Nucleotide-binding Oligomerization Domain-1 Ligand Induces Inflammation and Attenuates Glucose Uptake in Human Adipocytes△
Yi-jun Zhou,Ai Li,Yu-ling Song,Yan Li,and Hui Zhou
Department of Endocrinology and Metabolism,Fourth Affiliated Hospital of China Medical University,Shenyang 110032,China
INSULIN resistance,a hallmark of type 2 diabetes mellitus (T2DM),is associated with low-grade systemic inflammation characterized by up-regulated cytokine production and activated inflammatory signaling pathways.1,2Innate and adaptive immune systems have been implicated in the proinflammatory responses and have emerged as critical factors in the manifestation of insulin resistance.3,4Innate immune receptors are pattern recognition receptors (PRRs) that recognize the pathogen-associated molecular patterns(PAMPs) on invading pathogens and activate downstream signaling pathways leading to the up-regulation of diverse arrays of proinflammatory gene expression.Membrane-bound pattern recognition receptors,Toll-like receptor (TLR) 4 and TLR2 in particular,have been suggested to be involved in developing adipose inflammation and insulin resistance.5-8The recently identified nucleotide-binding oligomerization domain (NOD) proteins,as cytoplasmic PRRs,recognize conserved moieties of bacterial peptidoglycan (PGN) and activate proinflammatory signaling pathways.NOD1 recognizes PGN-related molecules containing the amino acid meso-diaminopimelic acid(meso-DAP) that are produced by most Gram-negative and certain Gram-positive bacteria.Similar to TLRs,NOD1 and NOD2 activate gene transcription through the nuclear factor-κB (NF-κB) transcription factor and the mitogen-activated protein kinase (MAPK) signaling pathwaysviathe adaptor molecules receptor-interacting protein(RIP)-like interacting caspase-like apoptosis regulatory protein kinase (RICK,also known as RIP2) and caspase-recruitment domains 9 (CARD9).9
Adipose tissue is a source of mediators of inflammation and insulin resistance.Recent researches have demonstrated that NOD1 and NOD2 mRNAs were markedly increased in differentiated murine 3T3-L1 adipocytes and human primary adipocyte culture upon adipocyte conversion.10,11However,the function of NOD1 in adipose tissue remains poorly understood.In the present study,we investigated the effects of NOD1 ligand on secretion of proinflammatory chemokine/cytokines and insulin-dependent glucose uptake in human mature adipocytes.
MATERIALS AND METHODS
Subjects
Abdominal subcutaneous white adipose tissue samples were obtained from overweight or obese patients who underwent liposuction under general anaesthesia for cosmetic purposes.The patients were randomly selected from Fourth Affiliated Hospital of China Medical University during the period of July 2010 and June 2011.A total of 14 samples were obtained from 14 patients.All patients were female (aged between 25 and 48 years,mean 37 years).Their mean body mass index was 27.1 ± 2.7 kg/m2.The study was performed with the approval of the Ethics Committee of China Medical University,and patients provided informed consents.
Primary human adipocytes culture
Adipose tissue was digested with collagenase (2 mg/mL;Worthington Biochemical,NJ,USA) to isolate the preadipocytes.Human preadipocytes were maintained in Dulbecco’s modified Eagle’s medium (DMEM;Invitrogen,Carlsbad,CA,USA) containing 10% fetal bovine serum (FBS;Zen-Bio Inc.,Research Triangle Park,NC,USA),100 U/mL penicillin,and 50 μg/mL streptomycin at 37°C in 5% CO2.In order to induce differentiation,confluent human preadipocytes were subsequently cultured in serum-free DMEM containing 50 nmol/L insulin,100 nmol/L dexamethasone (Sigma-Aldrich,St Louis,MO,USA),0.5 mmol/L 3-isobutyl-1-methylxanthine(Sigma-Aldrich),and 100 μmol/L rosiglitazone (Sigma-Aldrich).The medium was changed every 2 days for the first 4 days.Thereafter,the medium was replaced by serum-free DMEM containing 50 nmol/L insulin and 100 nmol/L dexamethasone,which was changed every 2 days until lipid droplets had accumulated (days 14-17).
NF-κB reporter assay
NF-κB is an inducible transcription factor that plays a central role in regulation of inflammatory.To evaluate whether NF-κB is activated by NOD1 ligand in human adipocytes,we determined the NF-κB transcriptional activity by NF-κB reporter plasmid assay.Four μg of a NF-κB-luciferase reporter plasmid (BD Pharmingen,CA,USA) were transfected into primary human adipocytes.After 24 hours of transfection,the cells were incubated with or without 10 μg/mL NOD1 synthetic ligand (D-lactyl-LAla-γ-D-Glu-meso-DAP,FK156;InvivoGen,San Diego,CA;n=10) or with 10 μg/mL NOD2 ligand (L18-muramyl dipeptide,L18-MDP;InvivoGen;n=10).After incubated with NOD1 ligand or NOD2 ligand for 24 hours,adipocytes were washed twice with PBS,and lysed with 80 μL of reporter lysis buffer (Promega,WI,USA).After quantification using Bradford reagent (Bio-Rad,CA,USA),the lysates(20 μL) were transferred into a 96-well plate for luciferase activity analysis using the dual luciferase assay kit (Promega).Luciferase activity was measured using a Mithras LB 940 luminescence reader (Berthold Technologies,Germany).NF-κB activity was estimated as relative luminescence unit (RLU) corresponding to equal protein amounts.
Enzyme-linked immunosorbent assay (ELISA)
To investigate whether activation of NOD1 by the synthetic ligands can induce a proinflammatory chemokine/cytokine response in human culture adipocytes,we analyzed interleukin (IL)-6,IL-8,and monocyte chemoattractant protein-1(MCP-1) secretion using ELISA.The mature adipocytes were treated (n=10) or not treated (n=10) with 10 μg/mL NOD1 ligand.The supernatant was collected at 0,12,24,36,and 48 hours of NOD1 ligand addition to the medium.The levels of MCP-1,IL-6,and IL-8 were determined by ELISA using Quantikine kits (R&D Systems,Minneapolis,MN,USA) according to the manufacturer’s instructions.
Glucose uptake assay
2-deoxy-D-[3H]-glucose uptake was assayed as described previously.12Briefly,the mature adipocytes were cultured with serum-free medium,followed by a 24-hour incubation without (Control,n=10) or with NOD1 ligand (1,5,or 10 μg/mL,n=10),or 10 μg/mL NOD1 ligand treatment for 0,6,12,and 24 hours at 37°C,and then by a 30-minute incubation with (Insulin,n=10) or without (Base,n=10) 100 nmol/L insulin at 37°C.Labeled 2-deoxy-D-[3H]-glucose(Amersham Biosciences,CA,USA) was added with a final concentration of 74 kBq/mL.After reaction for 10 minutes at 37°C,adipocytes were washed thrice with ice-cold PBS supplemented with 10 mmol/L D-glucose.After addition of 200 μL NaOH (1 mol/L) to each well,200-μL aliquots were transferred to scintillation vials for radioactivity measurement.The remaining 100 μL of cell lysate was used to determine the protein concentration.
Small RNA interference
Control non-coding small interfering RNA (siRNA) (sense,UUCUCCGAACGUGUCACGUTT;antisense,ACGUGACACGUUCGGAGGAGAATT) and siRNA targeting NOD1 (sense,GGGUGAGACCAUCUUCAUCTT;antisense,GAUGAAGAUGGUCUCACCCTG) were purchased from Ambion (Huntingdon,UK).The mature adipocytes were transfected with 2 μg NOD1-specific or non-coding control siRNAs using an Amaxa Nucleofector (Amaxa,Cologne,Germany) according to the manufacturer’s protocol.After 24 hours of transfection,cells were incubated with (n=10) or not (n=10)with 10 μg/mL of NOD1 ligand for an additional 24 hours.Then cells were collected for analysis of NF-κB activity,MCP-1,IL-6,and IL-8 secretions,as well as glucose uptake.
Statistical analysis
All data were presented as the means±SE unless otherwise stated.Data were analyzed by one-way analysis of variance (ANOVA) or the Student'st-test using the SPSS 11.5 statistical package (SPSS,Chicago,IL,USA).AP-value of <0.05 was considered to be statistically significant.
RESULTS
NOD1 ligand treatment stimulates NF-κB activity in human adipocytes
NF-κB transcriptional activity in NOD1 ligand treated adpocytes increased significantly,as compared with control(11.6×103RLUvs.4.1×103RLU,P<0.01).However,no NF-κB activation occurred in human adipocytes after stimulation with NOD2 ligand (4.7×103RLUvs.4.1×103RLU,P>0.05).
NOD1 ligand induces MCP-1,IL-6,and IL-8 secretions in human mature adipocytes
As shown in Fig.1A,MCP-1 production increased significantly in adipocytes treated with NOD1 ligand compared with untreated controls (allP<0.01).Similarly,IL-6 and IL-8 secretion rose significantly in adipocytes with NOD1 ligand treatment compared with untreated controls (allP<0.01,Figs.1B,1C).
NOD1 ligand attenuates insulin-stimulated glucose uptake in human adipocytes
For adipocytes without NOD1 ligand induction,insulin treatment increased their glucose uptake by 3.2 times (Fig.2A),but the ability of insulin-stimulated glucose uptake was suppressed by 40% (P<0.05) after 10 μg/mL NOD1 ligand stimulation for 24 hours (Fig.2B).Six-hour treatment of NOD1 ligand did not significantly suppress insulin-induced glucose uptake (P>0.05,Fig.2B).
NOD1 ligand induces proinflammatory response and suppresses glucose uptake in human adipocytes via NOD1
In comparison to non-coding siRNA,NOD1 specific siRNA achieved 70% knockdown of NOD1 mRNA as analyzed using quantitative PCR (data not shown).Cells transfected with the NOD1-specific siRNA and subsequently stimulated with NOD1 ligand showed a significant decrease in MCP-1,IL-6,and IL-8 production in comparison to NOD1 ligand stimulated cells transfected with a non-coding control siRNA (P<0.05,Fig.3A).The siRNA targeting NOD1 attenuated NOD1 ligandinduced NF-κB activity,as revealed by NF-κB reporter assay(P<0.05,Fig.3B).Furthermore,NOD1 ligand suppressed insulin-stimulated glucose uptake,however,the inhibitory effect was reversed by siRNA targeting NOD1 (Fig.3C).
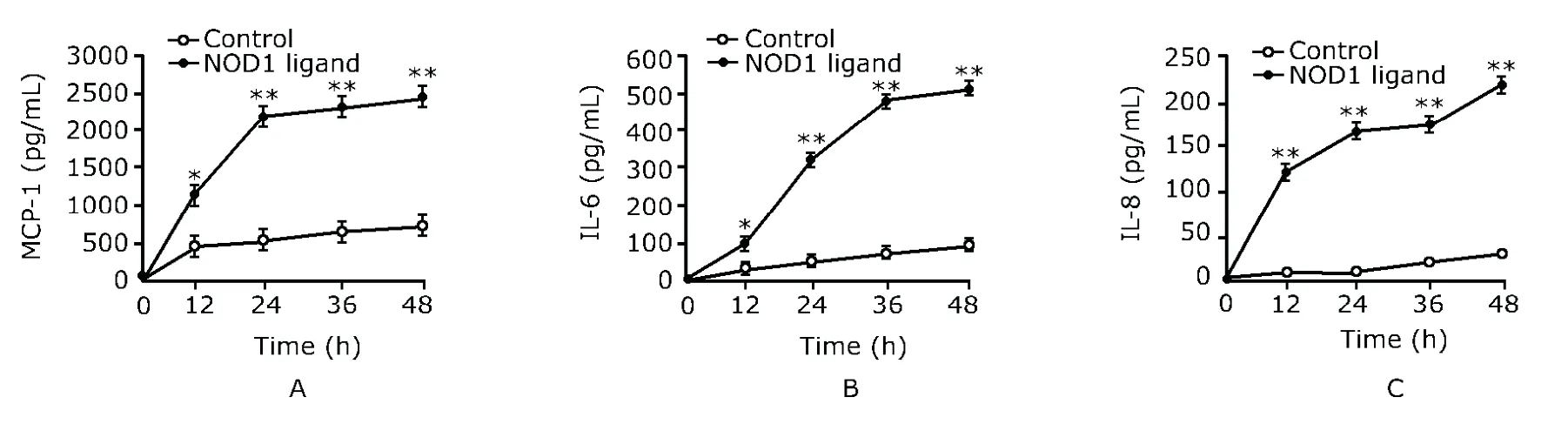
Figure 1.Secretion of MCP-1 (A),IL-6 (B),and IL-8 (C) by human mature adipocytes induced by NOD1 ligand.
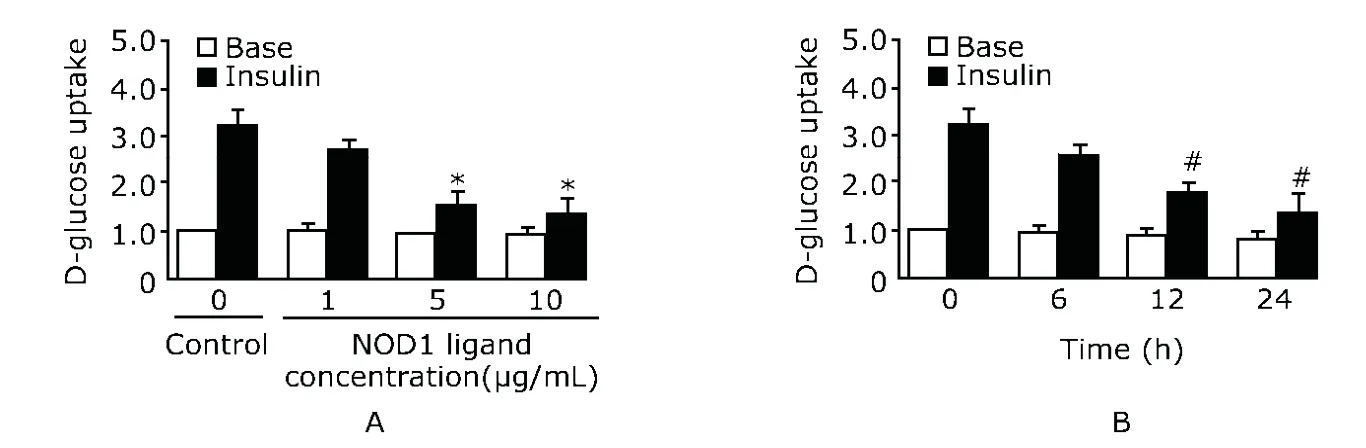
Figure 2.NOD1 ligand reduces insulin-stimulated glucose uptake in human adipocytes.
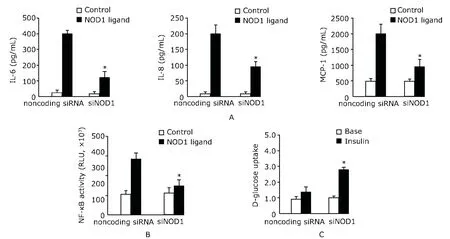
Figure 3.NOD1 ligand-induced proinflammatory response and decrease in insulin-induced glucose uptake in adipocytes are via NOD1.
DISCUSSION
The chronic inflammation observed in obesity appears to be a critical factor in the development of insulin resistance.Since insulin sensitivity is decreased in patients with endotoxemia,13it appears plausible that this response is mediated by PRRs in insulin target organs like muscles and fat tissue.14,15Adipose tissue is increasingly recognized as a key site of inflammation that can propagate responses leading to systemic insulin resistance.Here we investigated NOD1 proteins that recognize molecular patterns derived from pathogens other than Gram negative bacteria are also expressed inin vitromaturated human adipocytes and may contribute to insulin resistance.
In present study,we demonstrated that pure selective NOD1 ligand FK156 (a synthetic tetrapeptide originally isolated from culture filtrates ofStreptomycesstrains,with a molecular mass of 519.5 Da) induced a strong NF-κB activation.It is well known that NF-κB is a key regulator of inflammatory responses.16Following exposure to NOD1 ligand,adipocytes generated inflammatory cytokine IL-6 and chemokines such as MCP-1,IL-8.In agreement with our findings,NOD1 synthetic ligands Tri-DAP and iE-DAP have been shown to induce proinflammatory chemokine gene expression in differentiated human adipocytes through NOD1 and NF-κB action.10In this study,we additionally revealed that human adipocytes responded robustly to agonists stimulation such as NOD1 ligand.Recently,it has been reported that the proinflammatory chemokines are increased during insulin resistance in humans and rodents,and the cytokines have been directly linked to impaired insulin resistance.17,18We further found that NOD1 ligand suppressed the ability of insulin-stimulated glucose uptake in adipocytes,suggesting a possible role of bacterial components in the pathogenesis of insulin resistance.
To confirm the specificity of NOD1 in adipose inflammation and insulin resistance,we used siRNA targeting NOD1 to inhibit the effects of NOD1 ligand.The results revealed that NOD1 ligand-induced chemokine/cytokine protein expression are blocked by the siRNA targeting NOD1 but not by the non-coding siRNA.Moreover,NOD1 ligand-induced NF-κB activation and suppression of insulin-induced glucose uptake are attenuated by the siRNA targeting NOD1.Together,these results suggest that NOD1 might play an important role in adipose inflammation and insulin resistance.
We found here that NOD1 activation induces NF-κB activation and up-regulates cytokine release,which leads to suppression of insulin-induced glucose uptakes.But these data are not able to elucidate whether NOD1 ligand interacts directly with NOD1 in adipocytes.Therefore,it is necessary to further investigate the effects of NOD1 overexpression or NOD1 knock down in adipocytes in the absence or presence of its ligands.
In conclusion,we demonstrate that NOD1 ligand can activate the innate immune pathway in isolated human adipocytes to stimulate secretion of proinflammatory chemokine/cytokines.NOD1 activation by NOD1 ligand evidently reduced insulin-dependent glucose uptake in adipocytes,which eventually leads to insulin resistance.These results further strengthen the argument that NOD1 is a new modulator in the cross-talk between innate immunity and metabolic pathways.We,therefore,suggest that a selective interference with NOD1 might provide novel strategies to prevent insulin resistance.
1.Hotamisligil GS,Erbay E.Nutrient sensing and inflammation in metabolic diseases.Nat Rev Immunol 2008;8∶923-34.
2.Steinberg GR.Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance.Cell Cycle 2007;6∶888-94.
3.Cseh K,Baranyi E,Winkler G.The role of cytokines of the innate and adaptive immune system in the regulation of insulin resistance.Diabetologia 1999;42∶497-8.
4.Schertzer JD,Klip A.Give a NOD to insulin resistance.Am J Physiol Endocrinol Metab 2011;301∶E585-6.
5.Davis JE,Gabler NK,Walker-Daniels J,et al.The c-Jun N-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3T3-L1 adipocytes.Horm Metab Res 2009;41∶523-30.
6.Himes RW,Smith CW.Tlr2 is critical for diet-induced metabolic syndrome in a murine model.FASEB J 2010;24∶731-9.
7.Poggi M,Bastelica D,Gual P,et al.C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet.Diabetologia 2007;50∶1267-76.
8.Shi H,Kokoeva MV,Inouye K,et al.TLR4 links innate immunity and fatty acid-induced insulin resistance.J Clin Invest2006;116∶3015-25.
9.Magalhaes JG,Lee J,Geddes K,et al.Essential role of Rip2 in the modulation of innate and adaptive immunity triggered by Nod1 and Nod2 ligands.Eur J Immunol 2011;41∶1445-55.
10.Zhao L,Hu P,Zhou Y,et al.NOD1 activation induces proinflammatory gene expression and insulin resistance in 3T3-L1 adipocytes.Am J Physiol Endocrinol Metab 2011;301∶E587-98.
11.Stroh T,Batra A,Glauben R,et al.Nucleotide oligomerization domains 1 and 2∶regulation of expression and function in preadipocytes.J Immunol 2008;181∶3620-7.
12.Ceddia RB,Somwar R,Maida A,et al.Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells.Diabetologia 2005;48∶132-9.
13.Pickup JC.Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes.Diabetes Care 2004;27∶813-23.
14.Bes-Houtmann S,Roche R,Hoareau L,et al.Presence of functional TLR2 and TLR4 on human adipocytes.Histochem Cell Biol 2007;127∶131-7.
15.Kopp A,Buechler C,Neumeier M,et al.Innate immunity and adipocyte function∶ligand-specific activation of multiple Toll-like receptors modulates cytokine,adipokine,and chemokine secretion in adipocytes.Obesity (Silver Spring) 2009;17∶648-56.
16.Barnes PJ,Karin M.Nuclear factor-kappa B∶a pivotal transcription factor in chronic inflammatory diseases.N Engl J Med 1997;336∶1066-71.
17.Rotter V,Nagaev I,Smith U.Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is like IL-8 and tumor necrosis factor-α,overexpressed in human fat cells from insulin resistant subjects.J Biol Chem 2003;278∶45777-84.
18.Stienstra R,van Diepen JA,Tack CJ,et al.Inflammasome is a central player in the induction of obesity and insulin resistance.Proc Natl Acad Sci USA 2011;108∶15324-9.
 Chinese Medical Sciences Journal2012年3期
Chinese Medical Sciences Journal2012年3期
- Chinese Medical Sciences Journal的其它文章
- Hypercalcemia Appeared in a Patient with Glucagonoma Treated with Octreotide Acetate Long-acting Release
- Zinc Finger Protein-activating Transcription Factor Up-regulates Vascular Endothelial Growth Factor-A Expression in Vitro△
- Comparison of Clinical Effects of Au-Pt Based and Ni-Cr Based Porcelain Crowns
- Clinical Analysis of Placenta Previa Complicated with Previous Caesarean Section△
- Hipbone Biomechanical Finite Element Analysis and Clinical Study after the Resection of Ischiopubic Tumors△
- Accuracy Validation for Medical Image Registration Algorithms:a Review△
