Zinc Finger Protein-activating Transcription Factor Up-regulates Vascular Endothelial Growth Factor-A Expression in Vitro△
Li-shan Lian,Yao-guo Yang,Wei Liu,Li-long Guo,Heng Guan,Chang-wei Liu,and Yong-jun Li*
Department of Vascular Surgery,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100730,China
THE peripheral artery obstructive disease is common nowadays.Epidemiological research in Beijing showed the incidence rate of peripheral artery obstructive disease in people who were over the 60 years old is 15.3%.1In the diabetic patients being over 50 years,the incidence is as high as 19.47%.2Though the medical treatment can improve symptoms,it can not recanalize the occluded vessel.3At present,the effective treatment is still revascularization surgery.However,the restenosis would occur again after the angioplasty.Patients who suffer from the diabetes or have no vascular outflow tract can not undergo revascularization surgery.4Due to the limited effectiveness of revascularization surgery,therapeutic angiogenesis,which is a process of involving the growth and development of new capillaries from a pre-existing vasculature,is proposed by us.We seek to treat the disorders of inadequate tissue perfusion with this therapy.
Vascular endothelial growth factor (VEGF)-A is one of the VEGF families encoding a number of splice variants,which is a prototypic angiogenic growth factor.The functionally inequivalent and non-redundant protein isoforms of VEGF-A,5such as VEGF121,165,189,206,can promote the endothelial cell migration,proliferation,meanwhile,involve in the angiogenesis to increase the blood flow of the ischemic lower extremity.6However,single gene delivery of VEGF for therapeutic angiogenesis in ischemia differs from the normal endogenous VEGF expression process,and researches showed the clinic effects of VEGF on angiogenesis are not satisfactory.7,8The specifically designed zinc finger protein-activating transcription factor (ZFP-ATF)can interact with the unique sites in the genome and thus regulate the target gene expression.In this study,we delivered an engineered ZFP-ATF targeting VEGF genes to regulate the expression of natural array of splice variants,comparing with the single VEGF isoform,which is closer to the physiological processin vivoand can evoke a potentially therapeutic effect.9
MATERIALS AND METHODS
Cell culture
EY.HY926 endothelial cells (Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences) were maintained in DMEM medium (Invitrogen,CA,USA) supplemented with 10% fetal bovine serum and antibiotics(GIBCO,CA,USA) at 37°C in a humidified atmosphere with 5% CO2.
Construction and identification of ZFP-ATF plasmid
To induce up-regulation of endogenous VEGF expression,the coding sequences of three-finger proteins were engineered to bind to accessible regions in the VEGF promoter,which were determined by DNaseⅠhypersensitivity site mapping.The constructed zinc-finger DNA-binding domains were coupled to the VP16 or p65 activation domains from nuclear factor-kappa B (NF-κb),and nuclear translocation signal from the big T antigen of SV40.In addition,the oligomerization sites were involved in the spatial configuration in the transcription process.They were subcloned into the PVAX1 plasmid (Invitrogen) and expressed under direction of the CMV promoter.10
The product (5 μL) was then transferred to competent DH5 bacteria (Invitrogen).The positive recombinants were selected on a Luria-Bertain (LB) plate with kanamycin.The white bacterial colonies were amplified and plasmids were extracted and purified using the QIAquick DNA reagent kit(Qiagen,Germany).
The colony was further determined with theEcoRⅠrestriction enzyme (Invitrogen) and 1% agarose gel electrophoresis.The DNA of the positive recombinants was sequenced.The sequences were determined using BLAST analysis (IMGT).
Plasmid transfection
EY.HY926 endothelial cells were divided into four groups∶the ZFP-ATF,VEGF165,and two blank groups,with 6 wells in each group.One day before transfection,cells were plated into the 6-well plates at a concentration of 107cells per well.Four μg ZFP-ATF or VEGF165 plasmids (Invitrogen) were transfected into EY.HY926 cells using Lipofectamine2000 (Invitrogen) according to manufacturer’s instructions,respectively.The cells in the Blank 1 group were incubated with reduced serum media (250 μL/well,Opti-MEM,GIBCO-BRL Co.Ltd.,USA) and those in the Blank 2 group were cultured with media supplemented with Lipofectamine2000 (10 μL/well).The medium was replaced 4-6 hours after the transfection.
Western blot
At 48 hours after transfection,the cells were collected.Total cell lysates were prepared and analyzed by Western blot as previously described.11Briefly,rabbit antibody to VEGF (in 1∶1000 dilution,Abcam,Cambridge,UK) and mouse antibody to β-actin (in 1∶3000 dilution,Abcam)were used to detect VEGF and β-actin,respectively.After 2 hours of incubation with the first antibody,the membranes were incubated with the IgG antibody linked with horseradish peroxidase (1∶3000 dilution,Abcam) for 3 hours.Bands were visualized using ECL kit (Promega,USA) on a Kodak Image station 4000mm Pro System (Kodak,Rochester,NY,USA).The density of the bands was quantified by densitometric analysis.
RT-PCR
TRIzol reagent (Invitrogen) was used to extract the total RNA in cells 48 hours after transfection according to the manufacturer’s instructions.Prior to reverse transcription,the total RNA was digested with RNase-free DNase (Invitrogen) and the quality was determined by agarose gel electrophoresis and ultraviolet Spectrophotometer analysis.The cDNA was synthesized from the total RNA template using reverse transcriptase (Invitrogen) and Olig (dT)12-18primers (Promega,Germany).Primers for VEGF isoformsare shown in Table 1.The conditions for PCR amplification were as follows∶pre-denaturation at 95°C for 10 minutes,40 cycles of denaturation at 95°C for 15 seconds,annealing at 50°C for 2 minutes,extention at 60°C for 60 seconds.PCR products were purified using the QIAquick DNA reagent kit(Qiagen).The fragment length of amplified products was determined using an agarose gel electrophoresis.
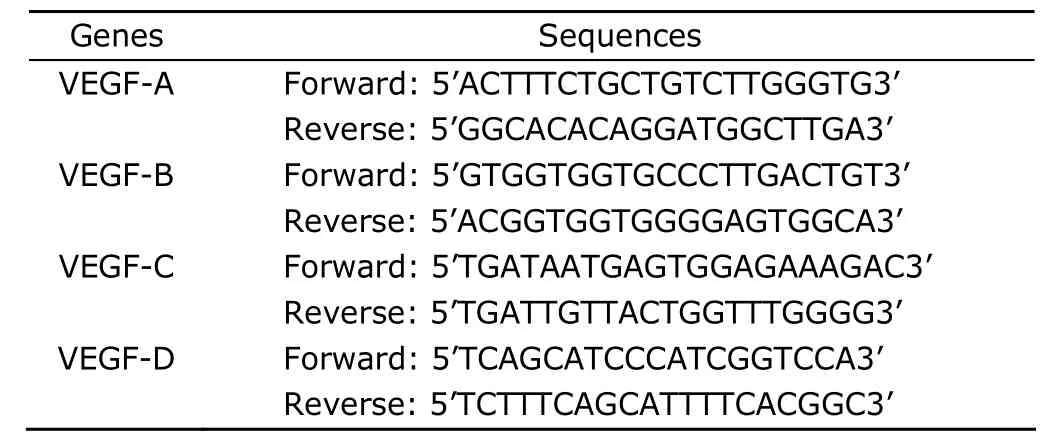
Table 1.Primer sequences used for PCR amplification
Statistical analysis
Microsoft Excel software was used for statistical analysis.All data were expressed as means±SD.Intra-group difference was determined by student’sttest.P<0.05 was considered to be statistically significant.
RESULTS
The nucleotide and deduced amino acid sequences of the ZFP-ATF are shown in Fig.1.BLAST analysis showed the ZFP-ATF had 807 bp in length and the total number of amino acids was 268.We found the specific targeted DNA sequences were located the multiclone sites of PVAX1 vector between the the site ofBamHⅠandXhol.
Western blot analysis showed ZFP-ATF and VEGF165 induced VEGF expression levels in EY.HY926 endothelial cells were 19.95±3.95 and 12.15±1.55 μg/μL,respectively.VEGF levels of the Blank 1 and Blank 2 groups were 1.72±1.33 and 2.12±1.21 μg/μL,respectively.VEGF expression in EY.HY926 endothelial cells transfected with ZFP-ATF plasmild was significantly higher than that transfected with VEGF165 as well as in cells of two blank groups (allP<0.01,Fig.2).
RT-PCR revealed ZFP-ATF only induced VEGF-A mRNA expression,and there was no difference in mRNA expression levels of VEGF-B,VEGF-C,and VEGF-D between ZFP-ATF and VEGF165 transfected EY.HY926 cells (Fig.3).
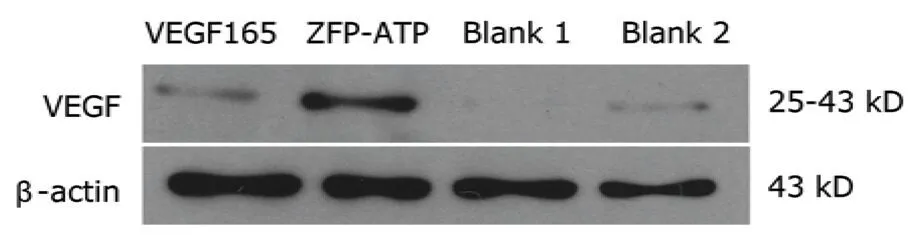
Figure 2.Western blot analysis result of VEGF expression in EY.HY926 endothelial cells after 48 hours of transfection with the designed ZFP-ATF binding to the targeted sequences in the VEGF promoter.β-actin was the loading control.
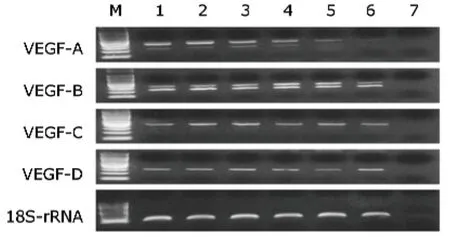
Figure 3.RT-PCR analysis result of VEGF mRNA isoform expression in EY.HY926 endothelial cells after transfection of ZFP-ATF or VEGF165.
DISCUSSION
Angiogenesis,firstly proposed by Folkman,includes two distinctly main mechanisms∶vasculogenesis and angiogenesis.12The angiogenesis we studied is stimulated by hypoxia,ischemia,and inflammation,finally,producing the vascular wall by the differentiation and migration of vascular endothelial cell.At present,a variety of angiogenesis-related growth factors have been identified,such as VEGF,fibroblast growth factor,and hepatocyte growth factor,which can respectively stimulate the angiogenesis and treat ischemic disease.However,the regional angiogenesis with VEGF (RAVE) trial carried out by Rajagopalanet al13showed that VEGF121 or VEGF165 alone can not effectively reduce the rate of amputation and improve patients' exercise capacity.
In this research,we constructed a novel transcription factor expression plasmid,namely ZFP-ATF,which was mutated at key positions in a given zinc-finger framework,making the deduced proteins specifically bind to VEGF promoter to up-regulate endogenous VEGF expression.14-16Because ZFP-ATF composed of human zinc fingers is less likely to induce a host immune response than mutated ZFP would be,17thus,it might be preferable in therapeutic applications.The constructed ZFP-ATF plasmid containing ZFP moieties and an activation domain can encode VEGF expression with great efficiencyin vitro.
Western blot results showed that the ZFP-ATF group,in contrast to the VEGF165 group,had a more powerful effect to promote VEGF protein expression.And RT-PCR results suggested that ZFP-ATF alone stimulated the VEGF-A expression and simultaneously increased the main VEGF splice variants expression.Though VEGF165 can stimulate the splice variants expression of VEGF,the effect was weak compared with the ZFP-ATF group.Our results demonstrated that the process of ZFP-ATF stimulating VEGF gene expression is closer to the normal physiological procedure in body.ZFP-ATF can meet the requirements for the angiogenesis which needs enough VEGF protein concentration and the major VEGF splice variants.
Taken together,these data further demonstrated that the ZFP-ATF plasmid can specifically interact with VEGF gene promoter,and increase VEGF expression,ultimately leading to the angiogenesis.In gene therapy applications,there are at least three major objectives which are of relevance to the use of artificial transcription factors∶maintaining a high level of DNA-binding specificity,achieving a desired level of gene expression in a target cell type and regulating the activity of the targeted gene,moreover,avoiding alteration in the expression of other genes.18-20Therefore,in addition to the VEGF gene,it is also feasible to design other kinds of ZFP for combination with different DNA sequences,which eventually will regulate the expression of other proteins in need.21,22
We have proven the ZFP-ATF might be effective in stimulating VEGF-A expressionin vitro.ZFP-ATF will become an effective and powerful factor in angiogenesis.However,the limitation of the ZFP-ATF is that it can only act on the VEGF gene.Because other factors are involved in the angiogenesis,such as fibroblast growth factor and nerve growth factor,23etc.Therefore,whether the ZEP-ATF combining with other growth factors will be more effective and practical in the treatment of ischemic disease deserves to be investigated in the future.
1.He Y,Jiang Y,Wang J,et al.Prevalence of peripheral arterial disease and its association with smoking in a population-based study in Beijing,China.J Vasc Surg 2006;44∶333-8.
2.Guan H,Liu ZM,Li GW,et al.The relative factor analysis of the peripheral artery obstructive disease in diabetes over 50 years.Chin J Med 2007;87∶23-7.
3.Clair D,Shah S,Weber J.Current state of diagnosis and management of critical limb ischemia.Curr Cardiol Rep 2012;14∶160-70.
4.Ruiz-Salmeron R,de la Cuesta-Diaz A,Constantino-Bermejo M,et al.Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia.Cell Transplant 2011;20∶1629-39.
5.Qazi Y,Stagg B,Singh N,et al.Nanoparticles-mediated delivery of shRNA VEGF-A plasmids regresses corneal neovascularization.Invest Ophthalmol Vis Sci 2012;53∶2837-44.
6.Thurston G,Rudqe JS,Zhou H,et al.Angiopoietin-1 protects the adult vasculature against plasma leakage.Nature Med 2000;6∶460-3.
7.Smith AH,Kuliszewski MA,Liao C,et al.Sustained improvement in perfusion and flow reserve after temporally separated delivery of VEGF and angiopoietin-1 plasmid deoxyribonucleic acid.J Am Coll Cardiol 2012;59∶1320-8.
8.Eduardo HY,Roberta SS,Samoto VY,et al.Treatment of mouse limb ischemia with an integrative hypoxiaresponsive vector expressing the vascular endothelial growth factor gene.PLoS One 2012;7∶1-6.
9.Rebar EJ,Huang Y,Hickey R,et al.Induction of angiogenesis in a mouse model using engineered transcription factors.Nat Med 2002;11∶1427-32.
10.Snowden AW,Zhang L,Urnov F,et al.Repression of vascular endothelial growth factor A in glioblastoma cells using engineered zinc finger transcription factors.Cancer Res 2003;63∶8968-76.
11.Chen G,Tian X,Liu Z,et al.Inhibition of endogenous SPARC enhances pancreatic cancer cell growth∶modulation by FGFR1-Ⅲ isoform expression.Br J Cancer 2010;102∶188-195.
12.Folkman J,Shing Y.Angiogenesis.J Biol Chem 1992;267∶10931-4.
13.Rajagopalan S,Mohler Ⅲ ER,Lederman RJ,et al.Regional angiogenesis with VEGF in peripheral arterial disease∶a phase Ⅱ randomized,double-blind,controlled study of adenoviral delivery of VEGF121 in patients with disabling intermittent claudication.Circulation 2003;108∶1933-8.
14.Kang YA,Shin HC,Yoo JY,et al.Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus.Mol Ther 2008;16∶1033-40.
15.Xie D,Li Y,Reed EA,et al.An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia.J Vasc Surg 2006;44∶166-75.
16.Yang YG,Guan H,Liu CW,et al.Reinstate the damaged VEGF signaling pathway with VEGF-activating transcription factor.Chin Med Sci J 2009;24∶186-90.
17.Kusumanto YH,Van Weel V,Mulder NH,et al.Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia∶a double-blind randomized trial.Hum Gene Ther 2006;17∶683-91.
18.Cristofaro B,Stone OA,Caporali A,et al.Neurotrophin-3 is a novel angiogenic factor capable of therapeutic neovascularization in a mouse model of limb ischemia.Arterioscler Thromb Vasc Biol 2010;30∶1143-50.
19.Liu Y,Figley S,Spratt SK,et al.An engineered transcription factor which activates VEGF-A enhances recovery after spinal cord injury.Neurobiol Dis 2010;37∶384-93.
20.Yu J,Lei L,Liang Y,et al.An engineered VEGF-activating zinc figure protein transcription factor improves blood flow and limb salvage in advanced-age mice.FASEB J 2006;20∶479-81.
21.Dent CL,Lau G,Drake EA,et al.Regulation of endogenous gene expression using small molecule-controlled engineered zinc-finger protein transcription factors.Gene Ther 2007;14∶1362-9.
22.Zhang HS,Liu D,Huang Y,et al.A designed zinc-finger transcriptional repressor of phospholamban improves function of the failing heart.Mol Ther 2012;20∶1508-15.
23.Makarevich P,Tsokolaeva Z,Shevelev A,et al.Combined transfer of human VEGF165 and HGF genes renders potent angiogenic effect in ischemic skeletal muscle.PLoS One 2012;7∶e38776.
 Chinese Medical Sciences Journal2012年3期
Chinese Medical Sciences Journal2012年3期
- Chinese Medical Sciences Journal的其它文章
- Hypercalcemia Appeared in a Patient with Glucagonoma Treated with Octreotide Acetate Long-acting Release
- Comparison of Clinical Effects of Au-Pt Based and Ni-Cr Based Porcelain Crowns
- Clinical Analysis of Placenta Previa Complicated with Previous Caesarean Section△
- Hipbone Biomechanical Finite Element Analysis and Clinical Study after the Resection of Ischiopubic Tumors△
- Accuracy Validation for Medical Image Registration Algorithms:a Review△
- Nucleotide-binding Oligomerization Domain-1 Ligand Induces Inflammation and Attenuates Glucose Uptake in Human Adipocytes△
