基于Keggin阴离子链镍化合物的结构和质子导电性
魏梅林王晓湘 李会华
(河南师范大学化学与环境科学学院,新乡 453007)
基于Keggin阴离子链镍化合物的结构和质子导电性
魏梅林*王晓湘 李会华
(河南师范大学化学与环境科学学院,新乡 453007)
由H+(H2O)2.5阳离子,[Ni(H2O)8]2+阳离子,[PW12O40]3-阴离子和异烟酸氮氧化物(HINO)自组装成一个具有质子导电性的化合物{[Ni(H2O)8][H(H2O)2.5](HINO)4(PW12O40)}n。293 K的单晶X-射线衍射分析表明标题化合物形成1个带有一维通道的三维氢键网络结构。[PW12O40]3-阴离子填充在一维通道内并且自组装成多阴离子链。热重分析表明在20~100℃范围内化合物没有失重,表明化合物结构单元内所有的水分子在100℃以下不易失去。标题化合物在85~100℃范围内表现出好的离子导电性(1×10-3~2× 10-3S·cm-1)。
杂多酸;晶体结构;镍(Ⅱ)配合物;异烟酸氮氧化物
Keggin-type heteropolyacids(HPAs),possessing a unique discrete ionic structure including heteropoly anions and countercations(H+,H3O+,H5O2+,etc.),are widely known as proton conducting electrolytes for low-temperaturehydrogen-oxygen fuel cells[1].However, the application of HPAs is limited by the extreme sensitivity of their conductivity to the relative humidity(RH)and the temperature of the surrounding atmosphere[2].To overcome these problems,various attempts have been made to immobilize HPAs in silica gel and to disperse it in an organicallymodified electrolyte membrane and organic/inorganic hybrid membranes[3-4].In addition,to enable fast ionic conduction in the hybrid materials,themolecularmodification of organic ligands to inorganic structures of HPAs has been continuously investigated[5].For a long time,we have focused on the proton conductivity of organic/ inorganic complexes based-on the transition metal salts of HPAs dispersing in self-ordered hydrogenbonded networks.Salts crystallize with fewer water molecules than the acids,and are more stable. Incorporation of salts into the self-ordered hydrogenbonded networks protects them from dehydration and enhanced their thermal stability.Moreover,each transition metal ion could form an ionized water cluster with a special hydration number and a special structure.In this work,we have succeeded in constructing a proton-conductive organic/inorganic hybrid complex by a self-assembly of protonated water clusters,transition metal aqua ions,[PW12O40]3-anions and isonicotinic acid N-oxide(HINO).Here,we report its synthesis,crystal structure,and proton conductivity as a function of temperature.
1 Experimental
1.1 M aterials and instruments
All organic solvents and material used for synthesis were of reagent grade and used without further purification.α-H3PW12O40·6H2O was prepared according to a literaturemethod[6]and characterized by IR spectra and TG analyses.HINO was synthesized according to a literaturemethod and characterized by IR spectra[7].Elemental analyses(C,H,and N)were carried out on a Perkin-Elmer 240C analyzer.X-ray powder diffraction(XRD)was performed on a Bruker D8 Advanced Instrument using Cu Kαradiation and a fixed power source(40 kV,40 mA).IR spectra were recorded on a VECTOR 22 Bruker spectrophotometer with KBr pellets in the 400~4 000 cm-1region at room temperature.Thermogravimetric analysis was performed on a Perkin-Elmer thermal analyzer under nitrogen at a heating rate of 10℃·min-1.For an electrical conductivity study,the powdered crystalline samples were compressed to about 1.0 mm in thickness and 12.0 mm in diameter under a pressure of 12~14 MPa.Ac impedance spectroscopy measurementwas performed on a chi660b(Shanghai chenhua) electrochemical impedance analyzer with copper electrodes[8](the purity of Cu ismore than 99.8%) over the frequency range from 105to 10 Hz.Samples were placed in a temperature-humidity controlled chamber(GT-TH-64Z,Dongwan Gaotian Corp).The conductivity was calculated asσ=(1/R)×(h/S),where R is the resistance,h is the thickness,and S is the area of the tablet.
1.2 Synthesis of the title com plex
The formation of heteropolyacid nickel salts was accomplished by neutralization of the acids.α-H3PW12O40·6H2O(180 mg,0.06 mmol)and adding NiCl2·6H2O(15 mg,0.06 mmol)dissolved in water(4 mL).The solution was heated at 80℃in a water bath.Light green crystals were formed by cooling the saturated solution and slow evaporation at room temperature,and characterized by IR spectrum.A mixture of result heteropolyacid nickel salts(90 mg, 0.03 mmol)and HINO(17 mg,0.12 mmol)was dissolved in enough acetonitrile/water(1∶1,V/V)to form a homogeneous solution.Finally,the solution was filtered and the solvent left to evaporate at room temperature.A week later,lightblue crystals appeared and were collected and dried in air after quickly being washed with water.Yield:76.4 mg,85%based onα-H3PW12O40·6H2O.Molecular formula is C24H42Ni W12N4O62.5P.Elemental analysis calcd.(%):C,7.83;H, 1.15;N,1.52.Found(%):C,7.92;H,1.28;N,1.61. Main IR bands(cm-1):four characteristic vibrations resulting from heteropolyanions with the Keggin structure:813ν(W-Oc),896ν(W-Ob),981ν(W=Ot), 1 081ν(P-Oa);and another vibrations resulting from theHINOmolecules:3 326ν(O-H),1704ν(C=O),1 618 ν(C=C),1 280ν(N-O),1 172δ(C-H,in plane).
1.3 X-ray diffraction analysis
Intensity data of the title complex was collected on a Bruker SMART-CCD area detector with graphitemonochromatic Mo Kαradiationλ=0.071 073 nm using SMARTand SAINTprograms[9].The structurewas solved by directmethods and refined on F2by using full-matrix least-squares methods with SHELXTL version 5.1[10].All non-hydrogen atoms except solvent water molecules were refined anisotropically.Two O (3W)centers are crystallographically disordered into four symmetrical positions with each oxygen site half-occupied,and two O(2W)centers are also crystallographically disordered into four symmetrical positions with each oxygen site half-occupied.The O(5W)with the occupancy of 50%is placed in a symmetry center and located in a tetragonal environment built from two watermolecules(O(6W)and O(7W)centers),which are crystallographically disordered into two symmetrical positions with each oxygen site half-occupied, respectively.Hydrogen atoms of the organicmolecules were localized in their calculated positions and refined using a ridingmodel.Hydrogen atoms ofwater molecules(O(1W)and O(4W))were localized by difference Fourier maps and refined by fixing the isotropic temperature factors 1.2 times that ofmother atoms attached.Hydrogen atoms of coordination water molecules(O(2W)and O(3W))and solvent water molecules were not treated because these oxygens are crystallographically disordered.The biggish absolute values of the final(Δρ)maxand(Δρ)minmight result from the many heavy metal atoms(W)in the title compound.The final(Δρ)maxand(Δρ)minare located the W atoms.The crystal parameters,data collection and refinement results for the title complex are summarized in Table 1,the selected bond lengths and bond angles are listed in Table 2,and the selected hydrogen bond parameters in Table 3.

Table 1 Crystal and structure refinement data for 1
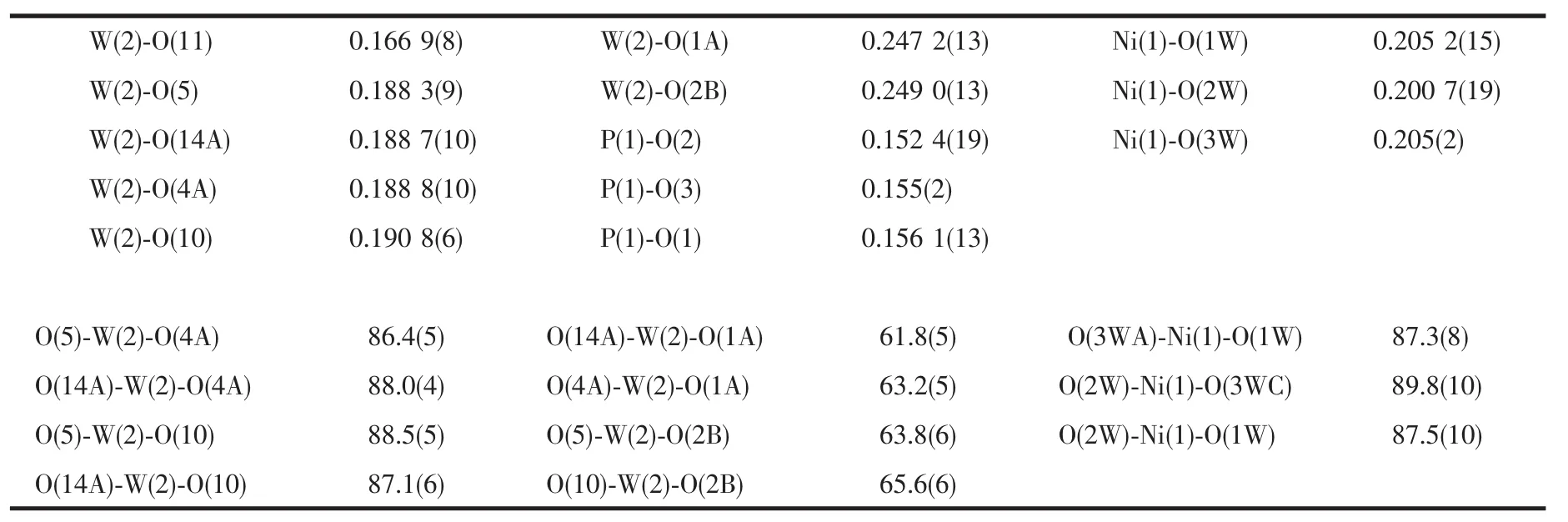
Table 2 Selected bond lengths(nm)and angles(°)for 1
CCDC:825709.
2 Results and discussion
2.1 IR spectra
Comparison of the IR spectra of heteropolyacid nickel salts,isonicotinic acid N-oxide and the studied crystal shows that theν(C=O)band at 1710 cm-1and theν(N-O)band around 1280 cm-1in spectrum of free HINOmolecules[11]remains around the similar position in the complex,indicating that these groups are not involved in complex formation.The IR spectroscopic studies show that there are not strong interactionsbetween themetal ions and the organic groups in the solid state.The proton polarizabilities are particularly large in the case of hydrogen-bonded chains because in such chains a collective proton-tunnelling occurs. In the FT-IR spectra,these hydrogen bonds or hydrogen-bonded chains are manifested as continua because they interact very strongly with their environments because of large proton polarizabilities, and vice versa hydrogen bonds and hydrogen-bonded chains with large proton polarizabilities are indicated by these IR continua.Thus,such hydrogen-bonded chains are very effective proton pathways.They conduct protons within picoseconds.Water molecules are highly ordered for the entropic reasons if they are in a hydrophobic environment.Therefore,they build up a particularly stable pathway.An IR continuum indicates that this system shows proton polarizability due to collective protonmotion.
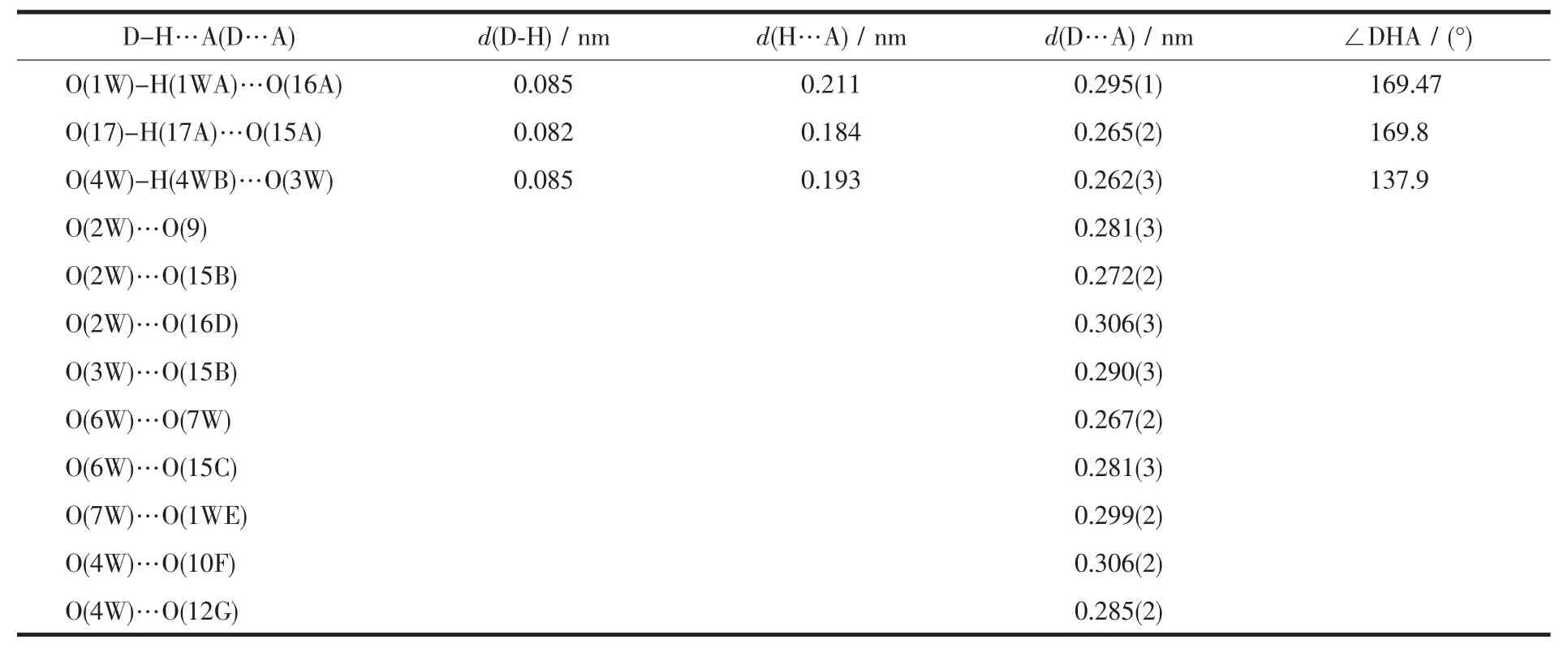
Table 3 Selected hydrogen bond lengths and bond angles for 1
2.2 Structure description
The title complex,{[Ni(H2O)8][H(H2O)2.5](HINO)4(PW12O40)}n,was synthesized by the reaction of NiHPW12O40·n H2O and HINO at room temperature.It was characterized by single-crystal X-ray diffraction, infrared spectroscopy,TG and elemental analyses.X-ray diffraction analysis at 293 K revealed that the title complex presented a 3D supramolecular framework built from non-covalent interactions among HINO molecules,[Ni(H2O)8]2+and H+(H2O)2.5cations,and [PW12O40]3-anions.Interestingly,[PW12O40]3-anions self-assembled into poly-Keggin-anion chains in the supramolecular framework.
In[Ni(H2O)8]2+cations(Fig.1a),the Ni2+ion is placed in a symmetry center and located in a coordination octahedral environment built from six water molecules(two O(1W)centers,two O(2W)centers and two O(3W)centers),as well as two O(4W)centers are situated outside the coordination shell through short hydrogen-bonding interaction with the coordination water molecule.It should be noted that two O(3W)centers are crystallographically disordered into four symme-trical positions with each oxygen site half-occupied,and two O(2W)centers are also crystallographically disordered into four symmetrical positions with each oxygen site half-occupied[12].In the H+(H2O)2.5(the proton added to balance the charge)[11](Fig.1b),2.5 watermolecules,O(5W),O(6W) and O(7W),formed an tetragon structure.
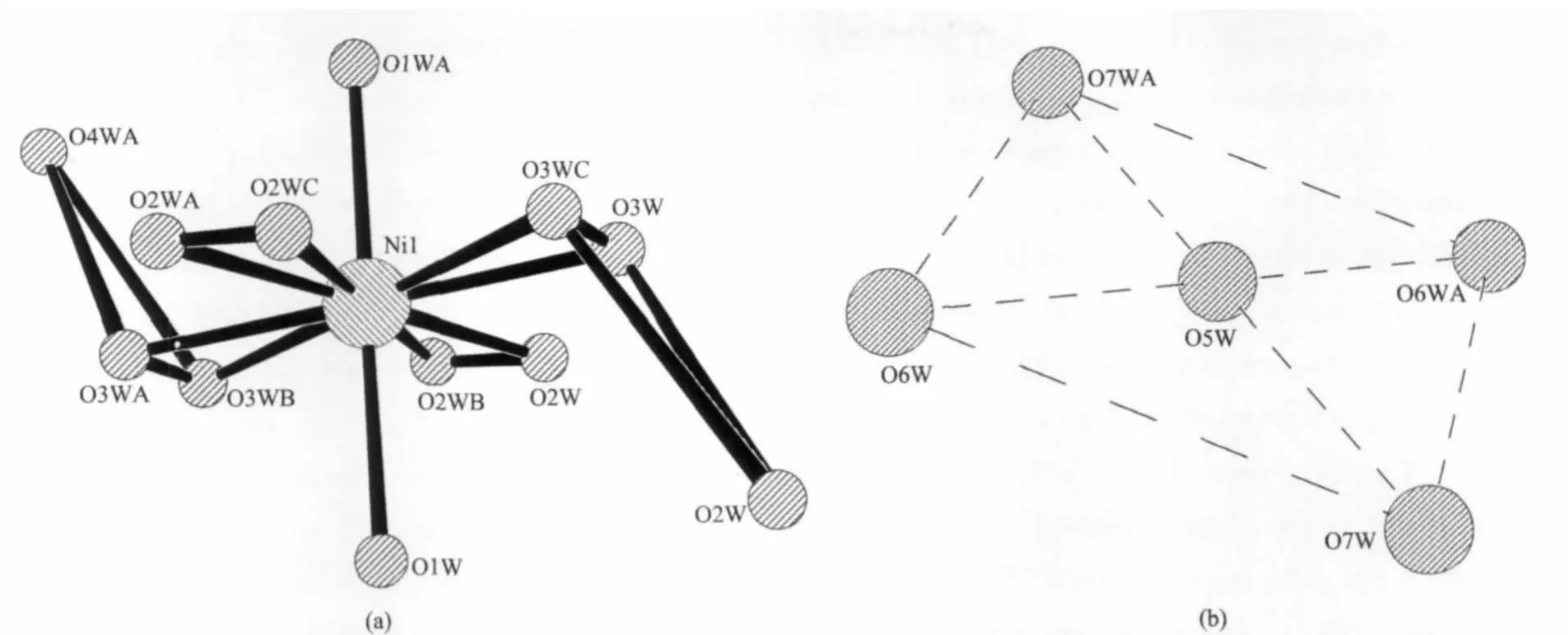
Fig.1 Views of[Ni(H2O)8]2+(a)and H+(H2O)2.5(b)cations
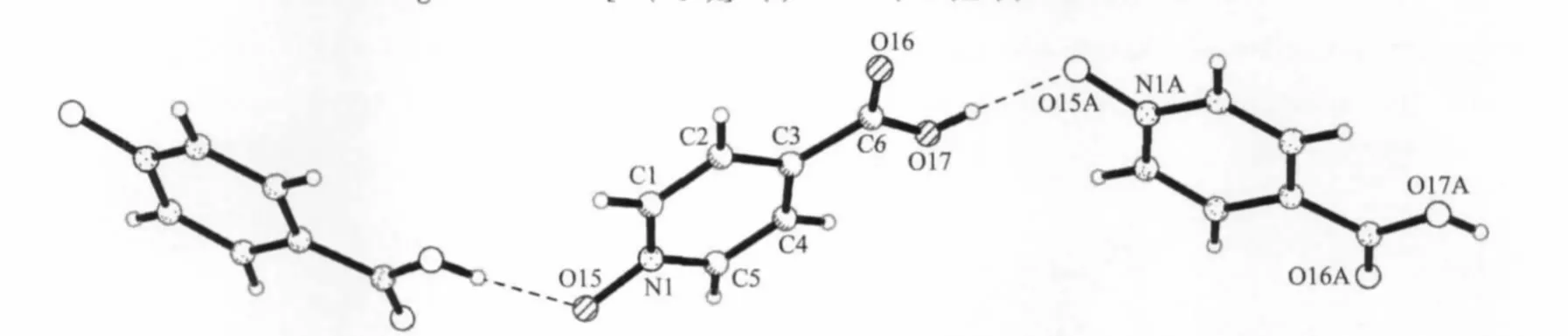
Fig.2 View of the 1D HINOs chain along the b axis
HINO is a goodmono-or bidentate ligand for the construction of supramolecular complexes with versatile bindingmodes.Until now,a large number of metal-organic framework structures containing HINO ligands have been reported[11].Interesting,in the title complex,HINO molecules are not bound to the Ni2+ion,but remaining outside the coordination shell to form hydrogen-bonded chains along the b axis(Fig.2). Two oxygen atoms O(15)(N-O)and O(17)(O-H)of each HINO molecule are involved in the hydrogen-bonded chains.As shown in Fig.3,these HINO hydrogenbonded chains are linked together by[Ni(H2O)8]2+aqua-complexes and small H+(H2O)2.5cations into a 3D cationic network with large 1D channels through hydrogen bonds between coordination watermoleculesO(2W)and oxygen atoms O(15)of HINO molecules, as well as through weak hydrogen bonds between coordination water molecules and oxygen atoms of HINO molecules.Thus,all O atoms of each HINO molecule are involved in the hydrogen bonds,creating a 3D supramolecular assembly with 1D channels. Moreover,there are weakly hydrogen-bonding interaction between watermolecules O(7W)in the H+(H2O)2.5cations and O(1W)center of the[Ni(H2O)8]2+cations. The section size of the channels based on the Ni…Ni separations is ca.1.04 nm×1.60 nm×1.98 nm for the title complex(these separations are equal to three axial lengths respectively),indicating that each pore could only accommodate a single Keggin anion. Interestingly,the adjacent Ni…Ni separation along the a axis ismuch shorter than other two separations along the b and c axes,and even shorter than the diameter of the discrete[PW12O40]3-anion(ca.1.05 nm),resul-ting in each cavity being heavily condensed along the a axis.In addition,the presence of positively charged species,[Ni(H2O)8]2+and H+(H2O)2.5cations,could attract the polyanions,as a result,the Keggin-type[PW12O40]3-anions for charge compensation are embedded in the voids of the 3D cationic framework and connect to one another leading to poly-Keggin-anion chains in the channel along the a axis(Fig.4).In the polymeric polyanion,there are some short atomatom separations of 0.279(2)nm,such as O(7A)…O(8BB),O(8A)…O(7BB),O(7AA)…O (8CB),O(7CB)…O(8AA).
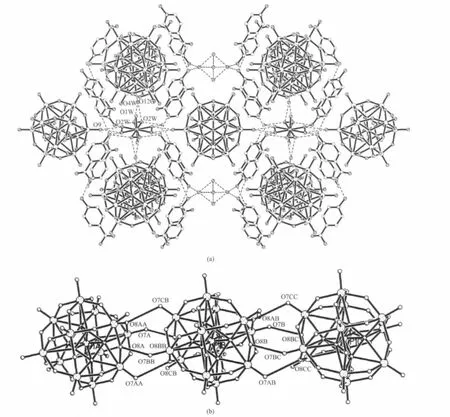
Fig.4 3D hydrogen-bonded network showing the 1D channels filled by poly-anion chains down the a axis(a),and view of the poly-anion chain along the a axis(b)
In the[PW12O40]3-unit,the central P atom is surrounded by a cube of eight oxygen atomswith each oxygen site half-occupied.These eight oxygen atoms are all crystallographically disordered,and this case can be found inmany compounds[13].In the title complex, the bond lengths of P-O and W-O are 0.151(2)~0.156 4(19)and 0.166 6(8)~0.249 6(18)nm respectively. The bond lengths of P-O and W-O in the title complex are respectively comparable to those in the 3D porous polyoxometalates-based organic-inorganic hybrid materials with Keggin anions as guests[13].In addition,the O-P-O anglesare in the range of108.3(6)° ~111.4(6)°for the title complex.All these results indicate that the[PW12O40]3-units have a normal Keggin structure in the polymeric-polyanion chains. The poly-Keggin-type anions play not only a chargecompensating role,but they can dramatically influence the overall solid-state architecture through their templating function,as well as the cationic framework with special channels also influences the polymerization of polyanions through its host function. In addition,several hydrogen bonds exist between the poly-Keggin-anion chain and the channel,such as between thewatermolecules(O(1W),O(2W)and O(4W)) belong to the[Ni(H2O)8]2+cations and oxygen atoms of the polyanions(O(9),O(10)and O(12)centers).As a result,based on the self-assembly of HINOmolecules, [Ni(H2O)8]2+and H+(H2O)2.5cations,the title complex form 3D hydrogen-bonding networkswith 1D channels along the a axis,in which poly-Keggin anions chains were formed and stabilized based on electrostatic and H-bonding interactions,resulting in[PW12O40]3-anions being not easy dissociated from the hybrid network. Moreover,the section size of the channels along the b and c axes is so larger than the diameter of the [PW12O40]3-anion(ca.1.05 nm)that there is enough space outside the poly-Keggin-anion chains to admit some small species,such as water molecules or hydronium ions,to transport along the channels.All these results indicate that the title complex can potentially be a new good proton-conductingmaterial.
2.3 TG Analysis
The complex is insoluble in water.Water retention in the hybrid at high temperature is a key factor for having fast protonic conduction[8-9].Thermogravimetric analysis of the powder of the crystalline sample of the complex in an atmosphere of N2(Fig.5) shows no weight loss in the temperature range of 20~ 100℃,indicating that allwatermolecules in the unit structure are involved in constructing the H-bonding network,which is consistent with the result of structural analysis,and are not easily lost below 100℃. This is not like that observed in the proton conductors including the quasi-liquid water clusters(which are generally loosely bonded in the structure)like pure tungstophosphoric acid with 26 water molecules (PWA-26)or molybdophosphoric acid with 26 water molecules(PMA-26),as well as many proton-conducting compositemembranes doped with HPAs[1-2].
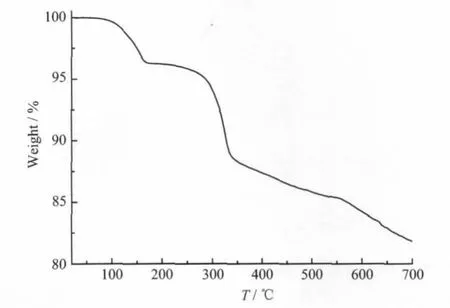
Fig.5 Curve of the thermogravimetric analysis of 1 in the atmosphere of N2
2.4 Proton conductivity
The proton conductivities of the title complex in the temperature range of 25~100℃under 98%RH conditions were evaluated by the ac impedance method using a compacted pellet of the powdered crystalline sample,which has the same structure as the single-crystal(Fig.6).Surprisingly,the title complex reached good proton conductivities of 1×10-3~2.1×10-3S·cm-1in the temperature range of 85~100℃,estimated from the Nyquist plots.
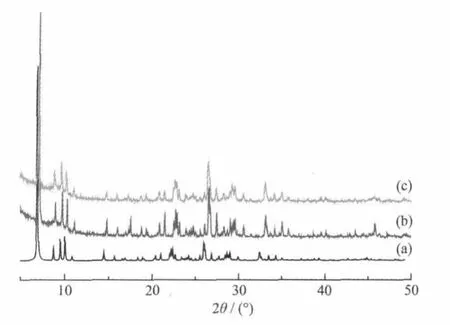
Fig.6 Powder X-ray diffraction data of the simulated powder pattern(a),the powder before the protonconductive measurement(b),and thepowder after the proton-conductivemeasurement(c)
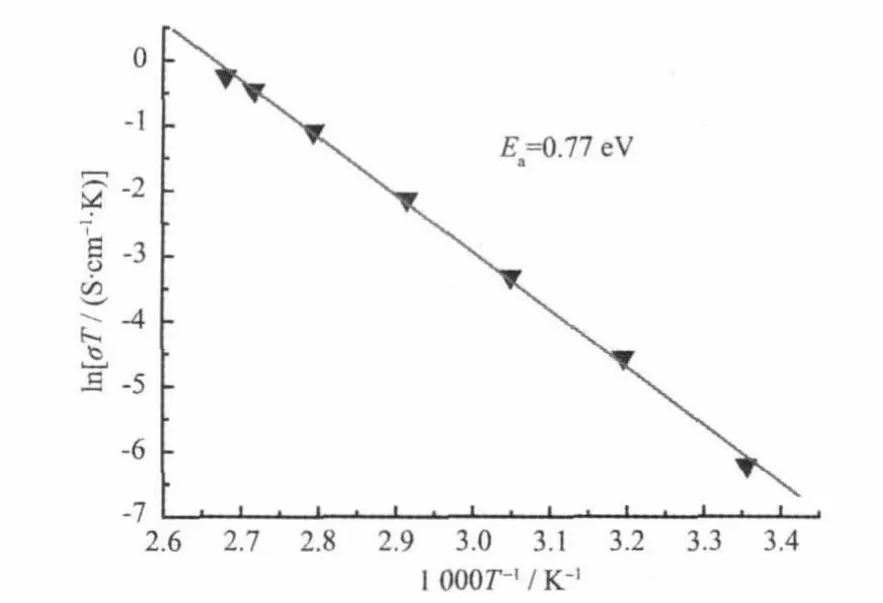
Fig.7 Arrhenius plotof the proton conductivity of 1

Fig.7 shows the Arrhenius plots of the proton conductivities of the title complex in the temperature range of 25~100℃under 98%RH conditions.The ln(σT)increases almost linearly with temperature range from 25 to 100℃,and the corresponding activation energy(Ea)of conductivity was estimated to be 0.77 eV for the title complex from the equation below[12-13e]. whereσis the ionic conductivity,σ0is the preexponential factor,kBis the Boltzmann constant,and T is the temperature.The Eavalue is high in the temperature range of 25~100℃.The results show that the general features of the changes in conductivities are different from that of PWA-26 or PMA-26,whose protonic conductivity decreased with the temperature from ambient to 60℃[1-2].However,the title complex has thermally activated protonic conductivities[11]from 25 to 100℃;as the temperature increases,the proton conductivities increase on a logarithmic scale even with almost saturated humidities.This is probably due to the fact that protons belong to the protonated water clusters and those originating from water molecules need a thermally activated process for dissociation as hydrated forms such as H+,H3O+or other proton species at a distance from[PW12O40]3-clusters[14].The mechanism of proton conduction of the title complex is,therefore,expected to be similar to that of the vehicle mechanism[15],that is,the direct diffusion of additional protonswith watermolecules.The existence of these half-occupied oxygen sites of watermolecules in[Ni(H2O)8]2+and H+(H2O)2.5cationsmay be derived from direct-jump diffusion and be conducive to formation of the H-bonding network.In addition,the existence of H-bonding network suggests that proton conduction in the title complex includes some other process such as proton transport of additional protons along H-bonding network(Grotthusmechanism)[16].It is possible that in higher temperature and humidity some of isonicotinic acid N-oxide molecules can be deprotonated and the protons can be incorporated by the watermolecules and therefore conductivity can be a result of dissociation of organic acid.The results of measurement of the proton conductivity of HINO molecules in the temperature range of 85~100℃at 98%RH showed the free HINO molecules reached proton conductivities of 10-5~10-4S·cm-1in the temperature range of85~100℃,these proton conductivities are about 1~2 orders ofmagnitude lower than that of the title complex,which shows conductivity of about 10-3S·cm-1in the conditions described.Therefore,the fact that the title complex exhibits good proton conductivities in the temperature range of 85~100℃is indicative of a high carrier concentration based on a thermally activated process,as well as the existence of the whole H-bonding networks.Moreover,there is the possibility of hydrolysis of the complex when it is held at 100℃with a RH higher than 98%(100%,or condensed water that attack the metal centres).The powder X-ray diffraction data in Fig.6 suggested that the powder sample after the proton-conductivemeasurement have the same supramolecular framework as that of complex 1.The proton conductivities of the title complex were alsomeasured at 100℃in the RH range 35%~98%by a complex-plane impedance method.Fig.8 shows the lgσversus RH plots of complex 1 at 100℃under 35%~98%RH.Theconductivity under 35% RH is~3.95×10-5S·cm-1, and the conductivity increases with RH to reach a high conductivity of 1.70×10-3~2.05×10-3S·cm-1in the range 60%~98%RH.
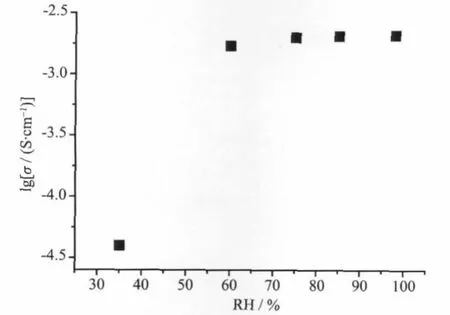
Fig.8 lgσversus RH plots of 1 at 100℃
[1](a)Misono M.Chem.Commun.,2001:1141-1152(b)Katsoulis D E.Chem.Rev.,1998,98:359-388(c)MARong-Hua(马荣华),WANGFu-Ping(王福平).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(3):445-450(d)FAN Ying(樊莹),LIU Shi-Zhong(柳士忠).Chinese J.Inorg.Chem.(WujiHuaxue Xuebao),2002,18(6):635-638
[2](a)AlbertiG,Casciola M,Costantino U,etal.J.Mater.Chem.,1995,5:1809-1812(b)Sang X G,Wu QY,PangW Q.Mater.Chem.Phys.,2003,82:405-409(c)Honma I,Nomura S,Nakajima H.J.Membr.Sci.,2001,185:83-94
[3](a)Kim Y S,Wang F,Hickner M,etal.J.Membr.Sci.,2003, 212:263-282(b)Malers J L,Sweikart M A,Horan J L,et al.J.PowerSources,2007,172:83-88
[4](a)Ramani V,Kunz H R,Fenton JM.J.Membr.Sci.,2004, 232:31-44(b)Verma A.,Scott K.J.Solid State Electrochem.,2010,14: 213-219
[5](a)Kim JD,Honma I.Solid State Ionics,2005,176:547-552 (b)Li M Q,Shao Z G,Scott K.J.Power Sources,2008,183: 69-75(c)Verma A,Scott K.J.Solid State Electrochem.,2010,14: 213-219
[6]Claude R D,Michel F,Raymonde F,et al.Inorg.Chem., 1983,22:207-216
[7]Simapson PG,Vinciguerra A,Quagliano JV.Inorg.Chem., 1963,2:282-286
[8]Wu QY,Zhao SL,Wang JM,etal.J.Solid State Electrochem., 2007,11:240-243
[9]SMART and SAINT;Area Detector Control and Integration Software,Madison,WI:Siemens Analytical X-ray Systems, Inc.,1996.
[10]Sheldrick GM.SHELXTL V5.1,Software Reference Manual, Madison,WI:Bruker AXS,Inc.,1997.
[11](a)Goher M A S,Mautmer F A.J.Mol.Struct.,2007,846: 153-156(b)Hong J.J.Mol.Struct.,2006,783:9-12(c)Zhang L P,Du M,Lu W,et al.Polyhedron,2004,23:857-863
[12](a)Sadakiyo M,Yamada T,Kitagawa H.J.Am.Chem.Soc., 2009,131:9906-9907(b)Yamada T,Sadakiyo M,Kitagawa H.J.Am.Chem.Soc.,2009,131:3144-3145(c)England W A,Cross M G,Hamnett A,et al.Solid StateIonics,1980,1:231-249
[13](a)Wei M L,He C,Hua W J,et al.J.Am.Chem.Soc., 2006,128:13318-13319(b)Wei M L,He C,Sun Q Z,et al.Inorg.Chem.,2007,46:5957-5966(c)Duan C Y,Wei M L,Guo D,et al.J.Am.Chem.Soc.,2010,132:3321-3330(d)WeiM L,Zhuang PF,LiH H,etal.Eur.J.Inorg.Chem.,2011:1473-1478
[14](a)Janic M J,Davis R J,Neurock M.J.Am.Chem.Soc., 2005,127:5238-5245(b)Hayashi EG,Moffat JB.J.Catal.,1983,83:192-204(c)Honma I,Nomura S,Nakajima H.J.Membr.Sci.,2001,185:83-94
[15]Kreuer K D,Rabenau A,Weppner W.Angew.Chem.Int. Ed.,1982,21:208-209
[16]Agmon N.Chem.Phys.Lett.,1995,244:456-462
Crystal Structure and Proton-Conductivity of a Nickel(Ⅱ)Comp lex Constructed by Poly-Keggin-anion Chains
WEIMei-Lin*WANG Xiao-Xiang LIHui-Hua
(College of Chemistry and Environmental Science,Henan Normal University,Xinxiang,Henan 453007,China)
A proton-conductive complex{[Ni(H2O)8][H(H2O)2.5](HINO)4(PW12O40)}n,wasconstructed by aself-assembly of cations,[Ni(H2O)8]2+cations,[PW12O40]3-anions and isonicotinic acid N-oxide(HINO).Single-crystal X-ray diffraction analysis at 293 K revealed that the title complex presented exactly a three-dimensional(3D)hydrogenbonded network with large one-dimensional(1D)channels.Interestingly,[PW12O40]3-anions just filled in the 1D channels and self-assembled into poly-Keggin-anion chains.Thermogravimetric analysis shows no weight loss in the temperature range of 20~100℃,indicating that all watermolecules in the unit structure are not easily lost below 100℃.The title complex was characterized by a satisfactory ionic conductivity(1×10-3~2×10-3S·cm-1)in the temperature range 85 to 100℃.CCDC:825709.
polyoxometalates;crystal structure;nickel(Ⅱ)complex;isonicotinic acid N-oxide
book=0,ebook=33
O614.81+3
A
1001-4861(2012)11-2485-09
2012-10-18。收修改稿日期:2012-08-03。
国家自然科学基金(No.20971038,21171050)资助项目。
*通讯联系人。E-mail:weimeilinhd@163.com

