一维链状铜(Ⅱ)配合物的合成、晶体结构及热稳定性研究
赵 丽董旭涛 孙银霞 程 倩 董秀延 王 莉
(兰州交通大学化学与生物工程学院,兰州 730070)
一维链状铜(Ⅱ)配合物的合成、晶体结构及热稳定性研究
赵 丽*董旭涛 孙银霞 程 倩 董秀延 王 莉
(兰州交通大学化学与生物工程学院,兰州 730070)
通过一种新的肟类配体HL(HL=1-(4-{[(E)-3-乙氧基-2-羟苯亚甲基]氨基}苯乙酮肟)与一水合乙酸铜反应,合成了一种铜(Ⅱ)配合物[Cu(L)2]·CH3OH。X射线单晶结构分析表明该配合物是一种单核配合物,其中铜(Ⅱ)原子以四配位的形式分别与2个单肟配体的酚氧原子和亚胺氮原子结合,形成稍微扭曲的平面四边形几何构型。O和N配位原子互为反式,所形成的Cu1N2O2平面和Cu1N4O5平面的二面角为23.33(3)°。在这个晶体结构中,每1个配合物分子分别与近邻的2个配合物分子通过O-H…O氢键连接,沿b轴形成了1个一维的无限延伸的链状结构。
肟类配体;铜(Ⅱ)配合物;合成;晶体结构;热性质
Oxime-type compounds are known to be versatile ligands.A large number of complexeswith oxime-type ligands have been reported because of their interesting structures and potential applications[1].Copper(Ⅱ)complexes with oxime-type ligands have been widely investigated in coordination chemistry and biological chemistry[2-5].In the last few years there has been a burgeoning effort to identify the biological activities of copper,primarily through techniques associated with the interface of biology/biochemistry/coordination chemistry[6-8].It appears that the biological role of copper is primarily in redox reactions and as a biological catalyst,although much remains to be understood[9].An extensive effort has been made to prepare and characterize a variety of Cu(Ⅱ)coordination complexes in an attempt to model the physical and chemical behavior of copper-containing enzymes[10].The peculiarity of copper lies in its abilityto form complexes with coordination number four,five or six[11-13].Asan extension of thework on the structural characterization of such complexes[14-19],the crystal structure of a new Cu(Ⅱ)complex,[Cu(L)2]·CH3OH,is reported in this paper.
1 Experimental
1.1 M aterials and instruments
3-Ethoxyl-2-hydroxybenzylidene was purchased from Alfa Aesar and used without further purification. 4-Aminophenylethanone oxime was synthesized according to an analogousmethod reported earlier[14-15].The other reagents and solvents were analytical grade reagents from Tianjin Chemical Reagent Factory.
Elemental analyses for Cu was detected by an IRIS ER/S·WP-1 ICP atomic emission spectrometer. C,H and N analyses were carried out with a GmbH VariuoEL V3.00 automatic elemental analyzer.IR spectra were recorded on a VERTEX70 FT-IR spectrophotometer,with samples prepared as KBr (500~4 000 cm-1)and CsI(100~500 cm-1)pellets.TGDTA analyses were carried out at a heating rate of 5℃·min-1on a ZRY-1P thermoanalyzer.Electrolytic conductancemeasurementwasmade with a DDS-11D type conductivity bridge using a 1.0mmol·L-1solution in DMF at room temperature.X-ray single crystal structure was determined on a Bruker Smart 1000 CCD area detector.Melting points were obtained by use of an X4 microscopic melting point apparatus made in Beijing Taike Instrument Limited Company and were uncorrected.
1.2 Preparation of the Cu(Ⅱ)complex
1.2.1 Synthesis of HL
HL(1-(4-{[(E)-3-ethoxyl-2-hydroxybenzylidene] amino}phenyl)ethanone oxime)was synthesized by modification of the reported method[14-15].To an ethanol solution of 2-hydroxy-3-ethoxybenzaldehyde(332.3 mg,2.00 mmol)was added an ethanol solution of 4-aminophenylethanone oxime(300.4 mg,2.00 mmol). The mixture solution was stirred at 328 K for 5 h. After cooling to room temperature,the precipitate was filtered,and washed successively with ethanol and nhexane,respectively.The product was dried under vacuum,and obtained orange red microcrystal.Yield, 75.1%.m.p.436~437 K.Anal.Calcd.for C17H18N2O3(%):C 68.44;H 6.08;N 9.39.Found(%):C,68.30;H, 6.02;N,9.52.
1.2.2 Synthesis of the complex[Cu(L)2]·CH3OH
A solution of copper(Ⅱ)acetatemonohydrate(2.4 mg,0.012 mmol)in methanol(2 mL)was added dropwise to a solution of HL(7.2mg,0.024mmol)in methanol(5mL)at room temperature.The color of the mixing solution turned brown immediately,and then continued to stirring for 4 h at room temperature.The mixture solution was filtered and the filtrate was allowed to stand at room temperature for about four weeks,the solvent was partially evaporated and obtained several green block-like single crystals suitable for X-ray crystallographic analysis.Anal. Calcd.for C35H38CuN4O7([Cu(L)2]·CH3OH)(%):C 60.52; H 5.46;N 8.35;Cu 9.41.Found(%):C 60.85;H 5.51;N 8.11;Cu 9.29.
1.3 Crystal structure determ ination
The X-ray diffraction measurement for the complex was performed on Bruker Smart 1000 CCD diffractometer with graphite monochromated Mo Kα radiation(λ=0.071 073 nm)at 298(2)K.Empirical absorption correction was applied to the data using SADABS program.The structure was solved by direct methods and refined by full-matrix least-squares method on F2using the SHELXL program[20].All nonhydrogen atoms were refined anisotropically.All the hydrogen atoms were generated geometrically and refined isotropically using the ridingmodel.Details of the crystal parameters,data collection and refinements for the complex are summarized in Table 1.
CCDC:871832.
2 Results and discussion
2.1 Crystal structure of[Cu(L)2]·CH3OH
X-ray crystallographic analysis of[Cu(L)2]· CH3OH reveals formation of a mononuclear structure. The Cu(Ⅱ)complex crystallizes in the monoclinic system,space group P21,and Z=2.The complex consists of one Cu(Ⅱ)atom,two deprotonated L-units and one crystallizing methanolmolecule,as expectedfrom the analytical data.The molecular structure of the Cu(Ⅱ)complex is shown in Fig.1,selected bond distances and angles are listed in Table 2.In asymmetric molecule unit of the Cu(Ⅱ)complex,the Cu(Ⅱ)center is tetra-coordinated by the phenolate O atoms and imine N atoms from two deprotonated oxime-type ligands,in a slightly distorted squareplanar geometry.The coordinate bond lengths and angles around the Cu atom(Table 1)are typical and comparable to the corresponding values observed in other Cu(Ⅱ)complexes with oxime-type ligands[21].The two deprotonated oxime-type ligands are coordinated in a trans fashion.The dihedral angle between the coordination plane of O2-Cu1-N2 and that of O5-Cu1-N4 is 23.33(3)°,while another dihedral angle between the coordination plane of N2-Cu1-O5 and that of O2-Cu1-N4 is23.27(3)°,indicating slightdistortion toward tetrahedral geometry from the square planar structure[22-23].

Table 1 Crystal data and structure refinement for the title comp lex

Table 2 Selected bond distances(nm)and bond angles(°)for the title com plex

Thermal ellipsoids are plotted at 30%probability level;Hydrogen atoms are omitted for clarityFig.1 Crystal structure of the Cu(Ⅱ)complex with theatom numbering
The introduction of one crystallizing methanol molecule in the Cu(Ⅱ)complex successfully leads to the assembly of these monomeric units by intermolecular hydrogen bonds.As illustrated in Fig. 2,The phenolate O2 and ethoxyl O3 atoms of one ligand molecule,respectively,and the O7 atom of the crystallizing methanol molecule is bonded to the hydroxyl-O4H4 group of the other ligand unit of the Cu(Ⅱ)complex.Simultaneous,the phenolate O5 and ethoxyl O6 atoms of the ligand unit in the Cu(Ⅱ)complex are hydrogen-bonded to the-O1H1 group of another adjacent Cu(Ⅱ)complex unit,respectively. Thus,each molecule links two other adjacent molecules into an infinite one-dimensional chain supramolecular structure through six intermolecular O-H…O hydrogen bonds.

Table 3 Intermolecular hydrogen bonds for the Cu(Ⅱ)complex

Symmetry codes:ix,y-1,z;iix,y+1,zFig.2 Intermolecular hydrogen bonds of the Cu(Ⅱ)complex
2.2 FTIR spectra
The FTIR spectra of HL and its corresponding Cu(Ⅱ)complex in the 400~4 000 cm-1region is given in Table 4.The free ligand HL exhibits characteristic C=N stretching band at 1 615 cm-1,while the C=N of the Cu(Ⅱ)complex was observed in the 1610 cm-1.The C=N stretching frequency is shifted to lower frequency by ca.5 cm-1upon complexation,indicating thebehavior between the free ligand and the corresponding Cu(Ⅱ)atom resulting in weakening the force constant of C=N bond[24-25].The Ar-O stretching frequency appear as a strong band in the 1 253 cm-1in the free ligand,and the toward lower wavenumber of the Ar-O absorption shift ca.18 cm-1indicating thatCu-O bonds are formed between the Cu(Ⅱ)atom and the oxygen atoms of the phenolic groups[26].
The N-O stretching frequencies of the oxime group in free ligand and the Cu(Ⅱ)complex allappearas a strong band in the 919 cm-1indicating that the oxime N atoms do not involve in coordination.In the 1460~ 1591 cm-1region,the observed bandswere attributed to aromatic C=C vibrations.Upon coordination these bands shift to lower frequencies for the Cu(Ⅱ)complex. In addition,theO-H stretching frequency of the free HL ligand appears at 3 289 and 3 241 cm-1.The out-ofplane bending vibration of phenolic alcohol in HL at 1 277 cm-1,which disappears in the complex,indicating the oxygen in the phenolic alcoholof the Cu(Ⅱ)complex hasbeen deprotoned and coordinated to the Cu(Ⅱ)atom. Meanwhile,infrared spectrum of the Cu(Ⅱ)complex shows the expected strong absorption band due toν(OH)at ca.3 248 cm-1,which is the evidence for the existenceofmethanolmolecule.
The far-infrared spectrum of the Cu(Ⅱ)complex was also obtained in the region 100~500 cm-1in order to identify frequencies due to the Cu-O and Cu-N bonds.The FT-IR spectrum of the complex showed ν(Cu-N)andν(Cu-O)vibration absorption frequencies at 515 and 440 cm-1,respectively[16].
2.3 UV-Vis absorption spectra
The UV-Vis absorption spectra of HL and its Cu(Ⅱ)complex in diluted chloroform solution are shown in Table 5.It can be seen that the absorption peaks of the Cu(Ⅱ)complex are obviously different from those of the HL upon complexation.Compared with the Cu(Ⅱ)complex,an important feature of the absorption spectrum of HL is shown that anabsorption peak is observed at 301 nm,which is absent in the spectrum of the Cu(Ⅱ)complex.The other feature is that the absorption peak at 235 nm in HL is shifted to 240 nm in the Cu(Ⅱ)complex,and two new absorption peaks at 280 and 328 nm are observed in the Cu(Ⅱ)complex,indicating that the coordination of the Cu(Ⅱ)atom with HL.
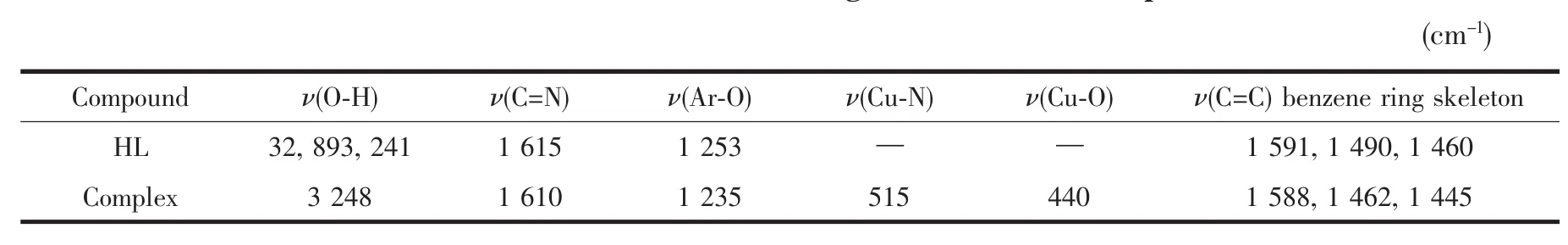
Table 4 M ain IR bands for the ligand HL and the com plex
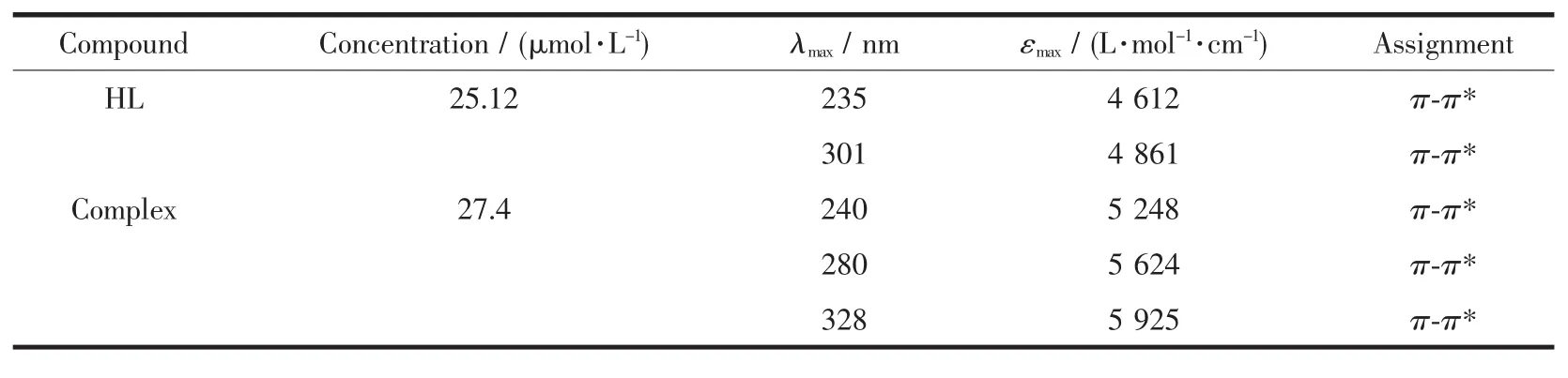
Table 5 UV-Vis data for the ligand HL and its Cu(Ⅱ)comp lex
2.4 M olar conductance of[Cu(L)2]·CH3OH
The Cu(Ⅱ)complex issoluble in ethanol,methanol, acetonitrile,acetone,THF,DMF,DMSO,but not soluble in diethyl ether and n-hexane.Molar conductance value of the Cu(Ⅱ)complex at 25℃of 1 mmol·dm-3DMF solutions is 2.8Ω-1·cm2·mol-1, indicating that the Cu(Ⅱ)complex is non-electrolyte. This implies that all the L-units in the Cu(Ⅱ)complex are always held in the coordination sphere in solution or solid state.
2.5 Thermal property of[Cu(L)2]·CH3OH
The thermal decomposition of the Cu(Ⅱ)complex can be mainly divided into two stages.First,the initial weight loss occurs in the range of 49.0 to 67.2℃,corresponding to an endothermic peak,and the TG curve shows that the weight loss corresponding to this temperature range is 4.3%that roughly coincides with the value of 4.6%,calculated for the loss of one crystallingmethanolmolecule;The Cu(Ⅱ)complex has nomelting point.The Cu(Ⅱ)complex ismore thermally stable than its ligand HL.Its second weight loss starts ataround 265.6℃.Subsequently,continuousmass loss wasobserved up to 650℃.At this temperature,CuO is formed.The total mass loss found(86.7%)was approximately consistentwith thatcalculated(88.5%).
[1]Adhikary C,Sen R,Bocelli G,et al.J.Coord.Chem.,2009, 62:3573-3582
[2]Rajasekar M,Sreedaran R,Prabu R,et al.J.Coord.Chem., 2010,63:136-146
[3]Qin DD,Yang ZY,Zhang FH,etal.Inorg.Chem.Commun., 2010,13:727-729
[4]Sang Y L,Lin X S.J.Coord.Chem.,2010,63:316-322
[5]Xiao JM,Zhang W.Inorg.Chem.Commun.,2009,12:1175-1178
[6]Dong W K,Duan J G,Liu G L.Transition Met.Chem., 2007,32:702-705
[7]DongW K,Ding Y J.Cryst.Res.Technol.,2007,43:321-326
[8]Tarafder M T H,Jin K T,Crouse K A,et al.Polyhedron, 2002,21:2547-2554
[9]Musie G T,Li X,Powell D R.Inorg.Chem.Acta,2003,348: 69-74
[10]Reddy P A N,Datta R,Chakravarty A R.Inorg.Chem. Commun.,2000,3:322-324
[11]Ray M S,Bhattacharya R B,Chaudhuri S,et al.Polyhedron, 2003,22:617-624
[12]Raptopoulou C P,Papadopoulos A N,Malamatari D A, etal.Inorg.Chem.Acta,1998,272:283-290
[13]Arnold P J,Davies S C,Durrant M C,et al.Inorg.Chem. Acta,2003,348:143-149
[14]Zhao L,Ng SW.Acta Cryst.,2010,E66:o2474
[15]Zhao L,Ng SW.Acta Cryst.,2010,E66:o2473
[16]DONG Wen-Kui(董文魁),GONG Shang-Sheng(宫尚生), TONG Jun-Feng(同俊锋),et al.Chinese J.Inorg.Chem. (WujiHuaxue Xuebao),2010,26(10):1868-1874
[17]DONG Wen-Kui(董文魁),TANG Xiao-Lu(唐晓璐),HE Xue-Ni(何雪妮),etal.Chinese JInorg.Chem.(WujiHuaxue Xuebao),2009,25(3):528-532
[18]Dong W K,Feng JH,Yang X Q.Synth.React.Inorg.Met-Org.Nano-Met.Chem.,2007,37:189-192
[19]DONGWen-Kui(董文魁),LV Zhong-Wu(吕忠武),SUN Yin -Xia(孙银霞),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(9):1627-1634
[20]Sheldrick G M.SHELXTL 5.10 for Windows NT,Structure Determination Software,Bruker Analytical X-Ray Systems, Inc.,Madison,WI,USA,1997.
[21]DongW K,Duan JG.J.Coord.Chem.,2008,61:781-788
[22]Wu H L,Liu JG,Liu P,et al.J.Coord.Chem.,2008,61: 1027-1035
[23]Wu H L,Yun R R,Wang K T,etal.Z.Anorg.Allg.Chem., 2010,636:629-633
[24]DONGWen-Kui(董文魁),SHI Jun-Yan(史军妍),ZHONG Jin-Kui(钟金魁),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(1):10-14
[25]DONGWen-Kui(董文魁),ZHANGYan-Ping(张艳萍),ZHAO Chun-Yu(赵春宇),etal.Chinese J.Chem.(ZhongguoHuaxue), 2008,26(10):1821-1825
[26]XU Li(许力),ZHANG Yan-Ping(张艳萍),SUN Yin-Xia(孙银霞),etal.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2007,23(11):1999-2002
Synthesis,Crystal Structure and Thermal Property of a 1D Chain-Like Copper(Ⅱ)Com plex
ZHAO Li*DONG Xu-Tao SUN Yin-Xia CHENG Qian DONG Xiu-Yan WANG Li
(School of Chemical and Biological Engineering,Lanzhou Jiaotong University,Lanzhou 730070,China)
A Cu(Ⅱ)complex,[Cu(L)2]·CH3OH,has been synthesized via the complexation of copper(Ⅱ)acetate monohydratewith a new oxime-type ligand(HL=1-(4-{[(E)-3-ethoxyl-2-hydroxybenzylidene]amino}phenyl)ethanone oxime).X-ray crystal structure determination of the Cu(Ⅱ)complex shows that isamononuclear complex.The Cu(Ⅱ)atom is four-coordinated by the phenolateO atomsand imine N atoms from two deprotonated oxime-type ligands,in a slightly distorted square-planar geometry.The O-and N-donor atoms are mutually trans and the dihedral angle between the two coordination planes(Cu1N2O2 and Cu1N4O5)is 23.33(3).In the crystal structure,each complex molecule links two other adjacent complexmolecules into an infinite one-dimensional structure along the b axis through intermolecularO-H…O hydrogen bonds.CCDC:871832.
oxime-type ligand;Cu(Ⅱ)complex;synthesis;crystal structure;thermal property
book=2494,ebook=42
O614.121
A
1001-4861(2012)11-2413-06
2012-04-02。收修改稿日期:2012-05-15。
甘肃省自然科学基金(No.210162)资助项目。
*通讯联系人。E-mail:zhaoli_72@163.com;会员登记号:S06N2551M1004。

