Role of ADAM10 and ADAM17 in CD16b Shedding Mediated by Different Stimulators△
Sha Guo,Min Peng,Qing Zhao,and Wei Zhang*
Department of Immunology,Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100005,China
CD16B (FcγRIIIb) is a low affinity IgG Fc receptor which attaches to cell membranes by glycosylphosphatidylinositol (GPI) anchor.Because of the obvious quantity advantage,CD16b is the dominant Fcγ receptor responsible for the immune complex(IC) induced oxidative burst and other inflammatory process.1Besides,studies also found increased level of soluble CD16b in inflammatory site.2Its functions include inhibitory effects on B cell proliferation and IgG/IgM production,prevention of Fc-mediated inflammation by competing for ligand with cell surface Fcγ receptor.3,4All of the above indicate that soluble CD16b does play an important role in Fc dependent immune responses.
Shedding has already been shown to play a crucial role in development,inflammation,and diseases.5As to CD16b,shedding is an effective regulation mechanism compared with polymorphism,glycosylationet al,since loose connection of GPI-linked proteins with membranes endows them with more liability to cleavage by enzymes.6Earlier studies indicated that both metalloproteinases and serine proteinases were involved in phorbol 12-myristate-13-acetate (PMA) induced shedding of CD16b.7,8But inhibitors of metalloproteinases were much more effective than inhibitors of serine proteinases in inhibiting CD16b shedding after stimulation with PMA.However,the shedding could not be rescued by tissue inhibitor of metalloproteinase 1(TIMP-1),the inhibitor of matrix metalloproteinases.7Therefore,other kind of metalloproteinases may be responsible for the shedding.
There are more than 30 a disintegrin and metalloproteinases (ADAMs) identified so far.Many of them are important for ectodomain shedding.Among them,ADAM10 and ADAM17 have received extensive attention.ADAM17 is capable of cleaving ectodomains of numerous proteins such as CD89,Mer tyrosine kinase receptor.9,10ADAM10 is closely related to ADAM17 in terms of structure,function,and substrates.Besides,ADAM10 possesses the ability to cleave GPI anchored proteins such as the prion protein(PrPc).5In this study,we investigated both ADAM10 and ADAM17 on shedding of CD16b.The methods used here were knock-in,knock-down and reconstitution,which were not applied to studies of CD16b shedding before.Besides,we have considered the influence of different stimulators on shedding process,which could replenish the substrate list of ADAMs under defined situations.In addition to the confirmation of functional selectivity for the two ADAMs,the potential value of this phenomenon was discussed.
MATERIALS AND METHODS
Antibodies and reagents
The murine monoclonal anti-CD16 antibody,3G8,was kindly donated by Dr.J.C.Unkeless (Mount Sinai School of Medicine,New York,USA).F(ab’)2fragments of 3G8 was made by digestion with pepsin (Sigma-Aldrich,St Louis,MO,USA).Fluorescein isothiocyanate (FITC,Sigma-Aldrich)-labeled F(ab’)2fragments of 3G8 and horseradish peroxidase (HRP)-labeled 3G8 were prepared as described previously.113G8 was also coupled to sepharose4B beads(GE Healthcare,Connecticut,USA).Goat polyclonal anti-ADAM10 (A10) and anti-ADAM17 (C15) antibodies were from Santa Cruz (California,USA).Rabbit-anti-goat IgG-HRP was from AbD SeroTek (Kidlington,UK).Mouse anti-GAPDH was from Bioon BioTek (Beijing,China).PMA and ionomycin were from Sigma-Aldrich.GM6001 was from Millipore (Massachusetts,USA).The plasmids,bovine ADAM10 (bADAM10) and murine ADAM17 (mADAM17) in pcDNA3.1,were provided by Zena werb.12
Construction of plasmid
The complementary DNA (cDNA) of CD16b was obtained by RT-PCR (TransGen BioTec,Beijing,China) from human peripheral white blood cells.The sense and anti-sense primers for CD16b were as follows∶sense,5’CGGGATCCACCATGTGGCAGCTGCTCCT3’;antisense,5’CGACGCGTCGAATGTTTGTCTTCACAGAG3’.
Thirty cycles of amplification (denaturation at 95°C for 5 minutes;annealing at 56°C for 45 seconds;extension at 72°C for 75 seconds) were carried out.The final extension was at 72°C for 10 minutes.CD16b cDNA was then inserted into a lentiviral expression vector pWPXL (donated by Dr.Didier Trono,Global Health Institute,School of Life Sciences and“Frontiers in Genetics”National Center for Competence in Research,Lausanne,Switzerland) betweenBamHIandMluIsites (pWPXL-CD16b).13The constructed plasmid was sequenced and was identical to the sequence of Genbank AJ581669.
Establishment of CD16b expressing cell line
293T cells and HEK293 cells (Cell Center,Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences)were cultured in DMEM containing 10% fetal bovine serum and antibiotics at 37°C in humidified atmosphere with 5%CO2.293T cells were co-transfected with packaging vector psPAX2,envelope vector pMD2.G and pWPXL-CD16b using Vigofect (Vigorous BioTec,Beijing,China).HEK293 cells stably expressing CD16b were produced by infection with the supernatants from 293T cells supplemented with 8-10 μg/mL of hexadimethrine bromide (Polybrene,Chemicon International Inc.,California,USA).HEK293 cells expressing CD16b were cloned by limited dilution.Monoclone3 (M3) was identified as a stable CD16b expressing cell line by RT-PCR,flow cytometry,and Western blot analyses.
Overexpression and knock-down of ADAM10 and ADAM17
To overexpress ADAM10 and ADAM17,M3 cells were transiently transfected with bADAM10 or mADAM17 using Vigofect 48 hours before detection and further treatment.
On the other hand,short hairpin RNAs (shRNAs) were used to interfere endogenous expression of ADAM10 or ADAM17.The target sequences against human ADAM10(GACATTTCAACCTACGAAT) and ADAM17 (CACATGTAGAAACACTACT) were referenced from previous publications.14,15The shRNAs (sh10 for ADAM10,sh17 for ADAM17)were inserted into lentiviral vector pLVTHM (LV,donated by Dr.Didier Trono,Global Health Institute,School of Life Sciences and“Frontiers in Genetics”National Center for Competence in Research,Lausanne,Switzerland) betweenMluIandClaIsites.Then M3 cells were infected with LV-sh10 or LV-sh17 using the same way for establishing CD16b cell line described above.
Measurement of CD16b,ADAM10,and ADAM17 in cell lysates
A total of 107M3 cells were lysed with Radio Immunoprecipitation Assay (RIPA) lysis buffer supplemented with 1 mmol/L of phenylmethanesulfonyl fluoride (PMSF),1 μg/mL of aprotinin,1 μg/mL of leupeptin,and 1 μg/mL of pepstatin to detect ADAM10 and ADAM17 levels in cell lysate using Western blot analysis.
After centrifugation (15 000 ×g,10 minutes,4°C) and quantification by BCA (Vigrous,Beijing,China),the lysates were run on 10% SDS-PAGE and transferred onto nitrocellulose (NC) membranes.After blocking with 5% skimmed milk for 2 hours at room temperature,the membranes were incubated with 3G8-HRP (1∶500),A10 (1∶200)or C15 (1∶200) at 4°C overnight,respectively.The latter two were then incubated with rabbit-anti-goat IgG-HRP for 1 hour at room temperature.The bands were visualized by ECL Western Blot Substrate (Pierce,Rockford,IL,USA).GAPDH was visualized as loading control.
Measurement of CD16b shedding by immunoprecipitation and immunoblot
For detection of CD16b shedding,5×107M3 cells after treatments (overexpression,knock-down or reconstitution)were seeded in 6-well plate in 2 mL Hanks balanced salt solutions (HBSS).PMA (10 ng/mL) and ionomycin (2 μmol/L) were used to stimulate the cells for 12 hours.The cell culture supernatants were harvested by centrifugation(15 000 ×g,10 minutes,4°C).Then soluble CD16b in supernatant was absorbed using 3G8-sepharose4B beads at 4°C overnight with rotation.The beads were washed three times with phosphate-buffered saline (PBS) and boiled in 2×SDS loading buffer for 5 minutes.3G8 bound protein was separated and detected by 10% SDS-PAGE as above.Same volumes of supernatants were also run on 10% SDS-PAGE and then stained by Coomassiebrilliantblue R-250 (Ausable BioTek,Beijing,China),among which the BSA bands were taken as loading control.Immunoblot results were scanned by BandScan 5.0 software.
Statistical analysis
Data were expressed as mean±SE.Quantification of CD16b shedding was done by taking mock as the basic level.Student’sttest was performed to determine statistical significance (SPSS 17.0) between mock and other transfections.Pvalues <0.05 were regarded as statistically significant.
RESULTS
Identification of CD16b stably expressing HEK293 cell line
The inserted CD16b had 702 bp in length (Fig.1A) and its expression could be detected by flow cytometry and Western blot using 3G8 which is specifically against CD16(Fig.1B,1C).The expressed CD16b possessed a molecular weight of 48-54 kDa,which could be explained by different rate of glycosylation.All of the above demonstrated the successful establishment of CD16b expressing cell line.
Both ADAM10 and ADAM17 contributed to CD16b shedding
After 48 hours of bADAM10 or mADAM17 transfection,Western blot analysis revealed higher expression of ADAM10 or ADAM17 in M3 cells (Fig.2A).As measured,1.5 to 1.7 (ADAM10) and 2.0 to 2.3 (ADAM17) times expression of their constitutive expression were reached.
Having confirmed that ADAM10 and ADAM17 could be overexpressed above their basal levels,we then used PMA or ionomycin to stimulate M3 cells for 12 hours to test their abilities to shed CD16b.Resultsshowed that overexpression of ADAM17 but not ADAM10 caused increased shedding of CD16b in PMA stimulated M3 (Fig.2B).In comparison,overexpression of ADAM10 but not ADAM17 caused increased shedding of CD16b in ionomycin stimulated M3 (Fig.2C).Therefore,both ADAM10 and ADAM17 contributed to CD16b shedding but their effects differed under different stimuli.
Inhibition of ADAM10 and ADAM17 expression reduced CD16b shedding
We infected M3 with shRNA specifically silencing ADAM10 or ADAM17 using the lentivirus system.Figure 3A shows that shRNA specifically inhibited ADAM10 or ADAM17 expression.The remained ADAM10 and ADAM17 were about 0.1-0.5 and 0.2-0.4 of the original counterparts.
In the presence of shRNA to ADAM17,shedding of CD16b was reduced in PMA stimulated M3 (Fig.3B-left)and in the presence of shRNA to ADAM10,shedding of CD16b was reduced in ionomycin stimulated M3 (Fig.3Bright).As expected,shRNA to ADAM10 had no diminishing effects on PMA stimulated M3 and shRNA to ADAM17 had no diminishing effects on ionomycin stimulated M3 either.
Reconstitution of ADAM10 and ADAM17 double-silenced M3 cells with wild type ADAM10 and ADAM17
In order to further determine the role of ADAM10 and ADAM17 in CD16b shedding under different stimuli,we did the reconstitution experiments.The M3 cells were firstly co-infected with sh10 and sh17 by lentivirus infection.After infection with the lentivirus system,M3 cells were then transiently transfected with mock vector,bADAM10 or mADAM17.At 48 hours post transfection,the cells were stimulated with PMA or ionomycin separately.The super-natants containing soluble CD16b were determined.In Figure 3C,we could see that reconstitution of ADAM17 enabled double-silenced cells to shed more CD16b under stimulation of PMA compared with both mock and ADAM10 reconstitution (left).Similarly,double-silenced cells reconstituted with ADAM10 shed more CD16b under stimulation of ionomycin compared with both mock and ADAM17 reconstitution (right).The experiments demonstrated the restoration ability of ADAM10 and ADAM17 to CD16b shedding under ionomycin and PMA stimulation,which is an additional evidence to validate the roles of ADAM10 and ADAM17 in shedding CD16b.

Figure 1.Identification of CD16b expressing HEK293 cell line (M3).

Figure 2.CD16b shedding from M3 cells transfected with bADAM10 or mADAM17.
DISCUSSION
In this study,by using CD16b transfected HEK293 cells,we demonstrated that members of the ADAM family,ADAM10 and ADAM17,could cleave extracellular domain of CD16b to generate soluble receptor.Furthermore,we found that ADAM17 was the main sheddase under stimulation of PMA and ADAM10 was the main sheddase under stimulation of ionomycin in HEK293 cells.
Though we demonstrated that ADAM10 and ADAM17 played different roles under stimulation by PMA (a PKC activator) or ionomycin (calcium ionophore) in shedding of CD16b,we were not clear how the specificity and selectivity were achieved.The functions of ADAM10 and ADAM17 have some overlapping since ADAM10 could shed some ADAM17 substrates under ionomycin stimulation in ADAM17-/-mouse embryonic fibroblasts,16indicating a functional intersection between the two pathways.Nevertheless the overlapping is not complete as they possess differed expression profile on cell surface,17,18which indicates that the two pathways could not totally compensate for each other and are relatively independent.This could also partially explain why shedding of CD16b still exists or even augments under stimulation of PMA when ADAM10 was knocked down,or under stimulation of ionomycin when ADAM17 was knocked down (Fig.3B).By and large,the preference of the two enzymes could ensure prompt and timely response to various stimuli,which fits the functions of CD16b.
Shedding is one of the regulatory mechanisms of CD16b function,but it seems not to be isolated.Copy number of Fcγ receptor 3B correlates with protein expression,IC uptake,as well as soluble CD16b level.Soluble CD16b decreases with lower copy number of Fcγ receptor 3B.In autoimmune diseases such as systemic lupus erythematosus,inefficient clearance of IC by Fcγ receptors on circulating cells and soluble Fcγ receptors,which correlates with low copy number of Fcγ receptor 3B,predisposes potential impairment mediated by deposition of IC onto tissues.19,20Therefore,the inadequacy of soluble CD16b is also one of the pathological factors in disease progression.In another aspect,membrane CD16b has been suggested to play an important role in intriguing cell activation and tissue damage.21To reduce inflammation,CD16b shedding is an attractive way in therapy of diseases such as rheumatoid arthritis,22which could both reduce the membrane expression and compete with the membrane form for binding to ICs.So determining the sheddases responsible for CD16b is necessary to illustrate this process.
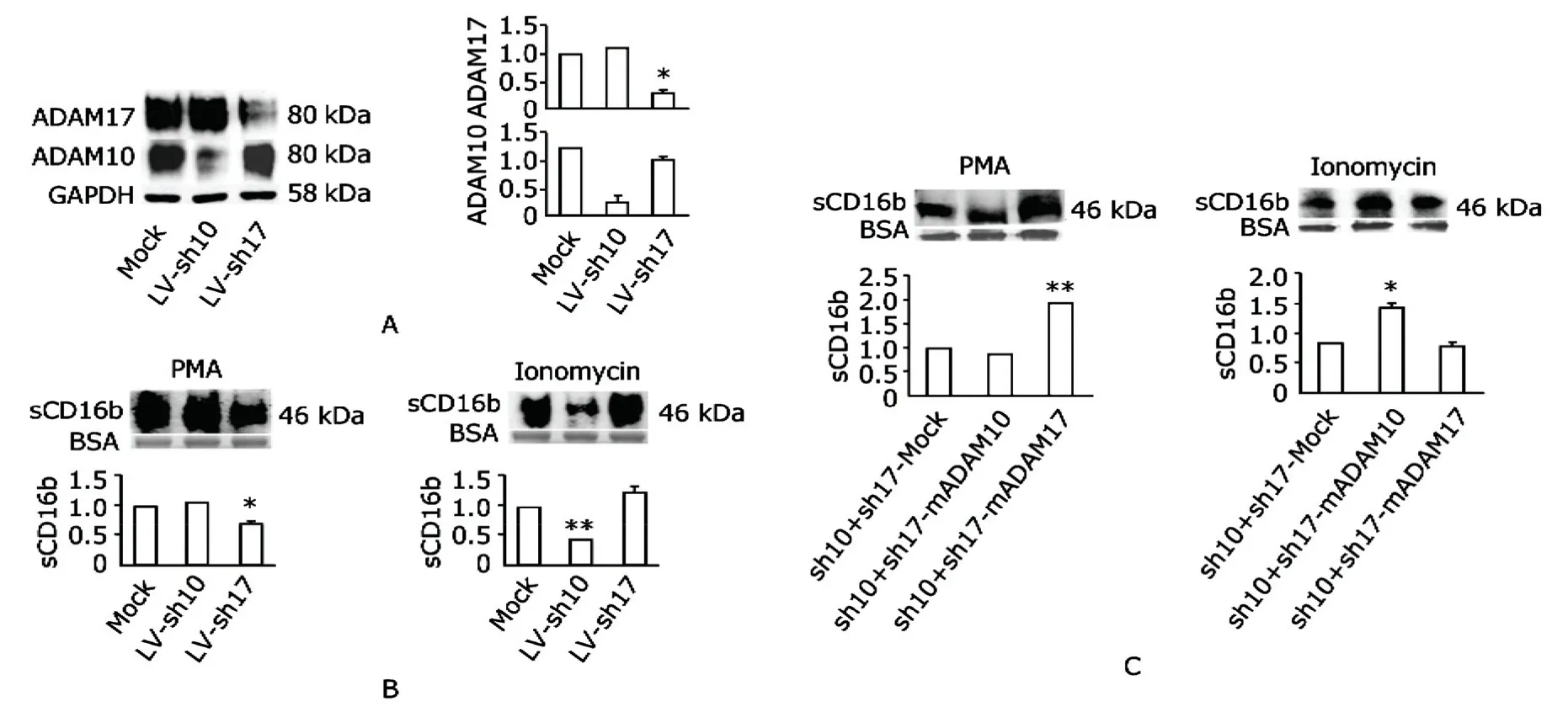
Figure 3.Effects of ADAM10 and ADAM17 knock-down and reconstitution on CD16b shedding from M3.
In conclusion,both ADAM10 and ADAM17 are sheddases of CD16b but with different preference to stimuli.ADAM17 is more potent under PMA stimulation and ADAM10 is more potent under ionomycin stimulation.Therefore,the two enzymes may play different roles in the regulation of CD16b shedding and if possible,the consequent applications of soluble CD16b.
ACKNOWLEDGMENT
We thank Jing Xue,Xi Yang,Lin Zhang,Xiao-yan Wang,Lian Shen,and Li-ping Zhu for technique supports and helpful discussions.
1.Jakus Z,Németh T,Verbeek JS,et al.Critical but overlapping role of FcgammaRIII and FcgammaRIV in activation of murine neutrophils by immobilized immune complexes.J Immunol 2008;180∶618-29.
2.Masuda M,Miyoshi H,Kobatake S,et al.Increased soluble FcgammaRIIIa(Mphi) in plasma from patients with coronary artery diseases.Atherosclerosis 2006;188∶377-83.
3.Li P,Nagarajan S,Zhu C,et al.Recombinant CD16A-Ig forms a homodimer and cross-blocks the ligand binding functions of neutrophil and monocyte Fcgamma receptors.Mol Immunol 2002;38∶527-38.
4.Siiman O,Burshteyn A,Concepcion O,et al.Competitive antibody binding to soluble CD16B antigen and CD16B antigen on neutrophils in whole blood by flow cytometry.Cytometry 2001;44∶30-7.
5.Taylor DR,Parkin ET,Cocklin SL,et al.Role of ADAMs in the ectodomain shedding and conformational conversion of the prion protein.J Biol Chem 2009;284∶22590-600.
6.Lauc G,Heffer-Lauc M.Shedding and uptake of gangliosides and glycosylphosphatidylinositol-anchored proteins.Biochim Biophys Acta 2006;1760∶584-602.
7.Middelhoven PJ,Van Buul JD,Hordijk PL,et al.Different proteolytic mechanisms involved in Fc gammaR IIIb shedding from human neutrophils.Clin Exp Immunol 2001;125∶169-75.
8.Moldovan I,Galon J,Maridonneau-Parini I,et al.Regulation of production of soluble Fc gamma receptors type III in normal and pathological conditions.Immunol Lett 1999;68∶125-34.
9.Peng M,Guo S,Yin N,et al.Ectodomain shedding of Fcα receptor is mediated by ADAM10 and ADAM17.Immunology 2010;130∶83-91.
10.Thorp E,Vaisar T,Subramanian M,et al.Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species,protein kinase Cδ,and p38 mitogen-activated protein kinase (MAPK).J Biol Chem 2011;286∶33335-44.
11.Yin N,Peng M,Xing Y,et al.Intracellular pools of FcαR(CD89) in human neutrophils are localized in tertiary granules and secretory vesicles,and two FcαR isoforms are found in tertiary granules.J Leukoc Biol 2007;82∶551-8.
12.Lemieux GA,Blumenkron F,Yeung N,et al.The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10.J Biol Chem 2007;282∶14836-44.
13.Xue J,Zhao Q,Zhu L,et al.Deglycosylation of FcalphaR at N58 increases its binding to IgA.Glycobiology 2010;20∶905-15.
14.Reiss K,Maretzky T,Ludwig A,et al.ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and betacatenin nuclear signalling.EMBO J 2005;24∶742-52.
15.Maretzky T,Schulte M,Ludwig A,et al.L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion,cell migration,and neurite outgrowth.Mol Cell Biol 2005;25∶9040-53.
16.Le Gall SM,Bobé P,Reiss K,et al.ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor alpha,L-selectin,and tumor necrosis factor alpha.Mol Biol Cell 2009;20∶1785-94.
17.Bret C,Hose D,Reme T,et al.Gene expression profile of ADAMs and ADAMTSs metalloproteinases in normal and malignant plasma cells and in the bone marrow environment.Exp Hematol 2011;39∶546-57.
18.Dehmel T,Janke A,Hartung HP,et al.The cell-specific expression of metalloproteinase-disintegrins (ADAMs) in inflammatory myopathies.Neurobiol Dis 2007;25∶665-74.
19.Willcocks LC,Lyons PA,Clatworthy MR,et al.Copy number of FCGR3B,which is associated with systemic lupus erythematosus,correlates with protein expression and immune complex uptake.J Exp Med 2008;205∶1573-82.
20.Morris DL,Roberts AL,Witherden AS,et al.Evidence for both copy number and allelic (NA1/NA2) risk at the FCGR3B locus in systemic lupus erythematosus.Eur J Hum Genet 2010;18∶1027-31.
21.Nagarajan S,Fifadara NH,Selvaraj P.Signal-specific activation and regulation of human neutrophil Fc gamma receptors.J Immunol 2005;174∶5423-32.
22.Masuda M,Morimoto T,Kobatake S,et al.Measurement of soluble Fcgamma receptor type IIIa derived from macrophages in plasma∶increase in patients with rheumatoid arthritis.Clin Exp Immunol 2003;132∶477-84.
 Chinese Medical Sciences Journal2012年2期
Chinese Medical Sciences Journal2012年2期
- Chinese Medical Sciences Journal的其它文章
- Status and Change Pattern of Kidney Transplantation:One Center Research
- Gastric Metastasis of Atypical Medullary Carcinoma from Breast:a Case Report
- Cardiac Electrophysiological Differences Between Kunming and C57BL6/J Mice△
- Bilateral Ureteral Fibroepithelial Polyps:a Case Report
- Multiple Coatings can Improve the Bond Durability of One-step Self-etching Adhesive to Primary Dentin
- Function of microRNA-346 and its Roles in Human Diseases
