Cardiac Electrophysiological Differences Between Kunming and C57BL6/J Mice△
Teng Wang*,Mu Qin,He Huang,Hong-liang Li,and Cong-xin Huang
Department of Cardiology,Renmin Hospital of Wuhan University,Cardiovascular Research Institute of Wuhan University,Wuhan 430060,China
AS powerful tools to study disease models,mice have been widely used in most disease research.1So not surprisingly,a lot of efforts have been invested to characterize the mechanisms underling the cardiac electrophysiology about Kunming(KM) mice,which are rather common to grow up in China.In the past decade,several techniques for the study of cardiac disease models in mice have been developed,including aortic banding for cardiac hypertrophy,anterior descending branch ligation for myocardial infarction,and genetically modified mice models.2Arrhythmia,the serious electrophysiological disorders of heart,is one of high risk factors to cardiac sudden death.Recently,many researchers have dedicated to the study of arrhythmia in mouse models.Over 10 000 different transgenic mouse models have been used to study electrical causes of heart diseases.3But properties of mouse heart including small volume,fast heart rate (HR),as well as lower T-wave,result to lower arrhythmic inducibility,unstable electrophysiological recording,and hard analysis.Thus,those negative factors restricted the development of researches.
Our currently experimental setup is to find the stable arrhythmia mouse model in KM and C57BL6/J (C57) mice and elucidate their electrophysiological properties alterations.It was noticed that the transient outward potassium current (Ito) as one of the main ion channels in 1 phase of action potential (AP) maybe played a radical role for the cardiac electrophysiological mechanisms of QT interval prolongation induced the cardiac arrhythmias in mice.4Here we systematically describe the cardiac electrophysiological parametersin vivoandin vitrothrough compared with the electrophysiological differences between KM and C57 mouse hearts.Especially,we answer the possible mechanism about the differences of cardiac electrophysiology with whole-cell patch-clamp technique.
MATERIALS AND METHODS
Electrocardiogram measurements and analysis
Six-lead surface-limb electrocardiogram measurements (I,II,III,aVF,aVL,and aVR) were recorded on 8-to 12-week-old male KM and C57 mice,which were provided by Experimental Animal Centre of Medical College of Wuhan University.After anesthetized with 60 mg/kg pentobarbital sodium,mice were prone positioned on a custom-made electrocardiogram recording platform.Body temperature was maintained at 37°C by use of a thermally controlled heating pad.Ag/AgCl gel-coated electrocardiogram electrodes were attached to the two front and left rear paws of the mouse.The electrodes were connected to a standard 6-lead electrocardiogram amplifier module(ADInstruments,Australia),which included high-and low pass filters (set to 0.3 Hz and 1 kHz,respectively) and a gain selection device (set to 1000-fold).Signals were digitized continuously at 1 kHz and recorded by using the data acquisition system (ADInstruments,Australia).For ach mice the data were collected twice with a time interval of 2 days (2×30 minutes of continuous recording).The software (LabChart Pro V7,ADInstruments,Australia) was used to analyze the electrocardiogram recorded data.For each mouse,analysis of the cardiac rhythm and measurements of HR were performed on 30 minutes of continuous experimental recording.The P-wave duration (Pd)was measured from the first deflection of the P-wave to the point where it rejoined the isoelectric line.The latter was set at the first deflection of the P-wave.The PR interval was measured from the beginning of the P-wave to the beginning of the QRS complex.QRS duration was measured from the first deflection of the Q-wave (or the R-wave when the Q-wave was absent) to the point where the negative part of the S-wave returned to the isoelectric line.The QT interval was measured from the beginning of the QRS complex to the end of the T-wave.T-end was defined as the point where the negative part of the T-wave returned to the isoelectric line.RR interval was determined automatically by averaging individual RR intervals for the 60-second period.5
Preparation of Langendorff-perfused hearts
The isolated heart was quickly excised and transferred to ice-cold bicarbonate-buffered Krebs-Henseleit solution(mmol/L∶NaCl 119,NaHCO325,KCl 4,KH2PO41.2,MgCl21,CaCl21.8,glucose 10,and sodium pyruvate 2;pH 7.4)bubbled with 95%O2-5%CO2.A small section of aorta (3-4 mm) was identified and cannulated with a tailor-made 21-gauge cannula that had been prefilled with ice-cold buffer.The aorta was secured onto the cannula with a micro-aneurysm clip.The heart was then rapidly transferred and fixed to the Langendorff perfusion system(ADInstruments,Australia).The perfusate was passed through 200 and 5 μm filters (Gene Corporation),and then warmed to 37°C by a water jacket and circulator.Perfusion was commenced in a retrograde manner through the aorta at 2-2.5 mL/min by a peristaltic pump.In this way,the heart was perfused by the Krebs-Henseleit solution passing through the aorta,into the coronary ostium,and then into the coronary arteries,draining into the right atrium.After the initiation of perfusion,hearts regained a pink colour and spontaneous rhythmic contractions.
Bipolar electrogram recording and arrhythmia induction
To examine the ventricular arrhythmia,the bipolar electrogram (BEG,ADInstruments,Australia) was recorded from the epicardial surface of the left ventricle using a silver chloride (2 mm tip diameter) recording electrode.The paired platinum stimulating electrodes paced the epicardial surface of the right ventricle and hearts were stimulated pulsesvia1 ms square-wave stimuli at two times excitation threshold (Grass,USA).These signals were amplified using an amplifier (ADInstruments,Australia) and band pass filtered between 0.3 Hz and 1 kHz.All digitized data was then captured and analyzed using LabChart Pro V7 software.
Monophasic AP (MAP) recording
MAP was recorded from the right and left ventricular epicardium by a custom-made MAP electrode,which composed of two strands of 0.25 mm Teflon-coated silver wire (99.99% purity).The tips of the MAP electrode had previously been galvanically chlorided to eliminate direct current offset.MAPs were amplified with an amplifier and band pass filtered between 0.3 Hz and 1 kHz.MAP waveforms were analyzed using LabChart Pro V7 software.
Electrical stimulated protocol
The programmed electrical stimulation (PES) and Burst pacing is thought to be the conventional method to induce arrhythmia.PES induction is thought to mimic physiological initiations of ventricular tachycardia (VT),for the premature ventricular contraction often provokes VT episodes.As the nonphysiological induction,the Burst pacing often reliably induce VTviacardiac electrical instability.
PES was made by cycles each consisting of a drive train of eight stimuli (S1) at a cycle length of 125 ms followed by the ninth extra-stimulus (S2).The first S1-S2interval equaled the pacing interval.Successive cycles progressively reduced the S1-S2interval by 1 ms until the S2stimulus could no longer evoke a ventricular deflection.6In arrhythmic induction,both PES and Burst pacing (2 ms pulses at 50 Hz,400 ms burst duration) were used to determine susceptibility to ventricular arrhythmia induction.7Burst pacing used up to 30 bursts of pacing in both ventricular locations.
The incidence of arrhythmias was analyzed by the inducibility and duration of arrhythmias based on PES and Burst pacing.According to the duration VT episode,the mice were divided into four groups∶no response,less than 10 seconds,between 10 and 30 seconds,and greater than 30 seconds groups.
Ventricular myocytes isolation
Single left and right ventricular myocytes were obtained from hearts of 12-to 14-week-old male mice as described previously.8,9The heart was rapidly removed and mounted on a Langendorff apparatus by aortic canulation,and perfused initially for 10 minutes with normal Tyrode's solution,and then switched to a nominally Ca2+-free solution for an additional 5 minutes.Tyrode's solution had the following composition (in mmol/L)∶NaCl 125,NaHCO324,NaH2PO40.42,KCl 5.4,CaCl21.8,MgCl21.05,glucose 11,and taurine 10.The all perfusion solutions were bubbled with 100% O2,and pH adjusted to 7.4 with NaOH.Thereafter,hearts were perfused for 30 minutes with a zerocalcium solution containing 0.08 mg/mL type II collagenase (Sigma Chemical Co,USA) and 0.05 mg/mL protease XIV (Sigma Chemical Co,USA).The enzymes were washed out by perfusion with a high-potassium,lowchloride saline (KB medium) for 5 minutes.The subepicardial layers were respectively dissected from the left and right ventricular free wall.10Single cells were maintained in a KB medium at 4°C for up to 2 hours before used by electrophysiological experiments.Normally,Ca2+-free solution was prepared by omitting CaCl2from the Tyrode's solution.The KB medium had the following composition (in mmol/L)∶potassium glutamate 80,KCl 40,taurine 20,KH2PO43,glucose 10,HEPES 10,and EGTA 0.2.The pH was adjusted to 7.4 with KOH.
Itoand current-voltage relationship (I-V) curve of Itorecordings
Itorecordings were performed on rod-shaped myocytes with clear cross striations and no spontaneous contractions using the whole-cell configuration of the patch-clamp technique.11During the experiments,the myocytes,which were placed in a 500 μL chamber on the stage of an inverted microscope (IX70-122,Olympus,Japan) and allowed to adhere onto the glass bottom of the chamber for 10-15 minutes,were continuously superfused with the extracellular solution (2 mL/min).And solution exchange of the bath was achieved within 30 seconds.The external solution had the following composition (in mmol/L)∶NaCl 140,KCl 5.4,CaCl20.1,CoCl20.4,MgCl21.0,HEPES 10,and glucose 11,and pH was adjusted to 7.4 with NaOH.The recording patch pipettes were pulled from borosilicate glass with a two-stage vertical puller (PP-83,Narishige,Japan).Resistances of pipettes ranged from 2.5 to 3.5 MΩ when filled with pipette solution.The pipette solution had the following composition (in mmol/L)∶potassium aspartate 90,KCl 54,KH2PO410,HEPES 5,EGTA 0.1,pH adjusted to 7.3 with KOH.Transmembrane currents were recorded by an EPC-9 amplifier (List Instruments,Germany),which received rectangular voltage pulses from a PC with running Pulse-pulsefit software interface (Version 8.31,HEKA Co.,Germany).Capacitance transients and leakage currents were corrected by the EPC-9 amplifier,which provided compensation for series resistance.Current signals were filtered at 3 kHz by a 8-pole Bessel filter,digitized at a sampling rate of 1 kHz,stored on the computer running Pulse-pulsefit software which was additionally used for the generation of voltage pulses and data analysis.
I-V curve of Itowere elicited by 500 ms depolarizing voltage pulses from a holding potential of -80 mV,to different test potential at 10 mV increased from -50 mV to+50 mV.It suggested that the current was the transient outward current,which was activated and inactivated rapidly as well as could be inhibited by 2 mmol/L 4-aminopyridine.The maintained component,which did not show time dependent current decay,activated at potential positive to -30 mV,and increased the current amplitude along with the test voltage increase.Ionic currents were corrected for cell capacitance and are expressed in terms of current density (in pA/pF).The experiments were performed at room temperature (22-25°C).
Statistical analysis
Measurement data were expressed as mean±SEM.Statistical analysis was performed withFisherexact test and Student’sttest by using SPSS 16.0.A value ofP<0.05 was considered statistically significant.Patch-clamp data were analyzed using Pulsfit and Origin 6.0 (Microcal,USA) for plotting graphs.
RESULTS
Surface electrocardiogram
To determine the cardiac electric differencein vivo,the surface electrocardiogram were recorded in KM (Fig.1A)and C57 mice (Fig.1B).Comparisons between KM and C57 mice showed that the QT interval was prolonged in KM mice relative to C57 mice (P<0.05,Table 1).In addition,the PR interval and QRS duration had significant difference between two groups (allP<0.05),but the P-wave duration was unchanged (Table 1).
Epicardial MAP recordings during regular pacing and PES
MAPs were recorded from the left and right ventricular epicardium during regular pacing to determine whether prolongation of the QT interval was associated with electrical modifications in KM and C57 mice.In isolated hearts of KM mice,we observed a significant prolongation of MAP duration recorded from the left ventricular epicardium at 50% repolarization and MAP duration of the right ventricular epicardium at 50% and 70% repolarization,compared with C57 mice,respectively (allP<0.05;Fig.2,Table 2).Moreover,it was not significantly altered on MAP amplitude and diastolic membrane potential in the left and right ventricular epicardium of both KM and C57 mice.
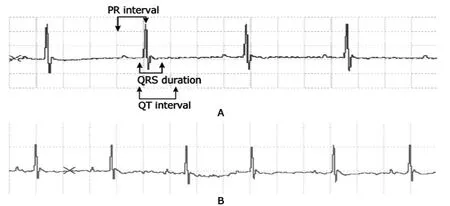
Figure 1.Recordings of surface lead Ⅱ of electrocardiogram in Kunming (KM,A) and C57BL6/J (C57) mice (B).

Table 1.Comparisons of main parameters of surface electrocardiogram in KM and C57 mice§ (ms)

Figure 2.Recordings of monophasic action potential (MAP) obtained from the left and right ventricular epicardium in KM and C57 mice.
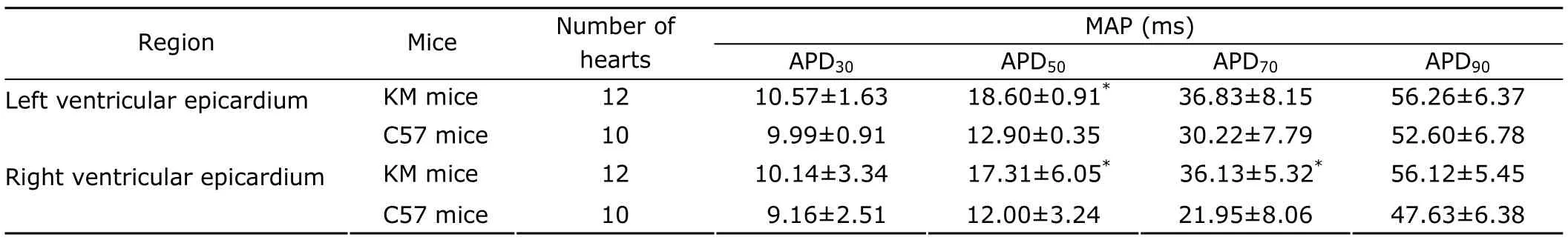
Table 2.Comparisons of MAP duration at 30%,50%,70%,and 90% full repolarization in the right and left ventricular epicardium of KM and C57 mice§
PES and Burst induced VT
The incidences of PES-induced VT and Burst-induced VT in KM mice (25%, 3 of 12 hearts and 50%, 6 of 12 hearts)were higher than those in C57 mice (20%, 2 of 10 hearts and 30%, 3 of 10 hearts, all P<0.05). The duration of induced VT was longer in KM mice in which VT lasted greater than 30 seconds (16.7%, 2 of 12 hearts) and between 10 and 30 seconds (8.3%, 1 of 12 hearts) than that in C57 mice (0, P<0.01; Table 3).
Recordings of Ito in the ventricular epicardial myocytes obtained from KM and C57 mice
Figure 3A-D displays representative recordings of Itocurrents of the left and right ventricular epicardial myocytes isolated from KM and C57 mice. At 50 mV, Itodensity of the left ventricular epicardial myocytes was significantly lower than that of the right ventricular epicardial myocytes for KM mice (11.8±4.5 pA/pF versus 17.8±3.3 pA/pF, P<0.05;Fig.3E).Compared with KM mice,Itodensity of C57 mice at 50 mV was significantly increased by about 45% in left ventricular epicardial myocytes and increased by 60% in the right ventricular epicardial myocytes,respectively (allP<0.01).Figure 3E shows the changes of I-V curve of Itoin left and right ventricular epicardial myocytes isolated from KM and C57 mice.For KM mice,Itodensities of the right ventricular epicardial myocytes were significantly altered upward compared to left ventricular epicardal myocytes;however for C57 mice,no significant difference was noted between right and left ventricular epicardial myocytes.But Itodensities of both the right and left ventricular epicardial myocytes in C57 mice were significantly higher than those in KM mice (allP<0.01).Fig.3E).But for C57 mice,the difference in Itodensity between the left and right ventricular epicardial myocytes had no significance (25.9±4.2 pA/pF,n=8 and 29.2±6.7 pA/pF,n=10;P>0.05;Fig.3E).Compared with KM mice,Itodensity of C57 mice at 50 mV was significantly increased by about 45% in left ventricular epicardial myocytes and increased by 60% in the right ventricular epicardial myocytes,respectively (allP<0.01).Figure 3E shows the changes of I-V curve of Itoin left and right ventricular epicardial myocytes isolated from KM and C57 mice.For KM mice,Itodensities of the right ventricular epicardial myocytes were significantly altered upward compared to left ventricular epicardal myocytes;however for C57 mice,no significant difference was noted between right and left ventricular epicardial myocytes.But Itodensities of both the right and left ventricular epicardial myocytes in C57 mice were significantly higher than those in KM mice (allP<0.01).

Table 3.Comparison of incidence of electrical stimulated ventricular tachycardia (VT) in KM and C57 mice (%)
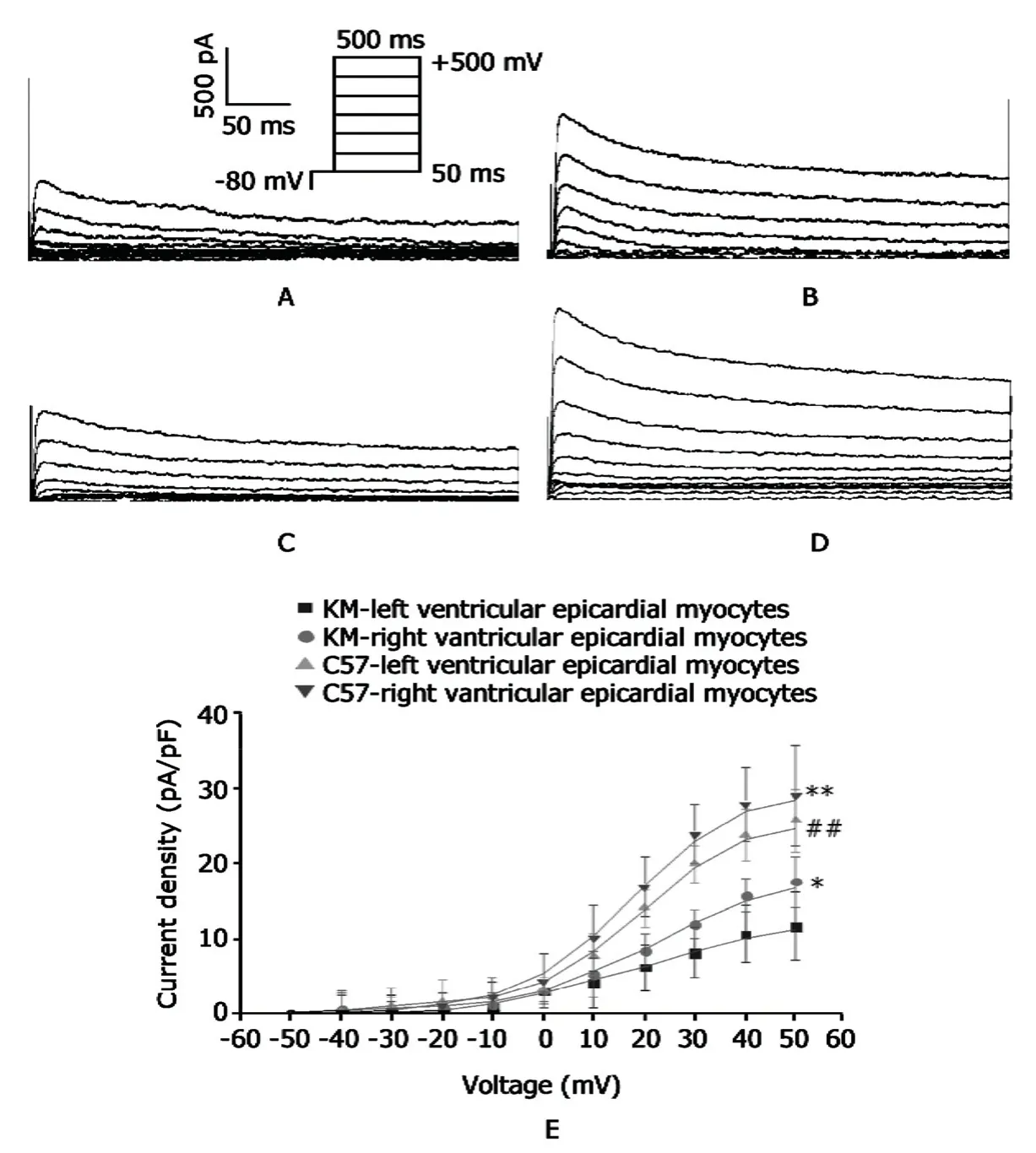
Figure 3.Recordings of transient outward potassium current (Ito) in the left and right ventricular epicardial myocytes isolated from KM and C57 mice.
DISCUSSION
In present study,our idea is triggered on that we observed KM mice,which are widely used as experimental preparations by scientific researchers in China,easily died of arrhythmias when we anesthetized them using common dose before we developed a model for cardiac electrophysiological experiments.But the phenomenon did not occur in C57 mice administrated with the same or larger dose of anesthetic.Sometimes,when we inject a drug to improve HR of KM mice,such as isoproterenol,we encounter a same problem that the KM mice experience ventricular fibrillation.To facilitate phenotypic analysis of gene-targeted animals,as well as to evaluated the potential and limitations of mouse models,the physiology of the normal mouse heart needs to be understood in detail.12,13The present electrophysiological research delineates the range of surface electrocardiogram and AP duration (APD)at normal KM and C57 mouse hearts,as well as the incidence of arrhythmias induced by PES and Burst pacing in KM and C57 mouse hearts.Moreover,the underlining mechanism was investigated using patch-clamp technique.The major findings of our study are∶(1) Compared with C57 mice,KM mice display prolonged QT interval in surface electrocardiogram recording.(2) Epicardial MAP duration in isolated KM mouse heart exhibits the significant delayed repolarization at 50% repolarization in the left ventricle and at 50% and 70% repolarization in the right ventricle compared with C57 mice.(3) Ventricular arrhythmia inducibility in heart isolated from KM mice seems higher than that in the heart obtained from C57 mice.(4) At last,significant differences of Itodensity in the left and right ventricular epicardial myocytes isolated from C57 and KM mice might increase the heterogeneity of QT intervaland APD,as well as elicit the occurrence of arrhythmias.
Our analysis identified C57 mice as a strain with shorter QT interval and APD,and KM mice,a Chinese special stain,with longer QT interval and APD,as well as higher inducibility of ventricular arrhythmias.The current findings are similar to the results described in previous researches.14,15To clarify the substantial ionic mechanism that KM mice were easily induced to occur VA,we chose Itoas the index and right and left ventricular epicardial myocytes obtained from C57 and KM mice as carriers.Because of bigger mammals with long APD in ventricular myocytes,Itoin ventricular myocytes of bigger mammals only composes the plateau of AP rather than determining the APD.In contrast,Itoin ventricular myocytes of mice contributes to every phase of the repolarisation during the entire AP.As soon as Itois altered,the APD is readily changed in mice hearts.16The increase in APD is mainly supported by a reduction of the Itodensity since no significant modification was observed in densities of L-type calcium channel (ICa-L),sodium-calcium exchange current(INCX),delayed rectifier potassium current (IK),and inward rectifier potassium current (IK1) in some mouse strains.The lower amplitude of Itocurrent was associated with a lower Kv4.3 protein expression both in the left and right ventricles while the amount Kv4.3 mRNA expression was decreased only in the left ventricle.17,18On the other hand,although an endo-epicardial gradient for K+outward currents obviously take place in the mouse right and left ventricular free wall,Itodensity is rather low and have no carried main role in endocardial than epicardial myocytes.While other potassium currents∶rapidly activating and very slowly inactivating K+current (IK,slow),slowly activating and noninactivating K+current (ISS),and IK1have no changes in endocardial myocytes compared with epicardial myocytes.19Therefore,Itoin epicardial myocytes really plays a key role in cardiac electrophysiological changes.In our work,we found that Itodensity of the left or right epicardial myocytes isolated from KM mice was significantly decreased in comparison to that of myocytes from C57 mice.Itodensity of the left epicardial myocytes was significantly lower than that of the right epicaridal myocytes obtained from KM mice,while for C57 mice Itodensity was also slightly decreased in the left epicardial myocytes compared with the right epicardial myocytes.To differ from HR of larger mammals,the HR of mouse is rather rapid to approximately arrive to 600 beats/min.Though there is only a bit of changes in APD in mice heart with a rapid stimulus,the VA might completely be triggered.There is reason to believe that Itoin cardiac myocytes of KM and C57 mice was distinctly altered.
Due to the mechanism of the ion channel’s difference between the two strains of mouse,the prolonged QT interval and vulnerability to VA in isolated KM mouse heart could be well explained.In human the long QT syndrome linked to the electric alteration of repolarization has been proposed as an important marker for the occurrence of ventricular tachycardia and a potential episodes for development of Torsade de pointes,20which is generally associated with life-threatening arrhythmias and sudden death.21The electrocardiogram and AP changes observed in this study could result to enhanced dispersion of refractoriness and variability of electrical activity,which could finally be contributed to high inducibility of VA in isolated KM mouse heart.
In conclusion,KM mice had the prolonged QT interval and APD,and were vulnerable to VA,which were attributed to lower Itodensities in ventricular myocytes of KM mice.
1.Nerbonne JM,Nichols CG,Schwarz TL,et al.Genetic manipulation of cardiac K+channel function in mice∶what have we learned and where do we go from here? Circ Res 2001;89∶944-56.
2.Domenighetti AA,Boixel C,Cefai D,et al.Chronic angiotensin II stimulation in the heart produces an acquired long QT syndrome associated with IK1potassium current downregulation.J Mol Cell Cardiol 2007;42∶63-70.
3.Casimiro MC,Knollmann BC,Ebert SN,et al.Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange–Nielsen Syndrome.Proc Natl Acad Sci U S A 2001;98∶2526-31.
4.Wagner M,Rudakova E,Schutz V,et al.Larger transient outward K+current and shorter action potential duration in Gα11mutant mice.Eur J Physiol 2010;459∶607-18.
5.Royer A,van Veen TA,Le Bouter S,et al.Mouse model of SCN5A-linked hereditary Lenègre's disease∶age-related conduction slowing and myocardial fibrosis.Circulation 2005;111∶1738-46.
6.Jones DL,Petrie JP,Li HG.Spontaneous,electrically,and cesium chloride induced arrhythmia and after depolarizations in the rapidly paced dog heart.Pacing Clin Electrophysiol 2001;24∶474-85.
7.Tuomi JM,Chidiac P,Jones DL.Evidence for enhanced M3 muscarinic receptor function and sensitivity to atrial arrhythmia in the RGS2-deficient mouse.Am J Physiol Heart Circ Physiol 2010;298∶H554-61.
8.Chouabe C,Ricci E,Kurdi M,et al.Evaluation of remodeling in left and right ventricular myocytes from heterozygous (mRen2)27 transgenic rats.Gen Physiol Biophys 2009;28∶24-38.
9.Li X,Huang CX,Jiang H,et al.The beta-adrenergic blocker carvedilol restores L-type calcium current in a myocardial infarction model of rabbit.Chin Med J 2005;118∶377-82.
10.Brunet S,Aimond F,Li H,et al.Heterogeneous expression of repolarizing,voltage-gated K+currentsin adult mouse ventricles.J Physiol 2004;559∶103-20.
11.Hamill OP,Marty A,Neher E,et al.Improved patch-clamp techniques for high resolution current recording from cells and cell free membrane patches.Pflugers Arch 1981;391∶85-100.
12.Nerbonne JM.Studying cardiac arrhythmias in the mouse—a reasonable model for probingmechanisms?Trends Cardiovasc Med 2004;14∶83-93.
13.Stables CL,Curtis MJ.Development and characterization of a mousein vitromodel of ischemia-induced ventricular fibrillation.Cardiovasc Res 2009;83∶397-404.
14.Waldeyer C,Fabritz L,Fortmueller L,et al.Regional,age-dependent,and genotype-dependent differences in ventricular action potential duration and activation time in 410 Langendorff-perfusedmouse hearts.Basic Res Cardiol 2009;104∶523-33.
15.Brouillette J,Rivard K,Lizotte E,et al.Sex and strain differences in adult mouse cardiac repolarization∶importance of androgens.Cardiovasc Res 2005;65∶148-57.
16.Sah R,Ramirez RJ,Oudit GY,et al.Regulation of cardiac excitation-contraction coupling by action potential repolarization∶role of the transient outward potassium current(Ito).J Physiol 2003;546∶5-18.
17.Nattel S,Maguy A,Le Bouter S,et al.Arrhythmogenic ion-channel remodeling in the heart∶heart failure,myocardial infarction,and atrial fibrillation.Physiol Rev 2007;87∶425-56.
18.Goltz D,Schultz JH,Stucke C,et al.Diminished Kv1.2/3 but not KChIP2 levels reduce the cardiac transient outward current in spontaneously hypertensive rats.Cardiovasc Res 2007;74∶85-95.
19.Xu H,Guo W,Nerbonne JM.Four kinetically distinct depolarization-activated K+currents in adult mouse ventricular myocytes.J Gen Physiol 1999;113∶661-78.
20.Shimizu W,Antzelevitch C.Cellular and ionic basis for T-wave alternans under long-QT conditions.Circulation 1999;99∶1499-507.
21.Maury P,Metzger J.Alternans in QRS amplitude during ventricular tachycardia.Pacing Clin Electrophysiol 2002;25∶142-50.
 Chinese Medical Sciences Journal2012年2期
Chinese Medical Sciences Journal2012年2期
- Chinese Medical Sciences Journal的其它文章
- Status and Change Pattern of Kidney Transplantation:One Center Research
- Gastric Metastasis of Atypical Medullary Carcinoma from Breast:a Case Report
- Role of ADAM10 and ADAM17 in CD16b Shedding Mediated by Different Stimulators△
- Bilateral Ureteral Fibroepithelial Polyps:a Case Report
- Multiple Coatings can Improve the Bond Durability of One-step Self-etching Adhesive to Primary Dentin
- Function of microRNA-346 and its Roles in Human Diseases
