Primary Meningeal Melanocytoma Located in Foramen Magnum:a Case Report and Review of the Literatures
Ming-chao Fan,Jing-feng WangWei-wei Fu,Ke LiuLian-di Liand Peng Sun*
1Department of Neurosurgery Intensive Care Unit,
2Department of Neurosurgery,
3Department of Pathology,the Affiliated Hospital of Medical College,Qingdao University,Qingdao 266003,China
LIMAS and Tio1proposed the term meningeal melanocytoma first time in 1972 to describe a primary melanotic tumor of the leptomeninges with prolonged clinical course and benign histology.Meningeal melanocytoma of the central nervous system is rare and benign primary meningeal melanocytoma (PMM) is more exceptional,and also less usual than the malignant types.2This rare tumor falls under the subclassification of primary melanocytic lesions in the World Health Organization’s classification of central nervous system tumors.3PMM located in the foramen magnum region is an unusual cause of bulbus medullae and fourth ventricle compression.Here we report a 48-year-old man with a PMM which is located in the foramen magnum inducing supratentorial obstructive hydrocephalus.
CASE DESCRIPTION
A previously healthy 48-year-old man presented with gradually aggravated headache,left body numbness and weakness more than one year admitted to our hospital in November 2010.Three months of generalized weakness and several days of aconuresis were also described.Physical examination and lab tests on admission were normal.Computed tomography (CT) scanning of the head revealed a high-density occupying lesion in the posterior cranial fossa (Fig.1A).Magnetic resonance imaging (MRI)showed that the lesion appeared slightly short-T1 and iso-T2 signal in the foramen magnum and the posterior cranial fossa,behind the bulbus medullae,55.2 mm×32.0 mm×49.9 mm in size.The signal was uneven,and short-T1 and long-T2 lamellar signal was found in the lesion (Fig.1B,1C).Contrast-enhanced T1-weighted MR image demonstrated that the supratentorial ventricular system was dilated and uneven short-T1 signal was displayed under enhancement.The inferior margin of the lesion was about 16 mm below the foramen magnum level(Fig.1D) preoperatively.The tentative diagnosis of meningeoma was made.
The patient underwent a suboccipital craniotomy.During the operation,a deep black,tenacious,rich blood supply tumor,which located in the pars dorsalis of foramen magnum with pseudo-capsule,was completely resected and the specimen was send to pathology department for histopathologic examination.
The patient’s postoperative course was uneventful.After surgery,the patient was sent to the neurosurgery intensive care unit for vital sign surveillance and neurological observation.Head CT scan was performed and showed that no residual tumor and the size of supratentorial ventricle was decreased.No radiation therapy or chemotherapy was given after surgery.
Haematoxylin &Eosin (H&E) staining showed the tumor was densely multicellular and composed of monomorphic spindle cells which formed whorls,nests,sheets,and interlacing bundles.Most of the cells contained a large amount of prominent dark-brown pigment granular in cell substance.The enlarged nuclei and prominent nucleoli were observed in some cells (Fig.2A,2B).Immunohistochemical analysis revealed the tumor was positive reactivity for human melanoma black-45 (HMB-45) (Fig.2C),melanin (Fig.2D),and vimentin (Fig.2E),whereas negative reactivity for S-100 and epithelial membrane antigen(EMA,Fig.2F),leading to the final diagnosis of meningeal melanocytoma.
Multiple and/or large,congenital,pigmented melanocytic nevi of the skin and mucosae where the melanoma occur frequently were unfound,so the foramen magnum neoplasm was considered as a PMM.The physical,neuroimaging,and hemadenology examinations were performed for this patient during follow-up,which were scheduled at 1-,6-,and 12-month.And he was still well after 12 months of follow-up.
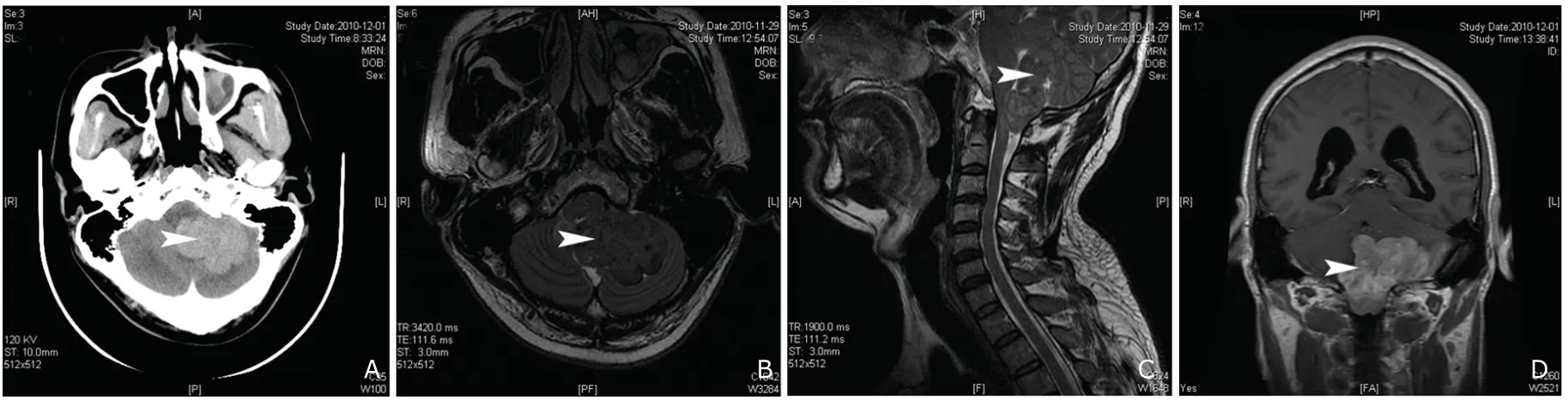
Figure 1.Routine CT scanning shows a dense-segmented inhomogeneous tumor in the left posterior cranial fossa,and computerized tomography number was 50 HU (A).MRI scanning reveals the segmented tumor is located in the left cerebellum and fourth ventricle.The lesion is iso-intensity on T2-weighted (B) and high intensity on T1-weighted MRI (C).The signal of the lesion is uneven,and lamellar shorter T1 signal and long T2 signal are discovered.The inhomogeneous contrast enhancement is seen on axial T1-weighted MR images with gadopentetate dimeglumine.The boundary of the tumor is marked.And the lesion extends into the foramen magnum.The ventricular system of supratentorial brain is ampliate (D).Arrows indicate location of the tumor.
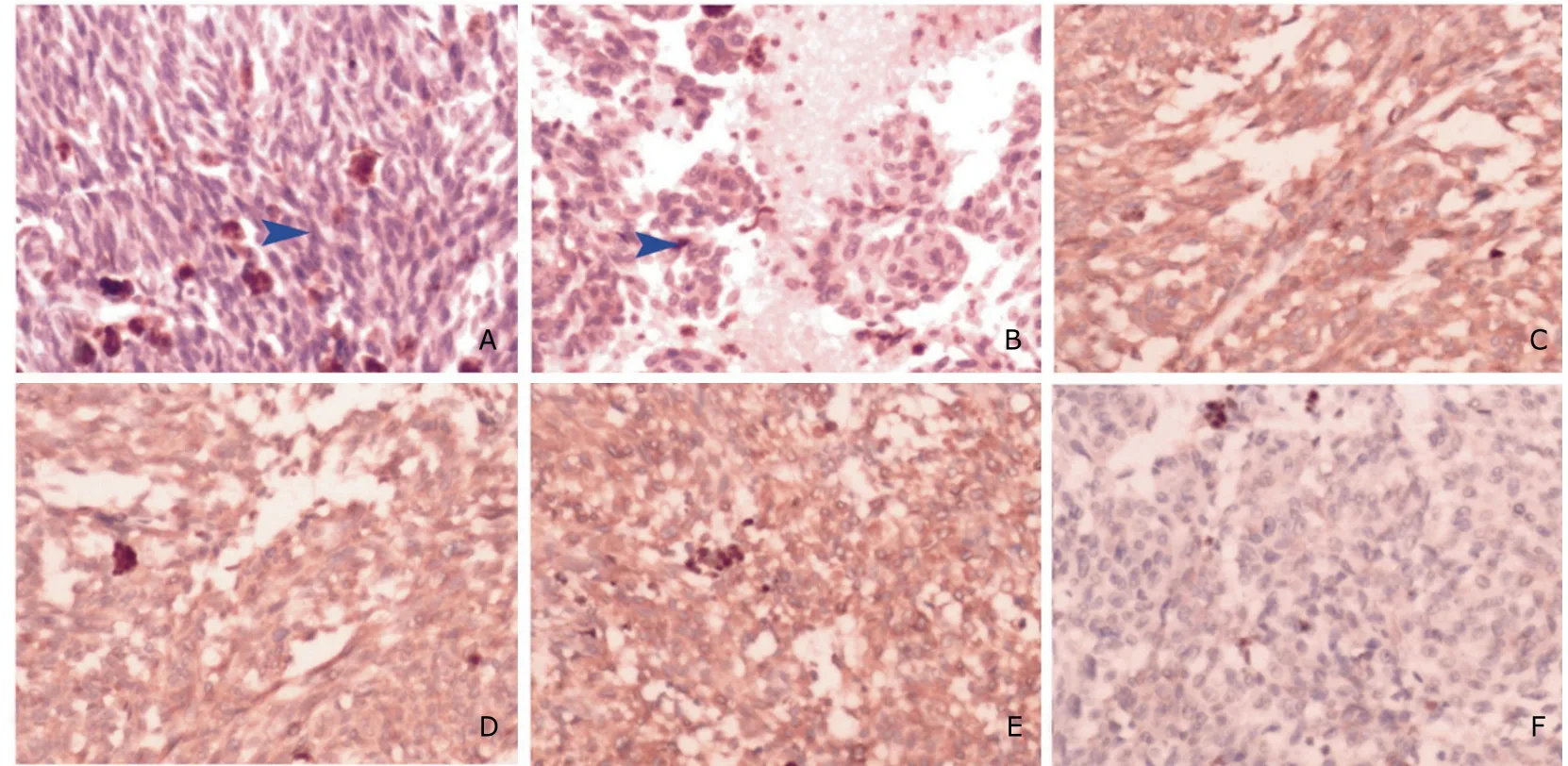
Figure 2.Histological and immunohistochemical findings.×200
DISCUSSION
The pathogenesis of PMM is uncertain.The melanocytic lesions of the nervous system are thought to arise from leptomeningeal melanocyte derived from the neural crest during early embryonic development,and produce melanin within melanosome from tyrosine precursors.4,5The term“meningeal melanocytoma”was introduced to classify benign pigmented leptomeningeal tumors which were originated from leptomeningeal melanocytes rather than from Schwann cells or meningeal fibroblasts.6PMM can arise from any meningeal location within the central nervous system and approximately 50% are intracranial lesions.7-9PMM is a slow growing tumor and accounts for less than 0.06%-0.1% of the brain tumors.10,11The mass effect rather than infiltrating adjacent tissue is the major reason that producing neurological deficit.4,12-14
The PMM is rare,and the lesion located in the foramen magnum region is more uncommon.On the basis of pathological findings,only 32 cases including the present case with sufficient evidence were confirmed as intracranial PMM since 1987 (Table 1).2,3,5-7,10,12,13,15-34The ages ranged from 0 to 71 years with a mean age of 41.6 years,and the sex preponderance was unfounded.The duration of symptoms ranged from 0 to 180 months,and the predominant symptoms was headache which was induced by mass effect.The Meckel’s cave and posterior cranial fossa were the frequently location where PMM occurred,followed by facies convexa cerebri and saddle area.So far,only 4 melanocytomas have been reported in the foramen magnum region in these 32 cases,so the case we describe is exceptional about its unusual display and location.19,27,28
Neuroimaging studies may be not specific for final diagnosis but useful to judge the pathogenetic condition.On CT scanning,PMM is iso-to hyper-density with variable contrast enhancement,9,13and usually indistinguishable from meningiomas.On MRI studies,most of these lesions appear iso-to short intense signal on T1-weighted and T2-weighted images,and usually show homogeneous enhancement after contrast administration.10,16,35For 11 cases undergoing MRI,the lesions of 10 cases exhibited iso-or short intense signal on T1-weighted images,7 cases showed iso-or short intense signal on T2-weighted images,and only 3 patients had long signal on T2-weighted images.The MR signal pattern of PMM is strongly related to the amount of melanin present,for example melanin can shorten T1 and T2 relaxation times.17,18Jaiswalet al4presumed that the effects of melanoma on MRI patterns depend not only on the content of free paramagnetic radicals from melanin,but also on the paramagnetic products from acute or chronic intratumoral hemorrhages and fat deposits.Uematsuet al19presumed that the PMM with tendency of hemorrhage might be easy to canceration.The imaging appearances of PMM varied with the different content of melanin and secondary changes of tumors.In our case,the PMM was hyper-dense on CT and short T1,iso-intense T2 signal on MRI,and a shorter T1.
Histopathologic and immunohistochemical examination,even electron microscopy,are the methods to make a definite diagnosis of PMM.On intraoperation inspection,PMM is a circumscribed pigmented lesion with increased vascularity,which was encapsulated by or occasionally adherent to the surrounding structures.20On light microscopy,PMM is multicellular and composed of uniform spindle or fusiform cells arrayed in whorls,sheets,bundles,or nests,and often surrounded by a fine network of reticular fibres.3The nucleoli in some cells are prominent and dominant granular black-brown pigment appears in the cytoplasm.9,25And they are lack of the evidence of significant mitotic activity,anaplasia,and atypical.15On immunohistochemistry,PMM is positive for S-100 protein,HMB-45,and vimentin,negative for EMA and glial fibrillary acidic protein.21-24In particular,HMB-45 positivity strongly supports the diagnosis of melanocytic tumors.36In our case,EMA and S-100 were negative,HMB-45 and vimentin were positive.
The recommended treatment option is complete tumor resection.For 26 patients receiving surgery treatment,22 lesions were completely removed and only 2 cases relapsed.For 4 cases undergoing subtotal tumor removal,1 patient relapsed.So if the tumors total removal to follow prodigious nerve injury,incomplete resection may be the better choice.The use of postoperative radiation therapy remains controversial.It has been recommended that radiation therapy be considered after surgery notwithstanding the PMM was benign lesions.14,16,25A total of 2 cases who underwent a complete tumor resection had radiotherapy after surgery,and 4 patients who received a subtotal tumor resection accepted radiotherapy.Three cases only received biopsy and radiotherapy,and one died after 6 months.We think that if the lesion is incompletely removed or the tumor has a tendency of canceration,postoperative radiotherapy should be considered.Despite the use of radiotherapy,recurrences have been observed in cases of incomplete lesion removal.10PMM has a benign histological appearance that includes lack of necrosis and mitotic activity,and these tumors do not metastasize but local recurrence may occur after incomplete surgical resection or even complete resection,so clinical follow-up is necessary.4,18,22,26Postoperative survival time rangedfrom 1 to 28 years.3,16The prognosis is quite satisfactory for those undergoing total tumor removal.21
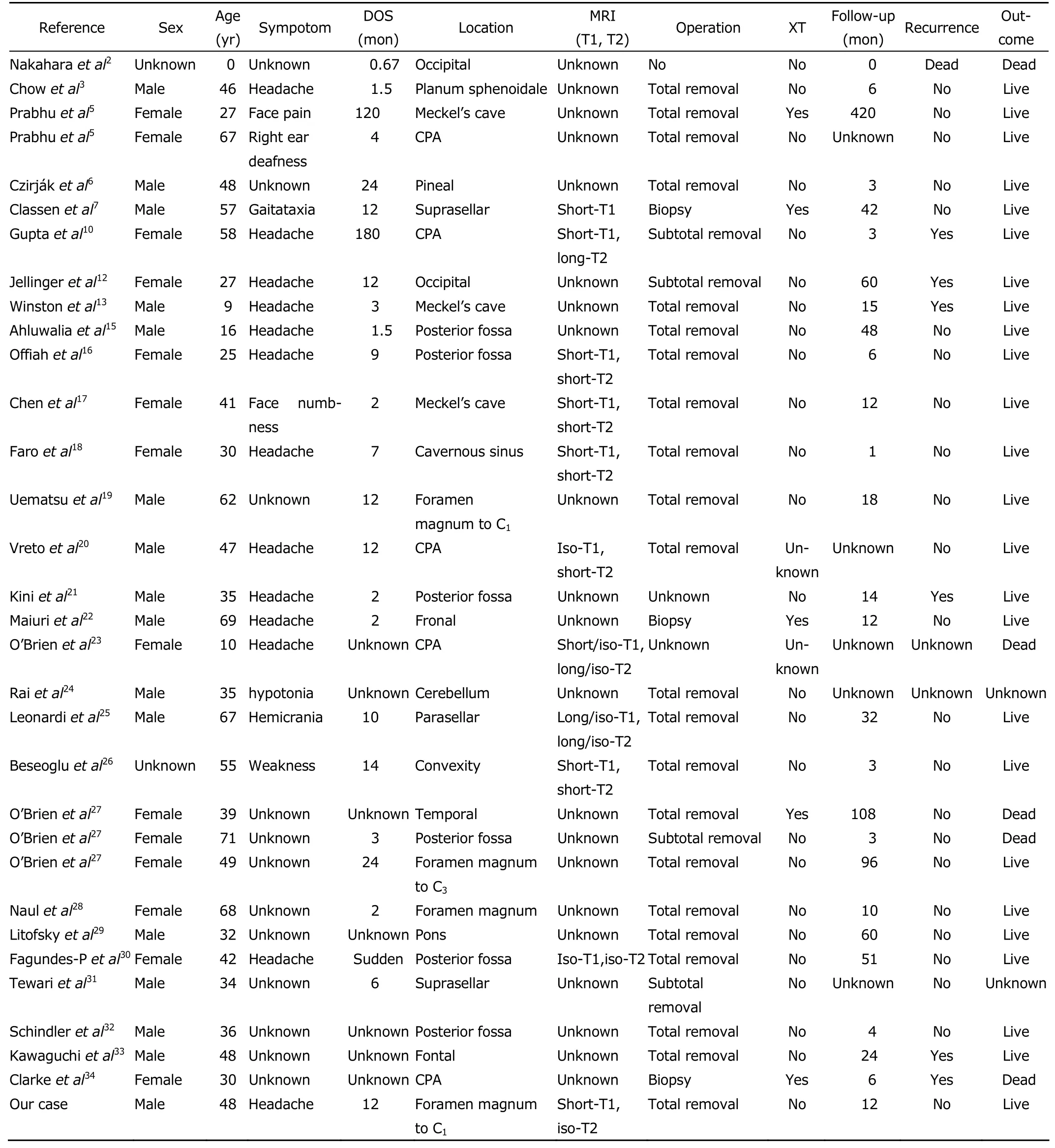
Table 1.Characteristics of 32 patients with intracranial meningeal melanocytoma reported during a period of 1987 to 2011
In summary,PMMs are rare tumors with variable biological appearance.Complete tumor resection is the recommended treatment option.Whether postoperative radio-therapeutics nominate or not remains controversial.PMM has a benign histologic appearance that includes lack of necrosis and mitotic activity,and the tumor does not metastasize but local recurrence may occur after incomplete surgical resection or even complete removal,so clinical follow-up is necessary.
1.Limas C,Tio FO.Meningeal melanocytoma (melanotic meningioma).Its melanocytic origin as revealed by electron microscopy.Cancer 1972;30:1286-94.
2.Nakahara K,Morota N,Ihara S,et al.Meningeal melanocytoma extruded from the skull of a neonate—case report.Neurol Med Chir (Tokyo) 2010;50:240-2.
3.Chow M,Clarke DB,Maloney WJ,et al.Meningeal melanocytoma of the planum sphenoidale:case report and review of the literature.J Neurosurg 2001;94:841-5.
4.Jaiswal S,Vij M,Tungria A,et al.Primary melanocytic tumors of the central nervous system:a neuroradiological and clinicopathological study of five cases and brief review of literature.Neurol India 2011;59:413-9.
5.Prabhu SS,Lynch PG,Keogh AJ,et al.Intracranial meningeal melanocytoma:a report of two cases and a review of the literature.Surg Neurol 1993;40:516-21.
6.Czirják S,Vitanovic D,Slowik F,et al.Primary meningeal melanocytoma of the pineal region:case report.J Neurosurg 2000;92:461-5.
7.Classen J,Hehr T,Paulus W,et al.Suprasellar melanocytoma:a case of primary radiotherapy and review of the literature.J Neuro Oncol 2002;58:39-46.
8.Eskandari R,Schmidt MH.Intramedullary spinal melanocytoma.Rare Tumors 2010;2:e24.
9.Merciadri P,Secci F,Sbaffi PF,et al.Multifocal meningeal melanocytoma of the conus medullaris.Acta Neurochir(Wien) 2011;153:2283-5.
10.Gupta A,Ahmad FU,Sharma MC,et al.Cerebellopontine angle meningeal melanocytoma:a rare tumor in uncommon location.Case report.J Neurosurg 2007;106:1094-7.
11.Liubinas SV,Maartens N,Drummond KJ.Primary melanocytic neoplasms of the central nervous system.J Clin Neurosci 2010;17:1227-32.
12.Jellinger K,Böck F,Brenner H.Meningeal melanocytoma.Report of a case and review of the literature.Acta Neurochir (Wien) 1988;94:78-87.
13.Winston KR,Sotrei A,Schnitt SJ.Meningeal melanocytoma.Case report and review of the clinical and histological features.J Neurosurg 1987;66:50-7.
14.Pan H,Wang H,Fan Y.Intracranial meningeal melanocytoma associated with nevus of Ota.J Clin Neurosci 2011;18:1548-50.
15.Ahluwalia S,Ashkan K,Casey AT.Meningeal melanocytoma:clinical features and review of the literature.Br J Neurosurg 2003;17:347-51.
16.Offiah CJ,Laitt RD.Intracranial meningeal melanocytoma:a cause of high signal on T1-and low signal on T2-weighted MRI.Clin Radiol 2006;61:294-8.
17.Chen CJ,Hsu YI,Ho YS,et al.Intracranial meningeal melanocytoma:CT and MRI.Neuroradiology 1997;39:811-4.
18.Faro SH,Koenigsberg RA,Turtz AR,et al.Melanocytoma of the cavernous sinus:CT and MR findings.AJNR Am J Neuroradiol 1996;17:1087-90.
19.Uematsu Y,Yukawa S,Yokote H,et al.Meningeal melanocytoma:magnetic resonance imaging characteristics and pathological features.Case report.J Neurosurg 1992;76:705-9.
20.Vreto G,Rroji A,Xhumari A,et al.Meningeal melanocytoma of the cerebellopontine angle as the unusual cause of superficial siderosis.Neuroradiology 2011;53:927-30.
21.Kini JR,Jeyraj V,Jayaprakash CS,et al.Intraoperative smear cytology of meningeal melanocytoma of the posterior fossa.Cytopathology 2009;20:59-62.
22.Maiuri F,Iaconetta G,Benvenuti D,et al.Intracranial meningeal melanocytoma:case report.Surg Neurol 1995;44:556-61.
23.O’Brien DF,Crooks D,Mallucci C,et al.Meningeal melanocytoma.Childs Nerv Syst 2006;22:556-61.
24.Rai S,Sharma M,Naik R,et al.Melanocytoma of cerebellum.Indian J Pathol Microbiol 2008;51:47-8.
25.Leonardi MA,Lumenta CB,Stölzle A,et al.Unusual clinical presentation of a meningeal melanocytoma with seizures:case report and review of the literature.Acta Neurochir(Wien) 1998;140:621-8.
26.Beseoglu K,Knobbe CB,Reifenberger G,et al.Supratentorial meningeal melanocytoma mimicking a convexity meningioma.Acta Neurochir (Wien) 2006;148:485-90.
27.O’Brien TF,Moan M,Miller JH,et al.Meningeal melanocytoma.An uncommon diagnostic pitfall in surgical neuropathology.Arch Pathol Lab Med 1995;119:542-6.
28.Naul LG,Hise JH,Bauserine SC,et al.CT and MRI of meningeal melanocytoma.AJNR Am J Neuroradiol 1991;12:315-6.
29.Litofsky NS,Zee CS,Breeeze RE,et al.Meningeal melanocytoma:diagnostic criteria for a rare lesion.Neurosurgery 1992,31:945-8.
30.Fagundes-Pereyra WJ,Sousa L,Carvalho GT,et al.Meningeal melanocytoma of the posterior fossa:case report and literature review.Surg Neurol 2005;63:269-74.
31.Tewari MK,Radotra BD,Sharma BS,et al.Meningeal melanocytoma:report of two cases.Indian J Cancer 1990;27:133-7.
32.Schindler CU,Kuchelmeister K,Richter HP,et al.Meningeal melanocytoma.Pathologe 1998;19:325-9.
33.Kawaguchi T,Kawano T,Kazekawa K,et al.Meningeal melanocytoma in the left frontal region.Brain Tumor Pathol 1998;15:58-62.
34.Clarke DB,Leblanc R,Bertrand G,et al.Meningeal melanocytoma:report of a case and a historical comparison.J Neurosurg 1998;88:116-21.
35.Hou GQ,Sun JC,Zhang XJ,et al.MR imaging findings of the intraspinal meningeal melanocytoma:correlation with histopathologic findings.AJNR Am J Neuroradiol 2012;[Epub ahead of print].
36.Matsumoto S,Kang Y,Sato S,et al.Spinal meningeal melanocytoma presenting with superficial siderosis of the central nervous system.J Neurosurg 1998;88:890-4.
 Chinese Medical Sciences Journal2012年2期
Chinese Medical Sciences Journal2012年2期
- Chinese Medical Sciences Journal的其它文章
- Hemi-semi Laminectomy Approach for the Microsurgical Treatment of Spinal Schwannomas
- Correlations Between Serum Uric Acid Level and Disease Activity,Intrathecal Inflammation Reactivity in Patients with Multiple Sclerosis
- Sternal Insufficiency Fractures of Post-menopausal Women:Retrospective Analysis of 17 Cases
- Fresh Frozen Plasma for the Treatment of Hereditary Angioedema Acute Attacks
- Function of microRNA-346 and its Roles in Human Diseases
- Multiple Coatings can Improve the Bond Durability of One-step Self-etching Adhesive to Primary Dentin
