The effect of feeding experience on the carboxylesterase activity of Bemisia tabaci B biotype(Homoptera:Aleyrodidae)
XU Qi-Yun,CHAI Fang-Hua,AN Xin-Cheng*(.Guangdong Entomological Institute,Guangzhou 5060,China;.South China Botanical Garden,Chinese Academy of Science,Guangzhou 5050,China)
1 Introduction
Bemisia tabaci(Hemiptera:Aleyrodidae)has several ecological advantages to invade and adapt to external environment,including small body size,short developmental cycle and rapid reproduction rate(Byrne and Bellows,1991).As a result, B. tabacibecomesan dangerous invasive agricultural pest in the past century.This is evidenced by its high adaptation to a wide range of climates in tropical and temperate area,ability to overcome physical barriers,and rapid development of pesticide resistance to a variety of host plants(Brown,1994;Oliveira et al.,2001).Frequent trading of plant materials across international border has also exacerbated the global distribution of B.tabaci over the past 20 years except in Antarctica(Brown,1994;de Barro,2000;Boykin,2007).The polyphagous nature of B.tabaci has been documented worldwide with about 24 biotypes recorded on more than 600 host plant species such as crops,vegetables,fruit trees and ornamental plants(Secker,1998;Oliveira et al.,2001;Perring,2001;de Barro et al.,2011).It was estimated that B.tabaci caused a severe loss of over one billion U.S.dollars every year(Brown,1994).
There was several literature supporting the influence of feeding experience to the reliance of herbivorous insects to its host plant(Zang et al.,2006;An et al.,2007;Mansaray and Sundufu,2009).In our previous study,host selection and oviposition preference were observed for B.tabaci population with feeding experiences of over 10 generations(An and Ren,2009).Therefore,it is believed that B.tabaci B biotype may be able to improve their physiological adaptation on new host plants quickly through feeding experience,instead of long-term genetic change.
Carboxylesterase is recognized as a major category among the family of detoxifying enzymes in B.tabaci with wide presence in animals, plants, insects and microbes(Bornscheuer,2002;Ranson et al.,2002).It plays an important role in xenobiotic detoxification,pheromone degradation, neurogenesis and regulating development(Bornscheuer,2002).Previous studies have indicated the involvement of CarE in host utility mechanism and pesticide resistance(Gao,1992;Byrne et al.,2000;Ranson et al.,2002).In this study,carboxylesterase activity was selected to verify the influence of feeding experience on the physiological adaptation of B.tabaci B biotype,as to selected,cucumber and bitten melon selected host plants had obviously different fitness for B.tabaci.
2 Materials and Methods
2.1 Whitefly collection
About 100 adults of Bemisia tabaci were originally collected from Poinsettia,Euphorbia pulcherrima,in the greenhouse of Dongsheng Gardern,Liwan District,Guangzhou,in 2010.This population was identified as biotype B using a mitochondrial DNA COI(Cytochrome Oxidase I)marker in the laboratory.Bemisia tabaci was reared on Cucumis sativus(cucumber)and Momondica charantia(bitter melon)for 3 generations in the greenhouse of Guangdong Entomological Institute,so that two populations had different feeding background.In total 13 plant species were collected from South China Botanical Garden,Chinese Academy of Science, Tianhe district, Guangzhou,in 2011.
2.2 Host plants
Leaves from collected host plants were placed(dorsal side down)on a water-agar(1% ~3%)mixture in a Petri dish(130 mm diameter)and covered with a plastic cup with several small holes.About 30 adult B.tabaci selected at random were transferred into these plastic cups.The apparatus was inverted to allow the leaf to be placed in a natural orientation and incubated at 25℃ and 60% relative humidity. For measurement of Carboxylesterase activity about 30 head adults per host plant were collected 24 h after transfer to host plants and 3 replicates were performed for each host plant.
2.3 Carboxylesterase activities measurement
Whitefly adults collected from 13 transferred host plants were placed in liquid nitrogen for 3 h and then transferred to-80℃ for later use.Carboxylesterase(CarE)activity was measured as described by Byrne et al.(2000),with slight modifications.About 30 B.tabaci adults were cleaned in phosphate buffered saline(PBS-0.04 mol/L),pH7.0,and homogenized in 1 mL PBS in a glass homogenizer.The homogenate was then centrifuged at 10,000 rpm for 10 min at 4oC and the supernatant was used for the enzyme assay.
For the bioassay 100 μL supernatant was added to 500 μL α-naphthyl acetate containing 1x10-5mol/L physostigmine in a 96-well microtitre plate and incubated in a 30℃ water bath.After 10 min,fast blue B salt was added and incubation was continued for an additional 30 min.The reaction was terminated by addition of 200 μL stop solution each and the OD600was determined on a microplate reader(Sunrise,Tecan,Austria).Three replicates were maintained for each sample and inactivated enzyme served as the negative control.Volume of α - naphthol produced was calculated using a standard curve.
The amount of protein in each sample was measured by adding 200 μL sample to 500 μL Coomassie brilliant blue G -250.After mixing,the OD595values were measured between 2min and 1h on a microplate reader.Protein content of each sample was then calculated according to the standard curve.
2.4 Data analysis
Statistical analyses were performed using SPSS(2005).Paired sample T test was used to detect difference between bitter melon and cucumber population of B.tabaci.
3 Results
CarE activity were measured in whitefly populations fed on cucumber and bitten melon,and data showed that B.tabaci performed lower CarE activity as fed on cucumber,which was regarded as more adaptive to whitefly,and higher CarE activity on bitter melon(Table 1).
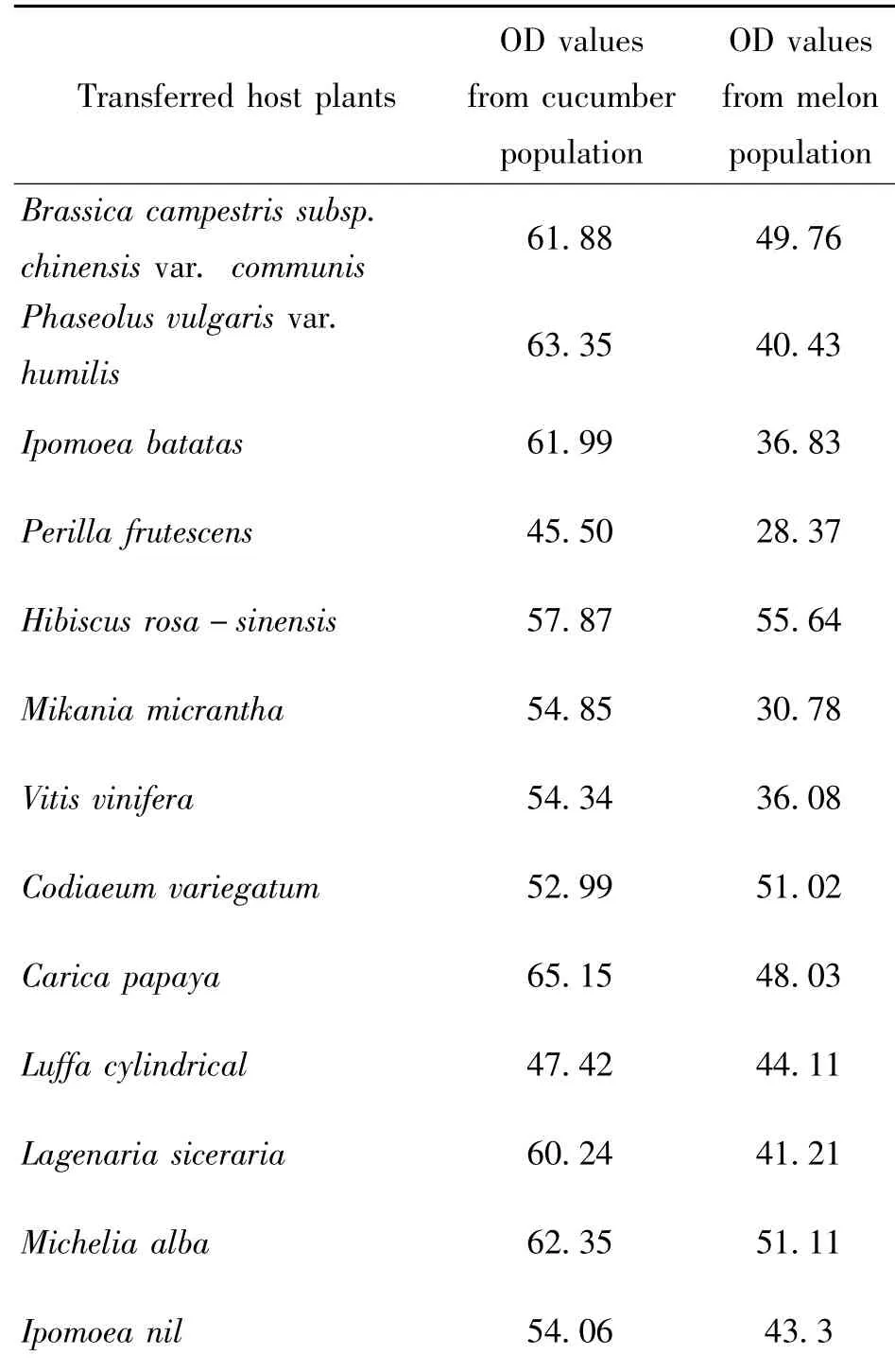
Table 1 OD values of CarE on 13 host plants from whitefly population feeding cucumber and bitter melon respectively
CarE activity were also measured when B.tabaci were transferred from cucumber and bitter melon to 13 new host plants as table 1.Paired sample analysis showed that OD value from different feeding background performed significant diffference each other(Table 2).Average of CarE activity from bitter melon was muchhigherthancucumber,whichimplied physiological effect of CarE activity happened at least a short time.
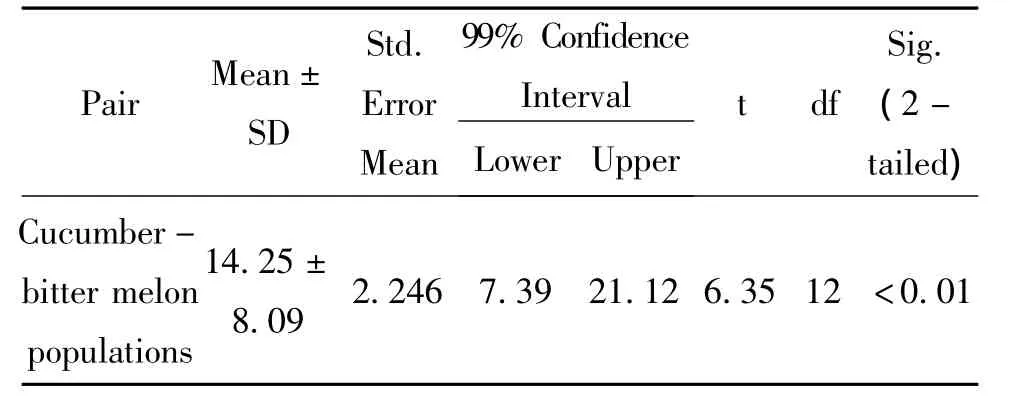
Table 2 Paired samples test
Result of Table 3 indicated that all of OD data from different feeding experience were according to the normality distribution typically.

Table 3 Test of Normality
4 Discussion
Bemisia tabaci is a rapidly evolving agricultural pest and is an extreme generalist foraging on a variety of host plants(de Barro,2011).However evolutionary interpretation on how it extends host niche rapidly is stillinsufficient. Apart from ecological factors,induction of detoxification enzymes were regarded to play a central role on host utility of B.tabaci,such as carboxylesterase,glutathione S-transferase,cytochrome-P450-dependent monooxygenase and so on(Terriere,1984;Liang et al.,2007;How and Jander,2008;Xie et al.,2010).We presumed that several benignant mutants were probably happened on cluster of detoxification enzymes,which improved entire ability on host utility for B.tabaci,especially for B and Q biotypes(Horowitz et al.,2005;Boykin et al,2007;Dennehy et al.,2010).
In this study,the data indicated that CarE of B.tabaci were significantly impacted by itsfeeding experience and the CarE activity of population feeding on unadaptivehostplantwasmuch higherthan adaptive one.Although the acquired improvement of CarE activity is unlikely to be involved in genetic structure,it should not be ignored because this kind of fitness enhancement was easy to be obtained from field and might play an important role as B.tabaci tries on new host plants.As expected,the acquired CarE's activity improvement was prolonged when B.tabaci were transferred to new host plants.This effect was verified statistically within 13 host plants on average level.It implied that physiological deviation induced by feeding experience might provide some advantages for B.tabaci population to unadaptive host range.It isstillunclearbutreasonable to presume that improvement of detoxification enzymes induction by feeding experience was popular phenomenon among herbivore populations.
We noticed that two groups of OD data from transferred hostplantsaccorded with the normal distribution,which implied thatdata ofsamples featured better symmetry and the mean values have higher stability.If the normal distribution pattern can be found in other detoxification enzymes,it could provide an effective way to detect them between biotypes.
References)
An XC,Ren SX,Hu QB,2007.The assessing system of host performance for herbivore populations.Environmental Entomology,36(4):694-699.
An XC and Ren SX,2009.The effect of feeding experiences on host adaptation of Bemisia tabaci(Gennaidus).Journal of South China Agricultural University,30(1):27-30.
Brown JK,1994.Current status of Bemisia tabaci as a plant pest and virus vector in agro - ecosystems worldwide. FAO Plant Prot.Bull.,42:3-32.
Boykin LM,Shatters Jr RG,Rosell RC,McKenzie CL,Bagnall RA,De Barro P,Frohlich DR,2007.Global relationships of Bemisia tabaci(Hemiptera:Aleyrodidae)revealed using Bayesian analysis of mitochondrial COI DNA sequences.Mol.Phylogenet.Evol.,44:1306-1319.
Bornscheuer UT,2002. Microbial carboxylesterases:classification,properties and application in biocatalysis.FEMS.Microbiol.Rev.,26:73-81.
Byrne DN and Bellows TS, 1991. Whitefly biology. Ann. Rev.Entomol.,36:431 -457.
Byrne FJ,Gorman KJ,Cahill M,Denholm I,Devonshire AL,2000.The role of B-type esterase in conferring insecticide resistance in the tobacco whitefly,Bemisia tabaci(Genn.).Pest Manag.Sci.,56:867-874.
de Barro PJ,Driver F,Trueman JWH,Curran J,2000.Phylogenetic relationships of world populations of Bemisia tabaci(Gennadius)using ribosomal ITS1.Mol.Phylogenet.Evol.16:29-36.
de Barro PJ,Liu SS,Boykin LM,Dinsdale AB,2011.Bemisia tabaci:A statement of species status.Annu.Rev.Entomol.,56:1 -19.
Dennehy TJ,Degain BA,Harpold VS,Zaborac M,Morin S,Fabrick JA,Nichols RL,Brown JK,Byrne,Li XC,2010.Extraordinary resistance to insecticides reveals exotic Q biotype of Bemisia tabaci in the new world.J.Econ.Entomol.,103:2174 -2186.
Gao XW,1992.Effect of host plant on carboxylesterase activity in cotton aphid Aphis gossypii Glov. Acta Entomologica Sinica,3:267-272.
Horowitz AR,Kontsedalov S,Khasdan V,Ishaaya I,2005.Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance.Arch.Insect Biochem.Physiol.,58:216-225.
Howe GA,Jander G,2008.Plant immunity to insect herbivores.Annu.Rev.Plant Biol.,59:41 -66.
Liang P,Cui JZ,Yang XQ,Gao XW,2007.Effects of host plants on insecticide susceptibility and carboxylesterase activity in Bemisia tabaci biotype B and greenhouse whitefly, Trialeurodes vaporariorum.Pest Manag.Sci.,63:365-371
Mansaray A, Sundufu AJ, 2009. Oviposition, developmentand survivorship of the sweetpotato whitefly Bemisia tabaci on soybean,Glycine max,and the garden bean,Phaseolus vulgaris.Journal of Insect Science,9:1-6.
Oliveira MV,Henneberry TJ,Anderson P,2001.History,current status,and collaborative research projects for Bemisia tabaci.Crop Protection,20:709 -723.
Ranson H, ClaudianosC, OrtelliF, AbgrallC, HemingwayJ,Sharakhova MV,Unger MF,Collins FH,Feyereisen R,2002.Evolution of multigene families associated with insecticide resistance.Science,298:179-781.
PerringTM, 2001. The Bemisia tabacispeciescomplex. Crop Protection,20:725 -737.
Secker A,Bedford I,Markham P,1998.Squash,a reliable field indicatior for the presence of B biotype of tabacoo whitefly,Bemisia tabaci.837 -842.British crop protection council.SPSS.2005.SPSS 13.0 for Windows.SPSS,Chicago,IL.
Terriere LC,1984.Induction of detoxification enzymes in insects.Ann.Rev.Entomol.,29:71-88.
Xie W,Wang SL,Wu QJ,Feng YT,Pan HP,Jiao XG,Zhou L,Yang X,Fu W,Teng HY,Xu BY,Zhang YJ,2010.Induction effects of host plants on insecticide susceptibility and detoxification enzymes of Bemisia tabaci(Hemiptera:Aleyrodidae).Pest Manag.Sci.,67:87-93.
Zang LS,Chen WQ,Liu SS,2006.Comparison of performance on different host plants between the B biotype and a non-B biotype of Bemisia tabaci from Zhejiang,China.Entomologia Experimentalis et Applicata,121:221-227.

