Effects of wind-dispelling drugs and deficiency-nourishing drugs of Houshiheisan compound prescription on astrocyte activation and inflammatory factor expression in the corpus striatum of cerebral ischemia rats****☆
Qiuxia Zhang, Hui Zhao, Lei Wang, Qi Zhang, Haizheng Wang
College of Traditional Chinese Medicine, Capital Medical University, Beijing 100069, China
Effects of wind-dispelling drugs and deficiency-nourishing drugs of Houshiheisan compound prescription on astrocyte activation and inflammatory factor expression in the corpus striatum of cerebral ischemia rats****☆
Qiuxia Zhang, Hui Zhao, Lei Wang, Qi Zhang, Haizheng Wang
College of Traditional Chinese Medicine, Capital Medical University, Beijing 100069, China
Abstract
This study explored protective effects of Houshiheisan and its compound prescription of wind-dispelling drugs and deficiency-nourishing drugs on cerebral ischemia in terms of astrocyte activation and inflammatory factor expression. Results suggested that Houshiheisan lessened neuronal degeneration in the corpus striatum on the ischemic side of rats following cerebral ischemia/reperfusion injury, contributed to astrocyte activation and glial fibrillary acidic protein expression in the corpus striatum and decreased the levels of interleukin-2, interleukin-6,interleukin-1β and tumor necrosis factor-α. Factor analysis results demonstrated that deficiency-nourishing drugs were more beneficial in protecting neurons and upregulating glial fibrillary acidic protein expression than wind-dispelling drugs. However, wind-dispelling drugs were more effective in increasing the number of glial fibrillary acidic protein-positive cells and reducing inflammatory factor expression than deficiency-nourishing drugs. These indicate that different ingredients of Houshiheisan suppress cerebral ischemic injury by promoting astrocyte activation and diminishing inflammatory factor expression.
Key Words
Houshiheisan; glial fibrillary acidic protein; corpus striatum; interleukin; tumor necrosis factor-α;cerebral ischemia; neuronal protection; neural regeneration
Research Highlights
(1) Houshiheisan inhibited acute cerebral ischemic injury by promoting astrocyte activation and decreasing inflammatory factor expression.
(2) The effects of Houshiheisan compound prescription were more beneficial than those of wind-dispelling drugs or deficiency-nourishing drugs alone.
Abbreviations
MCAO, middle cerebral artery occlusion; GFAP, glial fibrillary acidic protein
Qiuxia Zhang☆, M.D.,Associate professor, College of Traditional Chinese Medicine, Capital Medical University, Beijing 100069,China
Corresponding author: Hui Zhao, M.D., Associate professor, College of Traditional Chinese Medicine, Capital Medical University, Beijing 100069,China
zhaohui8957@sina.com
Received: 2012-03-06 Accepted: 2012-06-30(N20111020003/WLM)
Zhang QX, Zhao H, Wang L,Zhang Q, Wang HZ. Effects of wind-dispelling drugs and deficiency-nourishing drugs of Houshiheisan compound prescription on astrocyte activation and inflammatory factor expression in the corpus striatum of cerebral ischemia rats. Neural Regen Res. 2012;7(24):1851-1857.
www.crter.cn
www.nrronline.org
INTRODUCTION
Astrocytes rapidly became hypertrophic and swollen following cerebral ischemia[1].
Initially, ischemia-activated astrocytes released neurotrophic factors, enhanced neuronal tolerance to low glucose and hypoxia, and protected neurons by regulating extracellular fluid K+concentration and uptake of glutamic acid[1].Greatly affected astrocytes expressed various inflammatory mediators, caused an immune cascade reaction and intensified tissue damage, such as destruction of blood-brain barrier, brain edema, neural celldegeneration and death[2-3]. Therefore, it is important to investigate the changes in astrocytes and their inflammatory mediators to understand the mechanisms underlying cerebral ischemic injury/reperfusion and possible therapeutic pathways.
Houshiheisan produced by Zhongjing Zhang for the treatment of stroke, in accordance with the pathogenesis of deficiency of genuine qi and excess of pathogenic factor, has a proved valuable in clinical practice[4-6]. Our preliminary researches showed that Houshiheisan inhibited acute cerebral ischemic injury and protected neurons in the cortex and hippocampus[7-8]. The corpus striatum is often affected in cerebrovascular accidents, of which putamen hemorrhages accounted for 60%,resulting in severe dysfunction[9].
This study explored the neuroprotective effects of Houshiheisan in terms of astrocyte activation and inflammatory factor expression after cerebral ischemia.Factor analysis was conducted on the Houshiheisan wind-dispelling drugs, the deficiency-nourishing drugs and the Houshiheisan compound prescription to understand the effects of the combined wind-expelling and deficiency-nourishing drugs in the treatment of stroke.
RESULTS
Quantitative analysis of experimental animals
A total of 65 Sprague-Dawley male rats were equally and randomly assigned to five groups. In the sham surgery group, surgery just exposed the middle cerebral artery without occlusion; the model group had middle cerebral artery occlusion (MCAO) + saline; wind-dispelling drugs group, MCAO + wind-dispelling drugs; deficiencynourishing drugs group, MCAO + deficiency-nourishing drugs; and Houshiheisan group, MCAO + Houshiheisan.A total of 65 rats were included in the final analysis.
Houshiheisan relieves pathological injury to brain tissues of MCAO rats
Hematoxylin-eosin staining results exhibited intact brain tissues, abundant neurons with normal morphology,lightly stained cytoplasm, without edema in the sham surgery group. In contrast, 24 hours after cerebral ischemia/reperfusion, typical ischemic changes;obvious edema, scattered neurons, contracted neuronal cell bodies and pyknosis were visible in the right cerebral cortex and lateral corpus striatum of rats, and Nissl bodies and nuclei disappeared. Vascular endothelial cell swelling and blood vessel wall distortion were observed and the perivascular space became large. Pathological changes in brain tissues on the ischemic side were similar in each therapy group and the model group but the range of necrotic tissues was smaller and the pathological changes were less severe than in the model group. Although less edema formed in the neural cells and interstitial tissues of cortex,hippocampus and corpus striatum, moderate neuronal degeneration was clear in the wind-dispelling drugs group. On the other hand, there were fewer pyknotic neurons but vascular endothelial cell swelling, blood vessel wall distortion and large perivascular space were observed in the deficiency-nourishing drugs group compared with the model group. Most cells in the Houshiheisan group had clear nuclei, weakly stained cytoplasm and showed only slight neuronal degeneration; neuronal and interstitial edemas were significantly less and there were fewer pyknotic neurons than in the model group (Figure 1). Image analysis results demonstrated that Houshiheisan prevents the loss of neurons in rat corpus striatum and cortex after cerebral ischemia. Results of factor analysis indicated that wind-dispelling drugs and deficiency-nourishing drugs dramatically lessened neuronal degeneration,retaining more corpus striatum and cortical neurons in the ischemic side (P < 0.01).The protective effect of deficiency-nourishing drugs on neurons was greater than that of the wind-dispelling drugs (Table 1).

Figure 1 Pathological changes of corpus striatum on the ischemic side of rats from each group (hematoxylin-eosin staining, × 200).
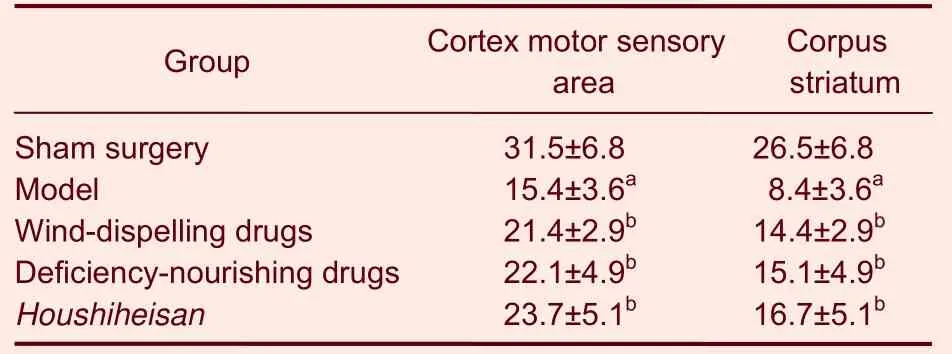
Table 1 The number of neurons (n/mm) in rat corpus striatum and cortex in the ischemic side of rats
Houshiheisan promotes astrocyte activation in the corpus striatum of MCAO rats
Immunohistochemical staining results showed a few glial fibrillary acidic protein (GFAP)-positive cells scattered in the cerebral cortex, corpus striatum,internal capsule, external capsule, corpus callosum and molecular layer in the sham surgery group. Fibrinolysis and a weak positive reaction to GFAP were observed in the center of the infarct region in the model group. A large body of GFAP-positive cells, brown cytoplasm and thick processes were clear in the corpus callosum,internal capsule and lateral ventricle. In each therapy group, GFAP expression was strong (Figure 2). Image analysis results suggested that GFAP expression was significantly more in the model group compared with the sham surgery group (P < 0.01). GFAP expression was significantly more in the wind-dispelling drugs group,the deficiency-nourishing drugs group and the Houshiheisan group compared with the model group(P < 0.05 or P < 0.01).The results of factor analysis indicated that the effects of wind-dispelling drugs on increasing GFAP-positive cell number were greater compared with those treated with deficiency-nourishing drugs (Table 2).
Houshiheisan elevates GFAP expression in the corpus striatum of MCAO rats
Results from Western blot assays were similar to those using immunohistochemical staining. GFAP expression was significantly greater in the wind-dispelling drugs group, deficiency-nourishing drugs group and Houshiheisan group compared with the model group (P <0.05 or P < 0.01). Factor analysis results showed that the upregulatory effects of deficiency-nourishing drugs on GFAP protein expression were larger than that of wind-dispelling drugs (Figure 3, Table 2).
Houshiheisan decreases inflammatory factor levels in the corpus striatum of MCAO rats
Levels of interleukin-1β, interleukin-2, interleukin-6 and tumor necrosis factor-α were significantly higher 24 hours after cerebral ischemia/reperfusion compared with those in the sham surgery group (P < 0.05 or P < 0.01).Two-way analysis of variance results confirmed that interleukin-1β, interleukin-2, interleukin-6 and tumor necrosis factor-α levels were significantly lower in the Houshiheisan group than those in the model group (P <0.05 or P < 0.01). Interleukin-1β, interleukin-2 and tumor necrosis factor-α levels were significantly lower in the wind-dispelling drugs group than those in the model group (P < 0.01). Interleukin-1β levels were significantly lower in the deficiency-nourishing drugs group than those in the model group (P < 0.01; Table 3). Effects of wind-dispelling drugs in decreasing inflammatory factor expression were greater than those of deficiencynourishing drugs.

Figure 2 Expression of glial fibrillary acidic protein (GFAP) in rat corpus striatum on the ischemic side (immunohistochemical staining, × 200).
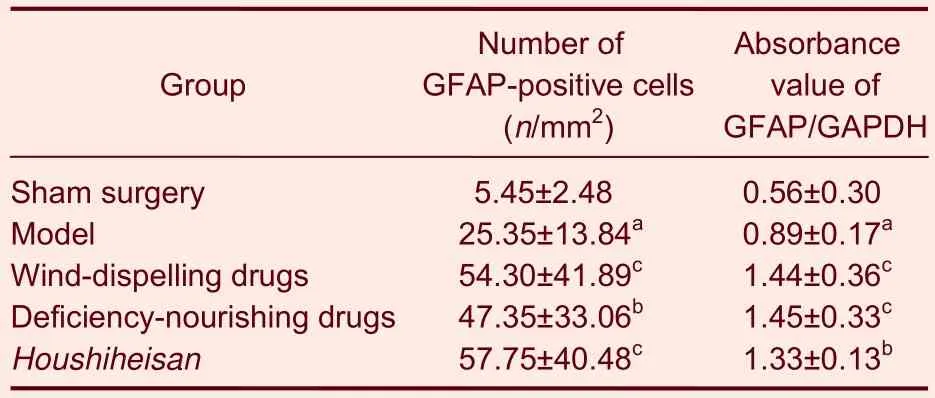
Table 2 Expression of glial fibrillary acidic protein (GFAP)in rat corpus striatum on the ischemic side in each group
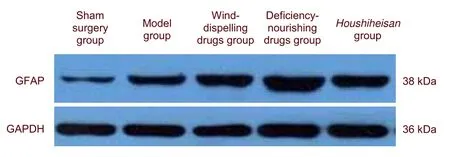
Figure 3 Glial fibrillary acidic protein (GFAP) protein expression in the rat corpus striatum on the ischemic side(western blot assay).
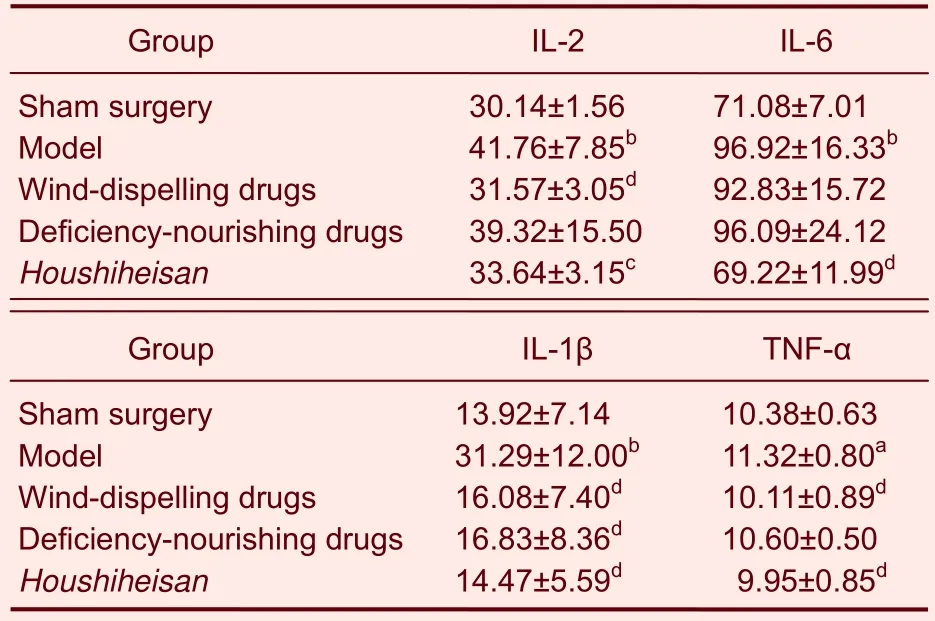
Table 3 Changes in interleukin-1β (IL-1β), IL-2, IL-6 and tumor necrosis factor-α (TNF-α) levels (ng/L) in the rat corpus striatum on the ischemic side
DISCUSSION
Astrocytes regulate the cerebral microenvironment and play an important role in neuronal survival, development,regeneration and differentiation[10]. A common response to central nervous system injury is reactive gliosis of astrocytes; the number of astrocytes increases, cells present more glial filaments and processes and they enhance their metabolism. Astrocytes and their processes could surround damaged and degenerated neurons, resulting in glial scar formation[11]. The present study mainly focused on how to regulate astrocyte function to protect neural cells and prevent glial scar formation[12].
Results from this study revealed that, 24 hours following cerebral ischemia/reperfusion, neuronal necrosis had occurred in the cortex and corpus striatum, there was astrocyte activation in the boundary of the necrotic region,and weak GFAP-positive reaction in the center of necrotic region. Astrocyte reaction is associated with neuronal damage and survival[13]. Houshiheisan relieved neuronal degeneration, promoted astrocyte activation in the cortex and corpus striatum and increased GFAP protein expression in the ischemic side of rat brains.
Factor analysis of the results showed that the protective effects of deficiency-nourishing drugs on neurons and its upregulatory effects on GFAP protein expression were larger than those of the wind-dispelling drugs. However,the effect of wind-dispelling drugs in increasing GFAP-positive cell number was larger than that of deficiency-nourishing drugs. Wind-dispelling drugs combined with deficiency-nourishing drugs supported,protected, separated and nourished neurons by activating astrocytes.
Secondary injury to brain tissues induced by local inflammatory reaction in astrocytes is an important reason for central nervous system injury[14]. After cerebral ischemia, astrocytes induced the production of many inflammatory mediators such as interleukin-1β,interleukin-2, interleukin-6 and tumor necrosis factor-α.
The interaction of these cytokines formed a complicated cytokine network that regulates cell function[15-17]. The cytokine network stimulated astrocyte division and proliferation by binding to corresponding receptors on astrocytes, participated in cascade reaction of inflammation, caused toxic effects on neural cells following ischemia, and resulted in neural cell necrosis and apoptosis[18]. Results from the present study showed that Houshiheisan reduced the levels of interleukin-1β,interleukin-2, interleukin-6 and tumor necrosis factor-α in the corpus striatum. Factor analysis of the results demonstrated that wind-dispelling drugs were more effective in decreasing inflammatory factor expression than deficiency-nourishing drugs. These indicated that wind-dispelling drugs reduced inflammatory factor release, lessened ischemic activated inflammatory factor-mediated immunologic injury and thus protected neurons and astrocytes.
In summary, wind-dispelling drugs combined with deficiency-nourishing drugs increased GFAP expression,contributed to astrocyte activation and protected neuronsagainst injury, probably by decreasing inflammatory factor expression. This shows that it is important to use wind-dispelling drugs to improve the clinical treatment of strokes.
MATERIALS AND METHODS
Design
This was a randomized controlled animal experiment.
Time and setting
Experiments were performed at the Experimental Animal Center, Capital Medical University, China from January to June 2011.
Materials
Experimental animals
A total of 65 specific pathogen free, male,Sprague-Dawley rats aged 3 months, weighing 280 ±20 g were supplied by Vital River, Beijing, China(Certificate No. SCXK (Jing) 2006-0009). The rats were allowed free access to food and water, and acclimatized for 3 days. Protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[19].
Drugs
Houshiheisan is composed of chrysanthemum flower 40 g, divaricate Saposhnikovia root 10 g, Cassia twig 3 g, Szechwan lovage rhizome 3 g, Manchurian wild ginger 3 g, Platycodon root 8 g, crude large-head Atractylodes rhizome 10 g, Indian bread 3 g, Zingiber 3 g, Chinese angelica 3 g, and red ginseng powder 3 g.Wind-dispelling drugs are composed of chrysanthemum flower 40 g, divaricate Saposhnikovia root 10 g, cassia twig 3 g, Szechwan lovage rhizome 3 g, Manchurian wild ginger 3 g and Platycodon root 8 g. Deficiencynourishing drugs are composed of crude large-head Atractylodes rhizome 10 g, Indian bread 3 g, Zingiber 3 g, Chinese angelica 3 g, and red ginseng powder 3 g.The amounts used of the above-mentioned drugs were in accordance with Synopsis of Golden Chamber[20].Although related to body surface area, the equivalent doses of Houshiheisan, wind-dispelling drugs and deficiency-nourishing drugs are respectively 10.5 g/kg,7.7 g/kg and 2.6 g/kg body weight. All Chinese medicinal materials were purchased from Beijing Tongrentang Pharmacy in China. The medicinal materials were decocted twice at 100°C under a normal pressure, each for 40 minutes. The physic liquor was mixed and filtrated. Wind-dispelling drugs,deficiency-nourishing drugs and Houshiheisan were condensed into crude drugs 0.77 g/mL, 0.26 g/mL and 1.05 g/mL, respectively.
Methods
Establishment of models of cerebral ischemia/reperfusion injury
In accordance with a previous method[18], the rats were anesthetized and secured. The right common carotid artery, internal carotid artery and external carotid artery were exposed, the external carotid artery and the common carotid artery were ligated and one end of the internal carotid artery, far from the heart, was occluded with a bulldog clamp. An incision was made at the crotch of the external carotid artery and internal carotid artery.An 18 mm long thread of 0.265 mm diameter was inserted for 2 hours. The thread was drawn back and reperfusion was completed. Under anesthesia, the rats from the sham surgery group only underwent exposure of common carotid artery, internal carotid artery and external carotid artery.
Administration method
The rats from the wind-dispelling, deficiency-nourishing and Houshiheisan groups were administered the drugs intragastrically at 10 mL/kg, once a day, for 3 days before surgery. Rats from the model group were established at 20 minutes following administration at day 4. They were administered once at 6 hours after surgery, and then administered at 20 hours after surgery. An equivalent volume of saline was given in the sham surgery and model groups.
Collection of brain tissues in the ischemic side
At 24 hours following cerebral ischemia/reperfusion, five rats were randomly taken from each group. The heart was exposed under anesthesia, followed by rapid heart cannulation. The heart was washed with saline at 37°C for 5 minutes and perfused with 4% paraformaldehyde and 0.1 M PBS (pH 7.4). The whole brain was then removed. 3-4 mm coronal tissue blocks were cut from the optic chiasma in a caudal direction, fixed at 4°C for 1 week, and used for hematoxylin-eosin staining and immunohistochemical staining. An additional eight rats were taken from each group, and then sacrificed. The corpus striatum in the ischemic side was rapidly isolated in an ice box. Tissue proteins were extracted using Tris-histone extraction reagent (Beijing Kangwei Shiji Biological Technology Co., Ltd., Beijing, China. Protein was quantified by bicinchoninic acid assay[21], and used for western blot assay.
Pathomorphological changes in brain tissues observed by hematoxylin-eosin staining
3-4 mm fixed coronal tissue blocks obtained from opticchiasma in the caudal direction were embedded in paraffin, dehydrated in gradient ethanol, and cleared in xylene. The specimens were serially sliced into 5 μmthick coronal sections with a microtome. The sections were baked dry and stained with hematoxylin and eosin.
GFAP expression in the corpus striatum of MCAO rats measured by immunohistochemistry
Substance P immunohistochemistry was used. The sections were deparaffinized, rehydrated, subjected to antigen retrieval in citric buffer (pH 6.0) by microwave.The sections were incubated in 3% hydrogen peroxide at room temperature in the dark, and processed in a wet box for 10 minutes to inactivate endogenous enzymes.The sections were then incubated in rabbit anti-rat GFAP monoclonal antibody 100 μL (1:500 of dilution; #2301-1;Epitomics, Burlingame, CA, USA) at 4°C for 40 hours,followed by rewarming for 1 hour. The sections were incubated in horseradish peroxidase-labeled goat anti-rabbit IgG (1:2 000; Epitomics) 80 μL at room temperature for 60 minutes. The staining was visualized with diaminobenzidine (1:20), followed by counterstain with hematoxylin. The sections were dehydrated and mounted.
Image processing and data analysis
Tissue sections were observed by light microscopy(Nikon, Tokyo, Japan), and images were captured using a digital microscope camera (Leica, Solms, Germany).For each rat, a total of three sections were selected, and four fields of each section were used. Cells were quantified using Image-Pro Plus version 5.1 software(Media Cybernetics, Bethesda, MD, USA). Results were expressed as the number of neurons, i.e. n/mm2.GFAP-positive cells in the corpus striatum were quantified by the same method.
GFAP expression in the corpus striatum of MCAO rats detected by western blot assay
A total of 20 μL protein extractives were added in SDS-PAGE loading buffer, followed by boiling for 5 minutes to denature proteins. Proteins were separated using 10% SDS-PAGE. The protein sample was transferred to a nitrocellulose filter at 4°C for 90 minutes and a 350 mA wet method was used. Membranes were blocked with 5% skim milk powder at room temperature for 1 hour, incubated with 5% skim milk powder-diluted rabbit anti-rat GFAP monoclonal antibody (1:20 000) at 4°C for 24 hours, washed with Tris-buffered saline/Tween, and incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (1:30 000) for 60 minutes. The membranes were washed and incubated in enhanced chemiluminescence to develop the membrane. With GAPDH as internal reference,images were analyzed using Image J software (National Institutes of Health, Bethesda, Maryland, USA).Absorbance values were read.
Enzyme-linked immunosorbent assay for levels of interleukin-1β, interleukin-2, interleukin-6 and tumor necrosis factor-α in the corpus striatum of MCAO rats
We used double antibody sandwich ABC-enzyme-linked immunosorbent assay[21]. 100 μL protein extracts were added on to a plate coated with rabbit anti-rat interleukin-1β (or interleukin-2, interleukin-6 and tumor necrosis factor-α) monoclonal antibody (Assay Designs,MI, USA). After mixing, the specimens were placed in a incubator for 120 minutes at 37°C, washed six times,dried on a filter paper. Then 50 μL of biotinylated anti-rat interleukin-1β/interleukin-2/interleukin-6/tumor necrosis factor-α antibody fluid was added to each well and incubated at 37°C for 60 minutes. Then 100 μL of antibody fluid was added at 37°C for 60 minutes. Finally 100 μL of tetramethylbenzidine was added at 37°C for 5 minutes until it turned blue. The reaction was terminated by adding 50 μL of sulfuric acid. There was a wash between each step. Absorbance values were measured at 450 nm. Levels of interleukin-1β,interleukin-2, interleukin-6 and tumor necrosis factor-α were proportional to the absorbance values. Using curve expert 1.3 software (Curveexpert, Hyams DG, Starkville,MS, USA), a standard curve was drawn utilizing absorbance values of standard preparations of 1 000,500, 250, 125, 62, 31, 16 and 0 pg/mL.
Statistical analysis
The data were analyzed using SPSS 10.0 software(SPSS, Chicago, IL, USA). Results were expressed as mean ± SD. Intergroup difference was compared by two-way analysis of variance. Least significant difference test was employed for paired comparison. A value of P <0.05 was considered statistically significant.
Funding: This project was funded by the National Natural
Science Foundation of China, No. 30973782; the Natural
Science Foundation of Beijing, No. 7102014; the Science and Technology Program of Chinese Medicine of Beijing City, No.JJ2008-042; the Chinese Medicine Nursing Special Foundation of Beijing Education Commission, No. 10ZYH04.
Author contributions: Qiuxia Zhang participated in animal
experimentation and data statistics. Hui Zhao, Qi Zhang and Haizheng Wang participated in immunohistochemistry and molecular biology experiments. Lei Wang was in charge of study design and manuscript authorization. Qiuxia Zhang and Hui Zhao obtained funding.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the AnimalEthics Committee, Capital Medical University, China.
REFERENCES
[1] Rouach N, Koulakoff A, Abudara V, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551-1555.
[2] Rouach N, Koulakoff A, Giaume C. Neurons set the tone of gap junctional communication in astrocytic networks.Neurochem Int. 2004;45(2-3):265-272.
[3] Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol.2010;79(2):77-89.
[4] Ding GD. Practical value of the Hou’s black powder and Feng Yin decoction. Jiangsu Zhongyiyao. 1983(1):51-53.
[5] Zhao LJ. The Hou’s black powder should be paid more attention in clinical application. Zhongwai Yiliao. 2008;30:114.
[6] Shi XH, Tan T, Li DD. Clinical Observation of the Hou’s black powder treatment of ischaemic stroke recovery belonging to Phlegm-stasis Blocking Collateral Type.Zhongyiyao Daobao. 2009;15(3):21-23.
[7] Mu Y, Zhao H, Zhang QX. Influence of Houshiheisan and its separate components on the neuropathology’s change of the MACO rats. Shandong Zhongyiyao Daxue Xuebao.2009;33(1):60-62, 65.
[8] Zhang QX, Zhao H, Wang L, et al. Mechanism of Hou Shi Hei San in accelerating repair of nerve after cerebral ischemia in rats. Shoudu Yike Daxue Xuebao. 2009;30(3):341-346.
[9] Caviness VS, Makris N, Montinaro E, et al. Anatomy of stroke, Part I: an MRI-based topographic and volumetric System of analysis. Stroke. 2002;33(11):2549-2556.
[10] Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, et al.Role of astrocytes in brain function and disease. Toxicol Pathol. 2011;39(1):115-123.
[11] Barreto G, White RE, Ouyang Y, et al. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. 2011;11(2):164-173.
[12] Pertusa M, García-Matas S, Rodríguez-Farré E, et al.Astrocytes aged in vitro show a decreased neuroprotective capacity. J Neurochem. 2007;101(3):794-805.
[13] Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science.2007;317(5841):1083-1086.
[14] Zhao Y, Rempe DA. Targeting astrocytes for stroke therapy. Neurotherapeutics. 2010;7(4):439-451.
[15] Yasuda Y, Shimoda T, Uno K, et al. Temporal and sequential changes of glial cells and cytokine expression during neuronal degeneration after transient global ischemia in rats. J Neuroinflammation. 2011;8:70.
[16] Strecker JK, Minnerup J, Gess B, et al. Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1β and G-CSF after transient focal ischemia in mice. PLoS One. 2011;6(10):e25863.
[17] Xia W, Han J, Huang G, et al. Inflammation in ischaemic brain injury: current advances and future perspectives.Clin Exp Pharmacol Physiol. 2010;37(2):253-258.
[18] Hata R, Mies G, Wiessner C, et al. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic,biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18(4):367-375.
[19] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[20] Zhang JL. Synopsis of Golden Chamber. Beijing: China Press of Traditional Chinese Medicine. 2008:95.
[21] Li JM. Clinical Enzyme Immunoassay Technology. Beijing:People's Military Medical Press. 2005.
(Edited by Zhang L, Sun JN/Qiu Y/Wang L)
10.3969/j.issn.1673-5374.2012.24.002
- 中国神经再生研究(英文版)的其它文章
- Acupuncture and moxibustion for visceral pain
- Therapeutic effect of nerve growth factor on cerebral infarction in dogs using the hemisphere anomalous volume ratio of diffusion-weighted magnetic resonance imaging*★
- Pre-ischemia electro-acupuncture potentiates the expression of Bcl-2 and transforming growth factor-beta 1 in rat brains*☆△◇
- Yizhijiannao Granule and a combination of its effective monomers, icariin and Panax notoginseng saponins, inhibit early PC12 cell apoptosis induced by beta-amyloid (25-35)☆
- Acupuncture activates signal transduction pathways related to brain-tissue restoration after ischemic injury**☆
- No association between a polymorphism of the adenylate cyclase type IX gene and major depressive disorder in the Chinese Han population*☆

