–2027.
[26]K.Kaack,K.-U.Schwarz,P.E.Brander,Variation in morphology,anatomy and chemistry of stems of Miscanthus genotypes differing in mechanical properties,Ind.Crops Prod.17(2003)131–142.
[27]V.K.Khanna,Relationship of lodging resistance and yield to anatomical characters of stem in wheat,triticale and rye, Wheat Inf.Serv.73(1991)19–24.
[28]D.G.Hamilton,Stem,crown and root development in oats as related to lodging,Sci.Agric.31(1951)286–315.
[29]M.D.Jellum,Relationships between lodging resistance and certain stem characters in oats,Crop Sci.2(1962)263–267.
[30]M.Li,Anatomical and morphological characteristics of maize genotypes varying in resistance to brittlesnap,1997.(MS Thesis of University of Nebraska,Lincoln,USA).
[31]E.Hondroyianni,D.K.Papakosta,A.A.Gagianas,K.A. Tsatsarelis,Corn stalk traits related to lodging resistance in two soils of differing quality,Maydica 45(2000)125–133.
[32]K.L.Niklas,Bending stiffness of cylindrical plant organs with a“core rind”construction:evidence from Juncus effuses leaves,Am.J.Bot.78(1991)561–568.
[33]A.R.Ennos,The mechanics of the flower stem of the sedge Carex acutiformis,Ann.Bot.72(1993)123–127.
[34]H.C.Spatz,C.Boomgaarden,T.Speck,Contribution to biomechanics of plants:III.Experimental and theoretical studies of local buckling,Bot.Acta 106(1993)254–264.
[35]W.L.Morrill,J.W.Gabor,G.D.Kushnak,Wheat stem sawfly (Hymenoptera:Cephidae):damage and detection,J.Ecol. Entomol.85(1992)2317–2413.
*Corresponding author at:The State Key laboratory of Plant Cell and Chromosome Engineering,Institute of Genetics and Developmental Biology,Chinese Academy of Sciences(CAS),Beijing 100101,China.Tel./fax:+86 10 64806618.
E-mail address:amzhang@genetics.ac.cn(A.Zhang).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
Solid stemmed wheat
Lodging resistance
Anatomical feature
- The Crop Journal的其它文章
- Zea mays(L.)P1 locus for cob glume color identified as a post-domestication selection target with an effect on temperate maize genomes
- Expression of an(E)-β-farnesene synthase gene from Asian peppermint in tobacco affected aphid infestation
- Difference between resistant and susceptible maize to systematic colonization as revealed by DsRed-labeled Fusarium verticillioides
- Identification and fine mapping of two blast resistance genes in rice cultivar 93-11
- Zea mays(L.) P1 locus for cob glume color identified as a post-domestication selection target with an effect ontemperate maize genomes
- Identification of unconditional and conditional QTL for oil,protein and starch content in maize

