铂/石墨烯氧还原电催化剂的共还原法制备及表征
王万丽 马紫峰
(上海交通大学化学工程系,上海200240)
铂/石墨烯氧还原电催化剂的共还原法制备及表征
王万丽 马紫峰*
(上海交通大学化学工程系,上海200240)
使用硼氢化钠共还原法制备40%(w)铂/石墨烯电催化剂用于氧还原反应.通过循环伏安测试发现,这种方法制备所得铂/石墨烯催化剂对氧还原反应活性较铂/碳催化剂差,但稳定性有所提高.在稳定性测试中,铂/石墨烯电催化性能衰减为50%,较铂/碳(79%)好.X射线衍射(XRD)和透射电子显微镜(TEM)表征发现在铂/石墨烯催化剂中两者存在明显交互作用,这可能是阻止石墨烯再堆垛和防止铂颗粒团聚的主要原因.通过对单电池性能测试也发现铂/石墨烯催化剂更有利于电池长期稳定.
石墨烯;共还原法;电催化剂;氧还原反应;质子交换膜燃料电池
1 Introduction
Gaphene has attracted great attention from researchers in both theoretical and applied chemistry in recent years.Its use has also been studied in capacitors,1,2lithium batteries,3-6and fuel cells7-9because of its interesting properties,such as ultrahigh surface area(there is a theoretical surface area of 2620 m2·g-1for an isolated graphene sheet),special quantum properties10-13and so on.
Proton exchange membrane(PEM)fuel cells have been developed as a promising energy technology because of their inherent advantages,such as simplicity,viability,and quick start-up,which give them of great potential in almost any con-ceivable application.14However,several problems still hinder its commercialization.One major problem is the corrosion of carbon support which results in the catalyst being degraded quickly.Much research has been done,however,to improve the durability of carbon support.It was found that carbon materials with nanostructure can improve catalytic properties significantly because of their special electronic properties.15Graphene has also been studied as one of the candidates with the most likely potentiality for its ultrahigh surface area and relatively high conductivity.16
The Pt/graphene nanocomposite has been identified as a material possibly able to play an important part in the development of low temperature fuel cells.9,17The nano composite has a large surface area,a high electrochemical surface area (ECSA),and well dispersity of platinum particles.However, its performance in a single cell,particularly the durability of the catalyst,still needs to be further researched.
In this study,Pt/graphene composite was co-reduced,characterized,and compared with Pt/C for oxygen reduction reaction. The electrochemical properties of Pt/graphene were discussed from several aspects,especially its durability.
2 Experimental
2.1 Material synthesis
All the chemicals used were analytical reagents,purchased from Sigma-Aldrich(USA).
Prior to Pt/gaphene preparation,graphite oxide(GO)was synthesized by the modified Hummers method.18,19In brief,the graphite powder(1 g)was stirred in concentrated sulfuric acid with potassium permanganate added gradually in a water bath till completely oxidized.The reaction was terminated by the addition of a large amount of distilled water and 30%(w)H2O2solution,and then the mixture was centrifuged and washed several times till a natural pH value.The dried film was stored and dispersed in solvents as needed.
The Pt/graphene was fabricated by a co-reduction process. 10 mg GO was exfoliated into 10 mL water by ultrasonication to form GO dispersion.Adding H2PtCl6·6H2O with stirring and adjusting its pH value to 13 by 1 mol·L-1potassium hydroxide,the dispersion was then reduced by 0.1 mol·L-1sodium borohydride added dropwise at room temperature for 4 h.Pt/graphene powder was gathered by filtration through a mixed cellulose ester membrane filter(0.45 μm pore size),and then it was dried in air.After grinding and sifting through a 200-mesh sieve,the powder was roasted at 200°C for 1.5 h.
For comparison,40%(w)Pt/C was fabricated using the same procedure using Vulcan XC-72R(Cabot Corporation, USA)instead of graphene.Pure graphene powder was prepared in the same procedure without the platinum precursor.
2.2 Characterization
The content of platinum in samples was analyzed by inductively coupled plasma mass spectrometer(ICP-MS,Agilent Technologies,USA).The powder X-ray diffraction(XRD) measurements of the samples were recorded on an X-ray powder diffractometer(D/max-2200/PC,Rigaku Corporation,Japan)using Cu Kαradiation(λ=0.15406 nm)with scattering angles(2θ)of 20°-80°.Scanning electron microscope(SEM, S-4800,Hitachi Corporation,Japan)and energy-dispersive X-ray(EDX)spectroscopy(Inca Oxford,U.K.)attached to the SEM were used to confirm the deposition of Pt on the graphene.The catalyst features were characterized by a transmission electron microscope(TEM,JEM-2010,Jeol,Japan).
2.3 Electrochemical measurements
The electrochemical measurements were conducted in a three-electrode cell recorded by a potentiostat/galvanostat model 273(EG&G Princeton Applied Research,USA).Platinum mesh and saturated calomel electrode(SCE)were used as the counter and reference electrodes,respectively.0.5 mol·L-1H2SO4were employed as the electrolyte.The sample inks were prepared by mixing 10 mg sample powder with 0.45 mL deionized water,0.03 mL isopropanol,and 0.02 mL Nafion®solution(5%(w),Dupont Company,USA),followed by sonication in a water bath for 10 min.20 μL ink was dispensed and dried in air on the glassy carbon electrode(φ=0.5 cm).The electrode was immersed in de-aerated electrolyte and pretreated in cycles between-0.24 and 1 V at a scan rate of 100 mV·s-1for 100 cycles.The anodic corrosion was measured by anodic linear sweep voltammetry from-0.05 V(vs open circuit voltage) to 1.76 V at a scan rate of 5 mV·s-1.The cyclic voltammetry (CV)performance of supported platinum catalyst was measured in a standard way between-0.24 and 0.96 V at a scan rate of 5 mV·s-1.The electrochemical surface area was calculated from the hydrogen adsorption-desorption peak of the CV profiles.20The accelerated durability was measured by cyclic voltammetry in the range of-0.24 to 0.96 V for 1000 cycles at a scan rate of 50 mV·s-1.Oxygen reduction reaction(ORR) performance was evaluated by rotating disc electrode(RDE) technique.The polarization curves for oxygen reduction reaction were measured in an oxygen saturated electrolyte by scanning the potential from 0.9 to 0.1 V at a scan rate of 5 mV·s-1. All potentials were reported with respect to the normal hydrogen electrode(NHE)scale.
The gas diffusion layer for the cathode and the gas diffusion electrode(1.0 mg·cm-2)for the anode were purchased from ElectroChem,Inc.The platinum loading at the anode side is relatively high,so the overpotential due to the anode half reaction can be neglected allowing the focus to be solely on the cathode side.21In this experiment,catalyst suspension was mixed by a supported platinum catalyst,Nafion(5%(w),Dupont Company,USA),and isopropanol.The mass ratio of catalyst to Nafion was 3:4.After being thoroughly dispersed,the suspension was brushed on the gas diffusion layer at the platinum loading until 0.4 mg·cm-2using as the cathode electrode. Nafion 117 membrane(Dupont Company,USA)was pretreated by boiling in 5%(w)hydrogen peroxide for 1 h,followed by treating in 1 mol·L-1sulfuric acid.After each step the Na-fion membrane was boiled in deionized water for 30 min.The prepared anode and cathode were positioned on the both sides of the membrane and hot pressed at 2 tons and 110°C for 4 min to form the membrane electrode assembly(MEA).
The PEM fuel cell was tested in a fuel cell testing unit with an active electrode area of 1.5 cm×1.5 cm.The cell was operated at atmospheric pressure.The oxygen and hydrogen flow rate was 200 mL·min-1.The temperature of the cell was 50°C. The current-voltage curves were collected after an activation process at a constant operation voltage of 0.6 V.
3 Results and discussion
3.1 Material properties
The amounts of platinum loaded on Pt/C and Pt/graphene were determined by ICP-MS showing that the contents of platinum were 39.62%(w)and 40.73%(w),respectively.XRD patterns of Pt/graphene and graphene are shown in Fig.1,which contain typical platinum diffraction peaks and a broadening C (002)peak.The peaks at the 2θ of 39.86°,46.25°,and 67.69° are assigned to(111),(200),and(220)facets of the face-centered cubic structure of platinum respectively,which are in agreement with the standard card of platinum(JCPDS No. 4-802).The crystallite size is calculated from Scherrer equation as following:
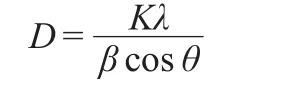
where,D is the crystallite size;K is the Scherrer constant(K= 0.89);β is the peak width at half height;θ is the angle of diffraction;λ is the wavelength of X-ray(λ=0.154056).The crystallite size of platinum is calculated as 7.7 nm,bigger than that of Pt/C(6.8 nm).The calculated lattice constants of platinum supported by graphene and carbon are a=b=c=0.3912 nm and a=b=c=0.3938 nm,respectively.The change between these values shows clearly that a stronger interaction exists when platinum particles are deposited onto graphene.22On the other hand, carbon diffraction peaks of Pt/graphene composite are broadened and weakened compared to that of pure graphene,which suggests that there might be a certain interaction between platinum particles and graphene support.It may be caused by platinum particles embedding into the spaces between graphene layers and leading to graphene lattices growing disorderly.8These results also confirm that the platinum precursor and graphite oxide are reduced to the composite of Pt/graphene.
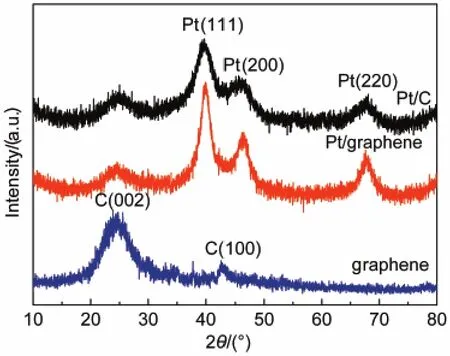
Fig.1 XRD patterns of Pt/C,Pt/graphene,and graphene
The features of pure graphene and Pt/graphene composite are shown in Fig.2.The monolayer graphene sheets are found in TEM images,supporting the platinum particles(black dots). The outstretched wrinkles of graphene sheets are clearly observed,which are obviously different from those of Vulcan XC-72.This might be attributed to the dispersion of platinum particles which also hinders the migration of the platinum particles.17The particle size of platinum observed is about 8 nm which agrees well with the results from XRD.The multi-crystal diffraction rings of platinum can be assigned to the(111), (200),and(220)facets of platinum in the selected area electron diffraction(SAED)pattern.However,Pt/graphene has a particular orientation which can be confirmed by the bright spots on the diffraction rings.This might be caused by the surface groups on the dispersed graphite oxide(epoxy and hydroxyl groups),acting as anchoring sites for platinum particles to deposit on.The platinum particles could only have adhered to the top or the bottom of the graphene sheets.The graphene sheets are reduced with wrinkles emerging in succession,leading to parts of the platinum particles changing their facet directions on the graphene sheets.23,24This reducing process might have contributed to the morphology of the platinum particlesʹdeposition.
The deposition of platinum particles is also confirmed by SEM images(Fig.3).From the top view of pure graphene (Fig.3(a)),the irregular wrinkles(bright lines)could be observed clearly on the surface,meanwhile,no crack could be found,which proving that the graphene is compact and flat. This suggests that it is both impermeable to gas and water resistant.The lamellar structure can be found even it is grinded to a powder.Fig.3(b)indicates that the platinum particles(bright dots)are deposited on the graphene sheets dispersedly.The graphene wrinkles are still formed but less than that of pure graphene film,which might be caused by preventing restacking of the graphene sheets from van der Waals forces when platinum particles deposited onto.The cross section of the film is a layer-by-layer structure as shown in the insert in Fig.3(a).The layers are stacked together closely;they are separated however from each other with only few layers stacking while platinum particles are deposited on the sheets as shown in the insert in Fig.3(b).The layer-by-layer structure is still maintained while the spaces between sheets are increased,suggesting the particles are acting as holders between layers hence resulting in a less layer-stacking film with a larger surface area,which is to its applicationʹs advantage.
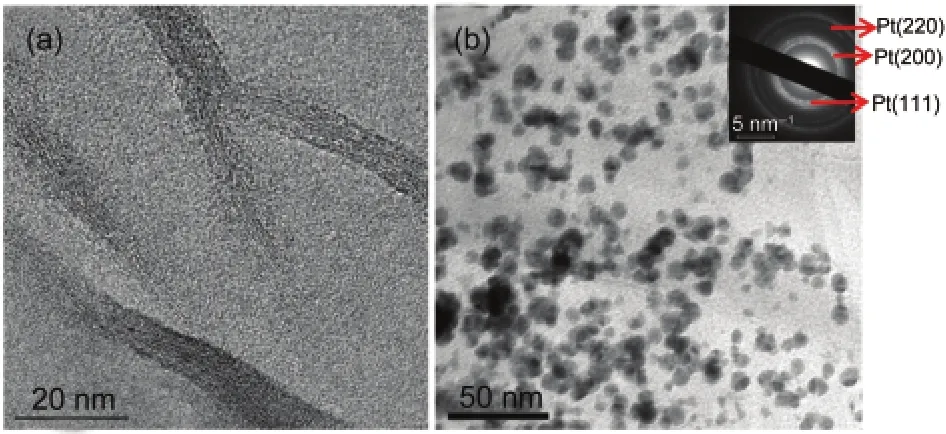
Fig.2 TEM images of(a)graphene and(b)Pt/grapheneInsert is the SAED pattern of Pt/graphene.

Fig.3 SEM images of(a)graphene and(b,c)Pt/grapheneInserts are the cross sections of(a)graphene and(b)Pt/graphene.
3.2 Electrochemical properties and durability
The electrochemical properties were characterized by cyclic voltammetry in 0.5 mol·L-1sulphuric acid system.Prepared Pt/ graphene and Pt/C catalysts exhibit the typical Pt peaks of hydrogen under-potential deposition and oxidation of hydrogen around 0-0.3 V in Fig.4(a,b).These peaks are similar to the published work25which indicates that the Pt-oxides formation and reduction appear at the same potentials for Pt/graphene and Pt/C.
The peaks also indicate that the platinum particles are active when supported by graphene,agreeing well with the reference.26The electrochemical surface area(ECSA)is calculated according to the equation:20

where,QHis the peak area of hydrogen adsorption-desorption, and[Pt]is the platinum loading.ECSA is calculated as 30.2 and 28.0 m2·g-1for Pt/graphene and Pt/C,respectively.It indicates that the numbers of platinum active sites of Pt/graphene and Pt/C are in the same order of magnitude.The ECSA value of Pt/C agrees well with the published data.27It suggests that the number of active sites is not affected when the feature of catalyst support changes into layer structure.
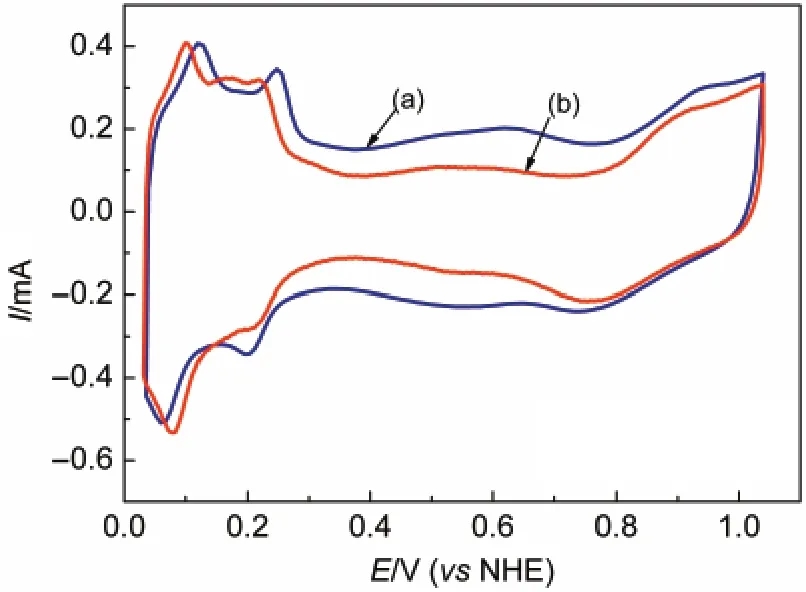
Fig.4 Cyclic voltammetry curves of(a)Pt/graphene and(b)Pt/C
The oxygen reduction reaction behavior was investigated by RDE method.Fig.5(a)shows the result of ORR polarization of Pt/graphene.The variation of the ORR current density(i) changes significantly with the RDE rotating speed(ω)in the diffusion region.The relationship between i-1vs ω-1/2can be expressed by the Koutecky-Levich equation:28

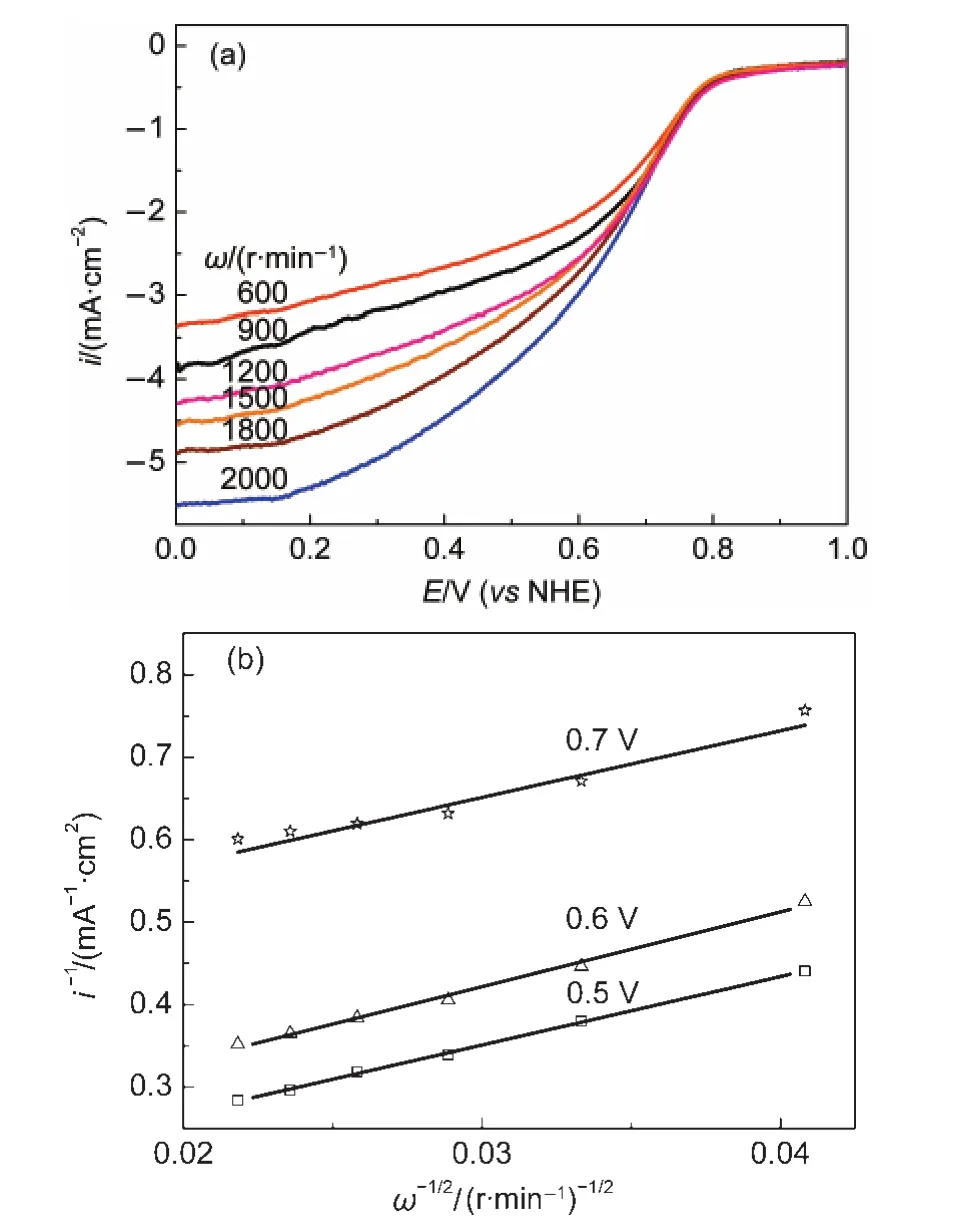
Fig.5 (a)ORR polarization curves and(b)Koutecky-Levich plots of Pt/graphene
where,i is the total ORR current density,ikis the kinetic current density(in the activation region),idis the diffusion limited current density(in the high ORR reduction potential)where the current is a plateau and its value changes with the RDE rotating speed(ω)(Fig.5(b)).K is the Levichʹs slope which contains the following parameters:n the number of electron transferred in ORR,F the Faraday constant,CO2the bulk concentration of oxygen(1.03×10-3mol·L-1),DO2the diffusion coefficient of oxygen in the bulk solution(2.1×10-5cm2·s-1),and v the kinematic viscosity of the solution(1.07×10-2cm2·s-1).29The calculated n values of the Pt/graphene and Pt/C are 3.86 and 3.90,respectively.This result indicates that the ORR on the Pt/graphene electro-catalyst proceeds via a four-electron transfer process.The kinetic current density ikis obtained by extrapolation of the Koutecky-Levich plots to ω-1/2to zero.The values are listed in Table 1.It is found that ikvalue of Pt/graphene is lower than that of Pt/C.This is an indication that the ORR kinetic rate on Pt/graphene might be lower than that of Pt/ C,even the reaction pathway is the same on both electrodes. The low kinetic rate on Pt/graphene might be due to the inhibition of the oxygen diffusion on its surface.Grapheneʹs layer structure that platinum particles embedded into it might impede the access of the platinum active sites to oxygen.However,the carbonʹs spherical structure encourages the oxygen diffusion.Tafel slopes(b)and current density in activation region of Pt/graphene and Pt/C are listed in Table 1.The larger Tafel slope and the lower current density of Pt/graphene than those of Pt/C also prove the less activity of Pt/graphene for ORR,although Pt/graphene has almost the same platinum active sites of hydrogen adsorption-desorption.

Table 1 Electron transfer number,dynamic current density,exchange current density,Tafel slope,and current density of Pt/graphene and Pt/C at 850 mV
The durabilities of both Pt/graphene and Pt/C were investigated under cyclic voltammetry for 1000 cycles in 0.5 mol·L-1sulphuric acid medium.The electrochemical surface area was calculated every hundred cycles.Fig.6 shows the normalized ECSA of Pt/graphene and Pt/C variation as a function of cycling number.Obvious degradations in ECSA values are found as 30.20 m2·g-1before cycles and 15.05 m2·g-1after cycles of Pt/graphene while 28.00 m2·g-1before cycles and 5.88 m2·g-1after cycles of Pt/C.The degradation of homemade Pt/graphene was slightly less than that in reference17where the decrease of activity for ORR was about 50%.17Meanwhile,the decrease of Pt/C was 79%,which was much bigger than that of Pt/graphene.Therefore,platinum on graphene was much more stable than that on carbon under the test condition.The degradation is mainly due to carbon corrosion.As to Pt/C,the carbon support is spherical which encourages the aggregation of platinum particles,leading to a decrease in the platinum surface area.However,the better durability of Pt/graphene could be attributed to the grapheneʹs interesting electronic properties that there is an interaction between Pt particles and the graphene surface,this hindering the metallic phase coalescence.30Moreover,it is more difficult for platinum particles to aggregate because the available particles are the surrounding ones in two dimensions,while the particles up or below are separated by graphene layers.

Fig.6 Normalized electrochemical surface area degradation of Pt/graphene and Pt/C
The performance and durability of Pt/graphene in PEM fuel cell were also investigated.Fig.7(a)shows the performance in a single cell that the open circuit voltage based on Pt/graphene of 0.975 V and the maximum power density of 158.65 mW· cm-2at 0.317 V are observed.The initial performance based on Pt/graphene is poorer than that of Pt/C,which might be due to the inhibition of oxygen diffusion on its catalyst surface as described above.However,a higher stability is observed in Fig.7 (b).The decrease of voltage at 400 mA·cm-2as a function of time based on Pt/graphene is less than that of Pt/C,which might be caused by the greater durability of the Pt/graphene catalyst.This result indicates that graphene might be more suitable for PEM fuel cell application than carbon.
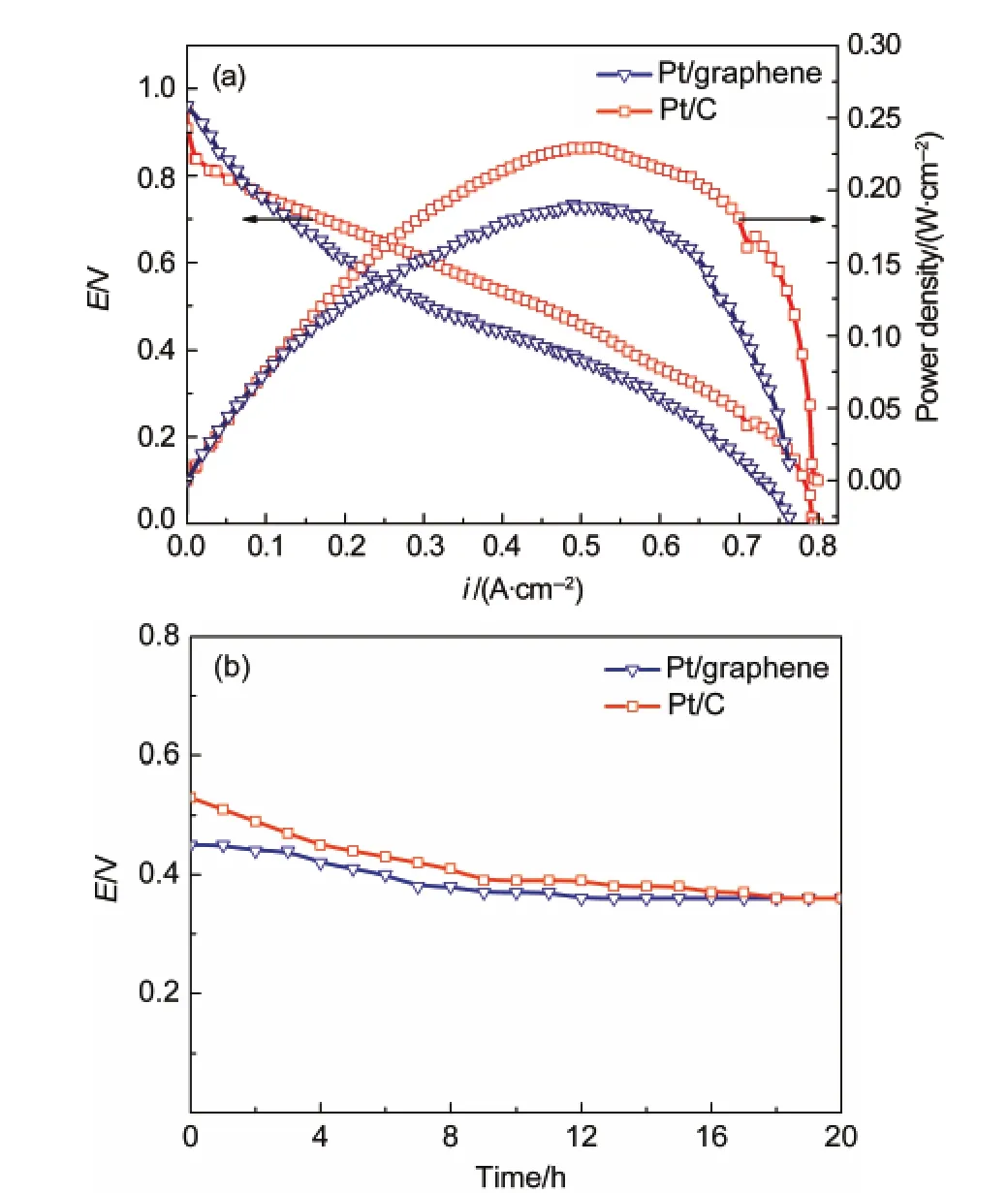
Fig.7 (a)Single cell performance and(b)stability of Pt/graphene and Pt/C
4 Conclusions
The 40%(w)Pt/graphene composite prepared by sodium borohydride chemical co-reduction was introduced as the electrocatalyst for oxygen reduction reaction.The electro catalytic activity and stability were evaluated.The results show that the layer structure of graphene maintains after reduction and the platinum particles with a particle size of 8 nm are dispersed onto graphene.The activity for ORR based on Pt/graphene is lower than that of Pt/C;however,better stability is observed in degradation test that the decrease of Pt/graphene is 50%,which is less than that of Pt/C(79%).On both electrodes,the ORR proceeds via a four-electron process.The performance of a single cell is also tested.The improvement in durability is confirmed by the delayed degradation of cell performance based on Pt/graphene.It might therefore be assumed that the grapheneʹs layer structure hinders the aggregation of platinum particles.Meanwhile,the platinum particles act as holders,which against the folding of graphene sheets.This result might be useful for the design of the catalyst with carbon support to improve the long-term performance in PEM fuel cells.
(1)Wang,Y.;Shi,Z.;Huang,Y.;Ma,Y.;Wang,C.;Chen,M.; Chen,Y.The Journal of Physical Chemistry C 2009,113(30), 13103.doi:10.1021/jp902214f
(2) Lu,X.J.;Dou,H.;Yang,S.D.;Hao,L.;Zhang,F.;Zhang,X. G.Acta Phys.-Chim.Sin.2011,27(10),2333.[卢向军,窦 辉,杨苏东,郝 亮,张 方,张校刚.物理化学学报, 2011,27(10),2333.]doi:10.3866/PKU.WHXB20111022
(3)Guo,P.;Song,H.;Chen,X.Electrochem.Commun.2009,11 (6),1320.doi:10.1016/j.elecom.2009.04.036
(4) Liang,M.;Zhi,L.J.Mater.Chem.2009,19(33),5871.doi: 10.1039/b901551e
(5)Xu,K.;Shen,L.F.;Mi,C.H.;Zhang,X.G.Acta Phys.-Chim. Sin.2012,28(1),105.[徐 科,申来法,米常焕,张校刚.物理化学学报,2012,28(1),105.]doi:10.3866/PKU. WHXB201228105
(6)Yang,X.W.;He,Y.S.;Liao,X.Z.;Ma,Z.F.Acta Phys.-Chim. Sin.2011,27(11),2583.[杨晓伟,何雨石,廖小珍,马紫峰.物理化学学报,2011,27(11),2583.]doi:10.3866/PKU. WHXB20111123
(7) Li,Y.;Tang,L.;Li,J.Electrochem.Commun.2009,11(4),846. doi:10.1016/j.elecom.2009.02.009
(8) Si,Y.;Samulski,E.T.Chem.Mater.2008,20(21),6792.doi: 10.1021/cm801356a
(9)Li,Y.X.;Wei,Z.D.;Zhao,Q.L.;Ding,W.;Zhang,Q.;Chen,S. G.Acta Phys.-Chim.Sin.2011,27(4),858. [李云霞,魏子栋,赵巧玲,丁 炜,张 骞,陈四国.物理化学学报,2011,27(4), 858.]doi:10.3866/PKU.WHXB20110411
(10) Castro Neto,A.H.;Kotov,V.N.;Nilsson,J.;Pereira,V.M.; Peres,N.M.R.;Uchoa,B.Solid State Commun.2009,149 (27-28),1094.doi:10.1016/j.ssc.2009.02.040
(11) Geim,A.K.Science 2009,324(5934),1530.doi:10.1126/ science.1158877
(12) Neto,A.H.C.;Guinea,F.;Peres,N.M.R.;Novoselov,K.S.; Geim,A.K.Reviews of Modern Physics 2009,81(1),109.doi: 10.1103/RevModPhys.81.109
(13)Rao,C.N.R.;Sood,A.K.;Subrahmanyam,K.S.;Govindaraj, A.Angewandte Chemie-International Edition 2009,48(42), 7752.doi:10.1002/anie.v48:42
(14) Barbir,F.PEM Fuel Cells——Theory and Practice;Elsevier Academic Press:Burlington,2005.
(15)Baughman,R.H.;Zakhidov,A.A.;De Heer,W.A.Science 2002,297(5582),787.doi:10.1126/science.1060928
(16) Chen,H.;Müller,M.B.;Gilmore,K.J.;Wallace,G.G.;Li,D. Adv.Mater.2008,20(18),3557.doi:10.1002/adma.200800757
(17)Kou,R.;Shao,Y.;Wang,D.;Engelhard,M.H.;Kwak,J.H.; Wang,J.;Viswanathan,V.V.;Wang,C.;Lin,Y.;Wang,Y.; Aksay,I.A.;Liu,J.Electrochem.Commun.2009,11(5),954. doi:10.1016/j.elecom.2009.02.033
(18) Huffman,G.P.;Shah,N.;Wang,Y.;Huggins,F.E.Prepr. Pap.-Am.Chem.Soc.,Div.Fuel Chem.2004,49(2),731.
(19) Kovtyukhova,N.I.Chem.Mater.1999,11(3),771.doi: 10.1021/cm981085u
(20) Fournier,J.;Faubert,G.;Tilquin,J.Y.;Cote,R.;Guay,D.; Dodelet,J.P.J.Electrochem.Soc.1997,144(1),145.doi: 10.1149/1.1837377
(21) Gasteiger,H.A.;Panels,J.E.;Yan,S.G.J.Power Sources 2004,127(1-2),162.doi:10.1016/j.jpowsour.2003.09.013
(22) Carmo,M.;Paganin,V.A.;Rosolen,J.M.;Gonzalez,E.R. J.Power Sources 2005,142(1-2),169.doi:10.1016/ j.jpowsour.2004.10.023
(23) Schniepp,H.C.;Li,J.L.;McAllister,M.J.;Sai,H.;Herrera-Alonso,M.;Adamson,D.H.;Prudʹhomme,R.K.;Car,R.; Saville,D.A.;Aksay,I.A.The Journal of Physical Chemistry B 2006,110(17),8535.doi:10.1021/jp060936f
(24) McAllister,M.J.;Li,J.L.;Adamson,D.H.;Schniepp,H.C.; Abdala,A.A.;Liu,J.;Herrera-Alonso,M.;Milius,D.L.;Car, R.;Prudʹhomme,R.K.;Aksay,I.A.Chem.Mater.2007,19 (18),4396.doi:10.1021/cm0630800
(25) Climent,V.;Marković,N.M.;Ross,P.N.The Journal of Physical Chemistry B 2000,104(14),3116.
(26)Takenaka,S.;Matsumori,H.;Matsune,H.;Tanabe,E.;Kishida, M.J.Electrochem.Soc.2008,155(9),B929.
(27) Cho,Y.H.;Park,H.S.;Cho,Y.H.;Jung,D.S.;Park,H.Y.; Sung,Y.E.J.Power Sources 2007,172(1),89.doi:10.1016/ j.jpowsour.2007.01.067
(28)Wang,C.;Waje,M.;Wang,X.;Tang,J.M.;Haddon,R.C.;Yan, Y.Nano Lett.2003,4(2),345.
(29)Chen,Z.;Waje,M.;Li,W.;Yan,Y.Angew.Chem.2007,119 (22),4138.doi:10.1002/ange.200700894
(30) Rochefort,A.;Yang,D.Q.;Sacher,E.Carbon 2009,47(9), 2233.doi:10.1016/j.carbon.2009.04.013
July 5,2012;Revised:September 13,2012;Published on Web:September 25,2012.
Synthesis and Characteristics of Pt/graphene by Co-Reduction Method for Oxygen Reduction Reactions
WANG Wan-Li MA Zi-Feng*
(Department of Chemical Engineering,Shanghai Jiao Tong University,Shanghai 200240,P.R.China)
40%(w)Pt/graphene composites were prepared by sodium borohydride chemical coreduction,and were subsequently used as an electrocatalyst for oxygen reduction reactions.The electrocatalytic activity and stability was evaluated by cyclic voltammetry.The results indicated that the initial activity of Pt/graphene was lower than that of Pt/C due to the oxygen diffusion inhibition;however,the Pt/graphene showed superior durability characteristics.Degradation tests showed a 50%degradation of Pt/ graphene,which was substantially less than that of Pt/C(79%).X-ray diffraction and transmission electron microscope results showed that the composite formed strong interactions between the platinum nanoparticles and the graphene supports.The graphene supports may also prevent the graphene sheets from folding or re-stacking,which would hinder platinum nanoparticlesʹaggregation.The performance of a single cell was also tested,confirming an improvement in durability.
Graphene;Co-reduction method;Electrocatalyst;Oxygen reduction reaction; Proton exchange membrane fuel cell
10.3866/PKU.WHXB201209252
∗Corresponding author.Email:zfma@sjtu.edu.cn;Tel:+86-21-54742894.
The project was supported by the National Natural Science Foundation of China(21073120,21176155)and Science and Technology Foundation of Shanghai Municipality,China(10JC1406900).
国家自然科学基金(21073120,21176155)及上海市自然科学基金(10JC1406900)资助项目
O646

