No association between a polymorphism of the adenylate cyclase type IX gene and major depressive disorder in the Chinese Han population*☆
Suxia Cao, Xiaofeng Zhao, Hengfen Li
Department of Psychiatry, First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China
No association between a polymorphism of the adenylate cyclase type IX gene and major depressive disorder in the Chinese Han population*☆
Suxia Cao, Xiaofeng Zhao, Hengfen Li
Department of Psychiatry, First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China
Previous studies have demonstrated that a missense single-nucleotide polymorphism variant (2316A>G; rs2230739) of the adenylate cyclase type IX gene was associated with bipolar disorder and affective disorder. We determined genotype and allele frequencies using a ligase detection reaction method in 315 patients with major depressive disorder and 278 unrelated, sex-matched healthy control subjects. We did not detect any statistically significant differences in genotype and allele frequencies between patients and healthy control subjects. Furthermore, we found no significant difference between genders in major depressive disorder, nor between patients and controls in the same gender. These results suggest that 2316A>G (rs2230739) may not be a risk factor for increasing susceptibility to major depressive disorder in the Chinese Han population.
adenylate cyclase type IX gene; 2316A>G; rs2230739; major depressive disorder; gene polymorphism
Research Highlights
Missense single-nucleotide polymorphism variant, 2316A>G (rs2230739), of the adenylate cyclase type IX gene is not associated with the onset of major depressive disorder in the Chinese Han population from Henan Province.
Abbreviations
ADCY9, adenylate cyclase type IX gene; MDD, major depressive disorder; cAMP, cyclic adenosine monophosphate; AC, adenylate cyclase; ATP, adenosine triphosphate
INTRODUCTION
Current evidence suggests that modifications in cyclic adenosine monophosphate (cAMP) signaling are involved in the pathophysiology and treatment of depression, with alterations in cAMP signaling pathways found in postmortem studies of brains from patients with depression[1-6].
Adenylate cyclase (AC) is the enzyme that synthesizes cAMP from adenosine triphosphate (ATP). Regulation of intracellular cAMP concentrations is large due to modulation of AC. Previous studies have shown that AC activities are decreased in platelets and postmortem brain from patients with depression[7-9].
There are at least nine membrane-bound isoforms of AC, which are regulated by neurotransmitters and drugs, actingviaG protein-coupled receptors to modulate intracellular cAMP levels. A number of AC isoforms have been linked to development of psychiatric disorders; for example, inhumans the adenylate cyclase type VII gene (ADCY7) is associated with major depressive disorder (MDD) in a sex-specific relationship.ADCY7is also associated with depressive-like behaviors in mice[10].
Additionally, in platelets from depressed patients, AC1, AC3 and AC8 show reduced activity[11-12], and AC5 is a pharmacological target for depression and anxiety disorders[13].
Of all the isoforms, AC9 shows the most sequence divergence, and is therefore a unique member of the mammalian AC family[14-15].
Of particular interest is the relatively abundant expression of AC9 mRNA and protein in the hippocampus, a region important for learning and memory, and also associated with depression[16-17].
Furthermore, the gene for the ADCY9 subunit is located on chromosome 16p13.3, a location that shows modest linkage signals for mood disorders[18-21].
Nevertheless, there are only a few studies focusing on the function of AC9 in the etiology of MDD. Recently, a missense single-nucleotide polymorphism variant (2316A>G; rs2230739) in exon 7 (aa 772) of the humanACgene,ADCY9, was identified[22]. This single-nucleotide polymorphism has previously been associated with bipolar disorder and mood disorder[23-24]. In this study, we investigated the potential role of the 2316A>G polymorphism in the etiology of MDD in the Chinese Han population.
RESULTS
Quantitative analysis of subjects
A total of 593 subjects (315 MDD patients and 278 healthy controls) were included in the study.
Subject baseline characteristics
315 (132 males, 183 females) MDD patients were recruited. The mean age was 37.5 ± 13.2 years, ranging from 18-60 years. The control group consisted of 278 (128 males, 150 females) sex-matched, healthy volunteers. The mean age was 39.7 ± 12.7 years, ranging from 18-60 years. The control group was recruited from the same region of the Chinese Han population.
There was no difference in the sex ratio between MDD patients and control subjects (χ2= 1.03,P= 0.31, Pearson chi-square). However, the mean age in the control group was significantly higher than that in the MDD group (t= -2.21,P= 0.03, independent samplest-test).
Changes in genotype and allele frequency of 2316A>G (rs2230739) in both groups
Genotype distributions ofADCY9were in Hardy-Weinberg equilibrium, in both the MDD group (P= 0.599) and control group (P= 0.132). The genotype distributions and allele frequencies for rs2230739 in both groups are shown in Table 1. No significant differences were detected in allele or genotype frequencies between MDD and control groups (genotype:χ2= 2.81,P= 0.25; allele:χ2= 2.12,P= 0.15).
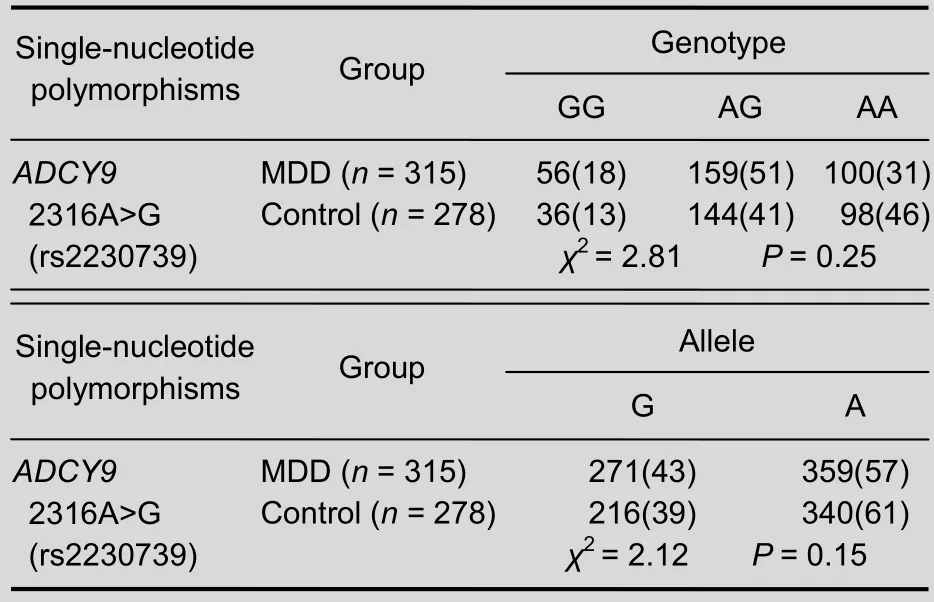
Table 1 Distribution of 2316A>G (rs2230739) variant of the adenylate cyclase type IX gene (ADCY9) in major depressive disorder (MDD) and control groups
Furthermore, no significant differences in allele or genotype frequencies were detected between males and females in the MDD group (genotype:χ2= 0.35,P= 0.84; allele:χ2= 0.34,P= 0.56) (Table 2).
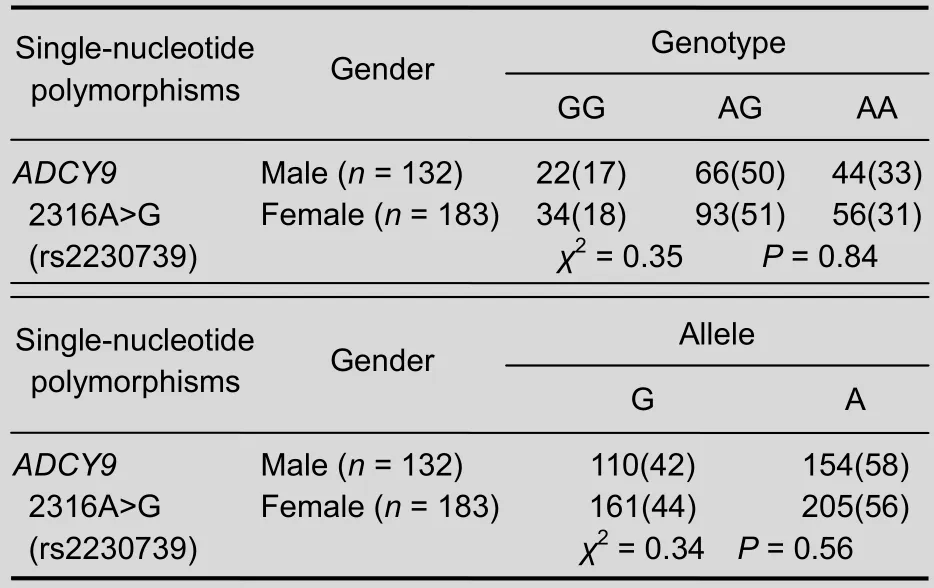
Table 2 Genotype and allele frequencies of 2316A>G (rs2230739) variant of the adenylate cyclase type IX gene (ADCY9) in major depressive disorder, between males and females
Genotype distributions and allele frequencies of rs2230739, between MDD and control groups from the same gender, are shown in Tables 3, 4. We detected no significant differences in the genotype distribution or allele frequencies for the same gender between MDD and control groups (males:χ2= 0.91,P= 0.64 (genotype);χ2= 0.49,P= 0.49 (allele); females:χ2= 1.856,P= 0.118 (genotype);χ2= 0.168,P= 0.19 (allele).

Table 3 Genotype and allele frequencies of 2316A>G (rs2230739) variant of the adenylate cyclase type IX gene (ADCY9) in major depressive disorder (MDD) and controls in males

Table 4 Genotype and allele frequencies of 2316A>G (rs2230739) variant of the adenylate cyclase type IX gene (ADCY9) in major depressive disorder (MDD) and controls in females
DISCUSSION
To our knowledge, this is the first study to examine the association between the AC9 polymorphism and MDD in a large case-control study in the Chinese Han population. The potential influence of this polymorphism on ADCY9 function has not been examined in detail, but its location within exon 7 leads to a missense mutation responsible for altering the activity of ADCY9 function, which may in turn increase susceptibility to certain psychiatric disorders. Our study failed to detect an association between this polymorphism and MDD. In contrast to our finding, there is the initial report of an association between this genetic variant and unipolar disorder in the Japanese population[24]. Toyotaet al[23]confirmed that theADCY9gene variation had a weak association with bipolar disorder (P= 0.033 2), but did not show which genotype or allele was the risk factor for bipolar disorder. The 2316A>G allele frequency in our samples (0.43) was not the same as Toyotaet al[24](0.36). The discrepancy in allele frequencies may be partly responsible for the divergent association results.
It has been suggested that focusing on specific subclinical phenotypes may increase the strength to detect genes involved in complex disorders, including affective disorders[25-26]. Given that in the United States, the lifetime prevalence of MDD in women is approximately twice that in men[2], we hypothesized that theADCY9polymorphism is associated with gender differences in MDD. Therefore, to reduce the clinical heterogeneity, we examined the patient and control subgroups according to gender. Thus, we compared genotype and allele frequencies of theADCY9single nucleotide polymorphism in MDD between genders but did not detect a significant relationship. Similarly, we compared genotype and allele frequencies of theADCY9single nucleotide polymorphism in MDD and control groups within the same gender, but again did not detect a relationship. It is not known whether these variants can alter ADCY9 function, although the substitution of isoleucine with methionine removes a bulky side chain that may change the conformation of the enzyme. Taken together, these results indicate thatADCY9does not have a genetic influence on MDD in the Chinese Han population. Therefore, our results suggest that the influence of the 2316A>G polymorphism on major depression may be ethnically dependent. Large replication studies with different ethnic groups are needed to determine whether there are ethnic differences in the influence of the 2316A>G polymorphism on major depression.
The main limitation of our study is the small sample size. It is generally accepted that case-control association studies are influenced by sample size and that small sample size can lead to false results. Another limitation of this study is that we only focused on the IX isoform of adenylate cyclase. It is possible that other isoforms of adenylate cyclase may be important in conferring susceptibility to MDD patients.
In conclusion, we report a negative association of anADCY9polymorphism with MDD. However, the result of our case-control gene association study should be interpreted with caution due to the study limitations discussed.
SUBJECTS AND METHODS
Design
A case-control human gene polymorphism association study.
Time and setting
The study was performed in the Laboratory of Zhengzhou University Medical Center, China, from October 2009 to March 2010.
Subjects
The study included 593 subjects (260 males, 333 females), with a mean age of 38.6 ± 12.9 years, ranging from 18-60 years. We recruited 315 MDD patients and 278 healthy controls, with no person declining to participate. The protocols were described to subjects, and written informed consent was obtained from each subject[27].
Inclusion criteria: patients from central China with major depression, who metDiagnostic and Statistical Manual of Mental Disorders, Fourth Edition[28]criteria. Additionally, a minimum baseline score of 15 on the 17-item Hamilton Rating Scale for depression. To reduce the influence of population stratification in gene frequencies, all participants in this study were from the Chinese Han population in Henan Province, located in central China. Exclusion criteria: patients with substance abuse, severe organic disorder, organic brain disease or any concomitant major psychiatric disorder were excluded following clinical interviews.
Healthy controls were recruited from the same region of the Chinese Han population as MDD patients. Subjects with a past or present psychiatric disorder, or a positive family history of a psychiatric disorder were excluded.
Methods
ADCY9 DNA extraction and PCR assay
Genomic DNA was extracted from ethylenediamine tetraacetic acid-treated venous blood samples obtained from the elbow, by standard techniques using a DNA extraction kit (Wizard Genomic DNA purification kit; Promega, Wisconsin, USA). Primer details are shown in Table 5.
The expected PCR product was 206 bp. PCRs were carried out in a final volume of 20 μL, containing 100 ng genomic DNA, 0.2 mM of each dNTP, 1.0 ng/μL template, 2.0 mM buffer, 0.6 mM Mg2+, 0.2 unit Hot Start Taq DNA polymerase, 9.8 μL H2O, 4 μL Q-solution and 0.4 pM primer mix. After an initial denaturation step of 95°C for 15 minutes, there were 35 cycles of denaturation at 94°C for 30 seconds, annealing at 59°C for 1 minute and extension at 72°C for 1 minute. A final step was performed at 72°C for 7 minutes. 2 μL of PCR mixture was run on Tris-borate-ethylenediamine tetraacetic acid gels to ensure successful amplification of products (Figure 1).

Table 5 Primer information for genotyping of adenylate cyclase type IX rs2230739
Figure 1 PCR gel of adenylate cyclase type IX rs2230739 amplification.
ADCY9 post-PCR ligase detection reaction genotyping assay
Genotyping was carried out in a final volume of 10 μL, containing 1 μL PCR mixture, 6.95 μL H2O, 0.05 μL ligase, 1 μL probe mix and 1 μL buffer. After an initial denaturation at 95°C for 2 minutes, there were 35 cycles of denaturation at 94°C for 30 seconds, with annealing and extension at 60°C for 20 minutes. Ligase detection reaction products were used for allele discrimination.
ADCY9 allele discrimination
Ligase detection reaction products were separated on 6% denaturing polyacrylamide gels using an ABI 377 automated sequencer (Applied Biosystems Incorporation, Maryland, USA). GenescanTM672 software (Applied Biosystems Incorporation) collected data, corrected the line of electrophoresis and measured different fragments. Genemapper software was used to analyze genotype distributions data (Figure 2).
Figure 2 Analysis of adenylate cyclase type IX rs2230739 nucleotide composition by PCR-ligase detection reaction.
Statistical analysis
Data were analyzed using SPSS 13.0 (SPSS, Chicago, IL, USA). The independent samplesttest was used to determine the average for continuous variables for parametric statistics. The Pearson chi-square test was used to compare gender differences. We determined the Hardy-Weinberg equilibrium for each group. The genotype and allele frequencies were compared between MDD and control groups, using the Pearson chi-square test when the sample size was smaller than expected (fewer than five subjects). All tests were two-tailed and alpha level set at 0.05.
Funding: This project was funded by Natural Science Foundation of Department of Education of Henan Province, No. 2009B20005.
Author contributions: Suxia Cao participated in manuscript writing and article authorization. Xiaofeng Zhao was in charge of statistical analysis. Hengfen Li was responsible for study design.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Ethics Committee, First Affiliated Hospital, Zhengzhou University, China.
[1] Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358(1):55-68.
[2] Bennett DS, Ambrosini PJ, Kudes D, et al. Gender differences in adolescent depression: do symptoms differ for boys and girls? J Affect Disord. 2005;89(1-3):35-44.
[3] Mizrahi C, Stojanovic A, Urbina M, et al. Differential cAMP levels and serotonin effects in blood peripheral mononuclear cells and lymphocytes from major depression patients. Int Immunopharmacol. 2004;4(8):1125-1133.
[4] Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378-2391.
[5] Whitaker CM, Wei H. An alternate cAMP pathway Epac promotes hippocampallong-term depression. J Physiol. 2009;587(Pt 13):3067-3068.
[6] Xiao Z, Liu W, Gao K, et al. Interaction between CRHR1 and BDNF genes increases the risk of recurrent major depressive disorder in Chinese population. PLoS One. 2011;6(12):e28733.
[7] Cowburn RF, Marcusson JO, Eriksson A, et al. Adenylyl cyclase activity and G-protein subunit levels in postmortem frontal cortex of suicide victims. Brain Res. 1994;633(1-2):297-304.
[8] Hines LM, Tabakoff B. WHO/ISBRA Study on State and Trait Markers of Alcohol Use and Dependence Investigators. Platelet adenylyl cyclase activity: a biological marker for major depression and recent drug use. Biol Psychiatry. 2005;58(12):955-962.
[9] Reiach JS, Li PP, Warsh JJ, et al. Reduced adenylyl cyclase immunolabeling and activity in postmortem temporal cortex of depressed suicide victims. J Affect Disord. 1999;56(2-3):141-151.
[10] Hines LM, Hoffman PL, Bhave S, et al. A sex-specific role of type VII adenylyl cyclase in depression. J Neurosci. 2006;26(48):12609-12619.
[11] Katsel PL, Tagliente TM, Schwarz TE, et al. Molecular and biochemical evidence for the presence of type III adenylyl cyclase in human platelets. Platelets. 2003;14(1):21-33.
[12] Willoughby D, Cooper DM. Organization and Ca2+regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87(3):965-1010.
[13] Krishnan V, Graham A, Mazei-Robison MS, et al. Calcium-sensitive adenylyl cyclases in depression and anxiety: behavioral and biochemical consequences of isoform targeting. Biol Psychiatry. 2008;64(4):336-343.
[14] Hacker BM, Tomlinson JE, Wayman GA, et al. Cloning, chromosomal mapping, and regulatory properties of the human type 9 adenylyl cyclase (ADCY9). Genomics. 1998;50(1):97-104.
[15] Yan SZ, Huang ZH, Andrews RK, et al. Conversion of forskolin-insensitive to forskolin-sensitive (mouse-type IX) adenylyl cyclase. Mol Pharmacol. 1998;53(2):182-187.
[16] Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression:a meta-analysis. Am J Psychiatry. 2004;161(4):598-607.
[17] Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957-1966.
[18] Byerley W, Badner JA. Strategies to identify genes for complex disorders: a focus on bipolar disorder and chromosome 16p. Psychiatr Genet. 2011;21(4):173-182.
[19] Kremeyer B, García J, Müller H, et al. Genome-wide linkage scan of bipolar disorder in a Colombian population isolate replicates Loci on chromosomes 7p21-22, 1p31, 16p12 and 21q21-22 and identifies a novel locus on chromosome 12q. Hum Hered. 2010;70(4):255-268.
[20] Mérette C, Roy MA, Bureau A, et al. Replication of linkage with bipolar disorder on chromosome 16p in the Eastern Quebec population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):737-744.
[21] Ross J, Berrettini W, Coryell W, et al. Genome-wide parametric linkage analyses of 644 bipolar pedigrees suggest susceptibility loci at chromosomes 16 and 20. Psychiatr Genet. 2008;18(4):191-198.
[22] Small KM, Brown KM, Theiss CT, et al. An Ile to Met polymorphism in the catalytic domain of adenylyl cyclase type 9 confers reduced beta2-adrenergic receptor stimulation. Pharmacogenetics. 2003;13(9):535-541.
[23] Toyota T, Hattori E, Meerabux J, et al. Molecular analysis, mutation screening, and association study of adenylate cyclase type 9 gene (ADCY9) in mood disorders. Am J Med Genet. 2002;114(1):84-92.
[24] Toyota T, Yamada K, Saito K, et al. Association analysis of adenylate cyclase type 9 gene using pedigree disequilibrium test in bipolar disorder. Mol Psychiatry. 2002;7(5):450-452.
[25] Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265(5181):2037-2048.
[26] Yanagisawa S, Kondo N, Miki A, et al. A common complement C3 variant is associated with protection against wet age-related macular degeneration in a Japanese population. PLoS One. 2011;6(12):e28847.
[27] State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994-09-01.
[28] American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4thed. Washington DC: American Psychiatric Association. 1994.
Cite this article as:Neural Regen Res. 2012;7(24):1914-1919.
Suxia Cao☆, Studying for doctorate, Attending physician, Department of Psychiatry, First Affiliated Hospital, Zhengzhou University, Zhengzhou
450052, Henan Province, China
Hengfen Li, M.D., Professor, Department of Psychiatry, First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China
li.hengfen@yahoo.com.cn
2012-03-15
2012-06-19
(N20111031003/WJ)
Cao SX, Zhao XF, Li HF. No association between a polymorphism of the adenylate cyclase type IX gene and major depressive disorder in the Chinese Han population. Neural Regen
Res. 2012;7(24):1914-1919.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.24.011
(Edited by Xie GR, Zhang KR/Qiu Y/Wang L)
- 中国神经再生研究(英文版)的其它文章
- Effects of wind-dispelling drugs and deficiency-nourishing drugs of Houshiheisan compound prescription on astrocyte activation and inflammatory factor expression in the corpus striatum of cerebral ischemia rats****☆
- Acupuncture and moxibustion for visceral pain
- Therapeutic effect of nerve growth factor on cerebral infarction in dogs using the hemisphere anomalous volume ratio of diffusion-weighted magnetic resonance imaging*★
- Pre-ischemia electro-acupuncture potentiates the expression of Bcl-2 and transforming growth factor-beta 1 in rat brains*☆△◇
- Yizhijiannao Granule and a combination of its effective monomers, icariin and Panax notoginseng saponins, inhibit early PC12 cell apoptosis induced by beta-amyloid (25-35)☆
- Acupuncture activates signal transduction pathways related to brain-tissue restoration after ischemic injury**☆

