Optimizing the Chemical Compositions of Protective Agents for Freeze-drying Bifidobacterium longum BIOMA 5920*
YANG Chanyuan (杨婵媛), ZHU Xiaoli (朱晓丽), FAN Daidi (范代娣),**, MI Yu (米钰),**,LUO Yan’e (骆艳娥), HUI Junfeng (惠俊峰) and SU Ran (苏然)1 Shaanxi Key Laboratory of Degradable Biomedical Materials, School of Chemical Engineering, Northwest University, Xi’an 710069, China
2 Shaanxi R&D Center of Biomaterials and Fermentation Engineering, School of Chemical Engineering, Northwest University, Xi’an 710069, China
3 Shaanxi R&D Center of Biomaterials and Fermentation Engineering, School of Urban and Environmental Science,Northwest University, Xi’an 710069, China
1 INTRODUCTION
For improving human health, probiotics gained more interests over the last few years. Bifidobacterium,one of the common probiotic microorganisms, has immune-enhancing and anti-cancer effects, as well as the ability to maintain the balance of intestinal microflora and to lower cholesterol level [1-3]. They have been widely employed in various dairy products including ice cream, frozen desserts, yoghurts, cheese and cultured milk drinks [4]. However, the reviving conditions of this strain, such as high nutritional requirement, hypersensibility to oxygen and poor resistance to low pH [5], are actually too harsh for its commercial circulation to meet. It is recommended that probiotics should be at least above the level of 106cfu·g-1when consumed [6]. Therefore, the concentrated starting culture of Bifidobacterium in freeze-dried form is greatly desired in order for its better performance to the host [7].
Freeze-drying is a common and effective method for the long-term preservation of biological materials with its application in microorganism preservation [8].Unfortunately, not all microorganisms could survive such a process [9]. The loss of cell viability is mainly due to hostile environmental conditions such as ice crystallization and high osmotic pressure [10]. Thus,the addition of lyoprotectant to guarantee a satisfactory level of cell viability is crucial. Many compounds have already been identified to enhance the survival ability of lactic acid bacteria [11], including those of permeable compounds (e.g. glycerol, a triol compound),impermeable compounds (e.g. polysaccharide, disaccharide), antioxidant compounds (e.g. VC, L-cysteine)and proteins (e.g. skim milk, soy protein) [12]. Generally, each single protectant has its own disadvantages which can be made up by other protectants. Therefore,several protectants are mixed together according to a certain formula for a better performance [13].
Human-like collagen (HLC), a water-soluble protein expressed by recombinant Escherichia coli BL 21 [14], is interestingly found with the protective effect for the freeze drying of Bifidobacterium. In this study,four most significant factors HLC, trehalose, L-cysteine and glycerol, for Bifidobacterium longum BIOMA 5920 viability during freeze drying are testified by response surface methodology (RSM). The optimal formula and the protective mechanisms are given as well.
2 MATERIALS AND METHOD
2.1 Microorganism and preparation of culture
Bifidobacterium longum BIOMA 5920 obtained from Biogas Institute of Ministry of Agriculture, P. R.China, was used in this study. This bacterium was anaerobically cultivated in deMann, Rogosa, Sharpe(MRS) broth at 37 ºC by using a bug box (80% N2, 10%CO2and 10% H2, Rusking, UK). The cells were harvested in the early stage of stationary phase by centrifugation at 1079 g for 10 min at 4 ºC (TGL-16M/No 2 Anger Rotor, Xiang Yi, China).
2.2 Preparation of freeze-dried powder
Harvested cells were washed twice in saline (0.85%NaCl) and blended with the complex protective medium: HLC, trehalose, L-cysteine and glycerol. Both two operations were carried out in a bug box. HLC(China patent number: ZL01106757.8, Mr=97000)was supplied by Xi’an Giant Biogene Technology Company. For the former three agents, they were autoclaved for 20 min at 121 ºC while HLC was sterilized by filtration through a 0.22 µm filter. Cells mixed with distilled water were considered as control.All mixtures were frozen at -20 ºC for 3 h and subsequently lyophilized with a freeze drier (FD 5-10, SIM,USA) at -50 ºC for 48 h under vacuum degree of 6.7-13.3 Pa (50-100 mTorr).
2.3 Determination of cell viability
To determine the viable counts of freeze-dried powder, 0.1 g sample was rehydrated with phosphate buffer (pH 7.0) followed by gentle shaking at room temperature for 20 min. The viability was determined by pour plate method and the cell suspension was then spread onto MRS agar plates. After cultivated at 37 ºC for 48 h, viable cells were enumerated before (initial count) and after freeze drying. Colonies at the interval of 30-300 colony forming units per plate were counted.All experiments were performed in triplicate. Viability was calculated by using the following equation:
where N and N0represent the viable counts (cfu·g-1)after freeze-drying and the initial count (cfu·g-1) before freeze-drying, respectively.
2.4 Experimental design
2.4.1 Design of the single protectant
HLC, trehalose, L-cysteine and glycerol were investigated separately to determine their individual protective effects on the survival of Bifidobacterium during freeze drying. Both HLC and glycerol were used on four different levels: 0.5%, 1%, 2% and 3%,trehalose on 6%, 8%, 10% and 12%, L-cysteine on 0.05%, 0.1%, 0.15% and 0.2%. Statistical analysis was performed by one-way analysis of variance(ANOVA) from the SPSS software (SPSS Statistics,version 17.0). Significance differences between values were at P<0.05 levels.
2.4.2 Design of the complex protectants
RSM was employed to design this experiment with the aid of Minitab software. Cell viability was set as the response value. Plackett-Burman Design was to identify the specific compound with significant effect on the viability of Bifidobacterium. The steepest ascent design was then conducted to get the response value close to the optimized area. Finally, a mathematic model was developed through the Box-Behnken design(BBD) to find out the optimal point in the pre-optimized area. In this experiment, each significant factor was represented at three levels: low (-1), medium (0) and high (+1). It was made up of totally 15 experiments with twelve B-B design and three central points design.A second degree polynomial equation was employed as follows:
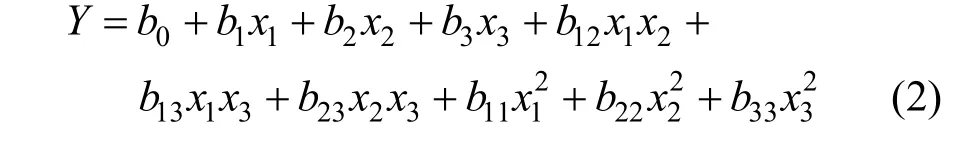
where Y is the predicted response of cell viability, x1,x2and x3are the values of significant variables, b0is the offset term, b1, b2and b3are the linear coefficients,b12, b13and b23are the interaction coefficients, and b11,b22and b33are the quadratic coefficients.
Basing on the results of RSM, the optimal formula of the protective agents was then obtained. In order to verify this predictive value, an experiment was conducted under the set condition, while the control was implemented by employing 2% HLC in addition to 10% trehalose and 2% glycerol as the protective agent according to the mathematic model.
3 RESULTS AND DISCUSSION
3.1 Analysis of the single protectant
Lyophilization has several defects such as the formation of ice crystal, changing permeability of the cell membrane, denaturation and devitalization of sensitive proteins. Therefore, it comes to the point of lyoprotectants to prevent these adverse impacts. In this work HLC, trehalose, L-cysteine and glycerol are adopted as the protective additives.
Table 1 shows the effects of different protectants on the survival of Bifidobacterium. The initial viable count prior to freeze-drying is 1.21×109cfu·g-1. After freeze-drying, cells exhibit higher viabilities than that of the control and are irrelevant with which specific protectant is adopted. Fortunately, this operation fulfills the requirement (106cfu·g-1) for the commercial destination.
In terms of percentage survival, the highest viability of 53.22% is obtained when 10% trehalose is used as protectant. Trehalose is a non-reducing disaccharide with its characteristic of anti-freezing, high heat-resistance and stability. It increases the stability of cellular protein by forming hydrogen bonds, thus reducing the risk of exposure to stressful conditions[11]. And also, with its high viscosity, it restricts the molecule interactions by forming a glass structure,hence the substance is in a frozen state [15]. Zayedet al. [16] had found that trehalose greatly enhanced the survival rate ofLactobacillus salivariuswhen used alone or together with other protective agents.
In comparison with 10% trehalose, the viability of 2% HLC can reach up to 39.09%. This data indicates that HLC might also protectBifidobacteriumeffectively. HLC, expressed by recombinantEscherichiacolicontaining human-like collagen cDNA,has good hydrophily and facilitates rehydration by creating a porous structure in the freeze-dried powder[9, 17, 18]. On the other hand, electric double layer and hydration shell are formed around the cell surface,which reduce the revelation of intracellular materials.Caoet al. [19] had studied the macromolecules protectants on the survival ofBifidobacterium. They concluded that skim milk in addition to sucrose and glycerol had better protective effects.
As to glycerol, it is easy to access intracellular and extracellular of microorganisms. When glycerol is used as a lyoprotectant, hydration is formed by binding water molecules, and then the viscosity of the solution increases. In this case, the formation of excessive ice crystal is prevented [20]. When 2% glycerol is used, the viability can reach 32.15%. It is complied with Trsic-milanovic’s report, showing that a cryoprotective medium containing glycerol remarkably affected the lyophilization ofBifidobacterium[21].
WhenBifidobacteriumis freeze-dried in L-cysteine protectant, the highest viability is 25.54%. Because of anaerobic characteristic ofBifidobacterium, L-cysteine,a reducing agent to lower the oxidation-reduction potential and to consume the oxygen in experiment, is applied in the previous research [22]. It had also been treated as a protectant ofLactobacillus acidophilusfor freeze drying [23]. However, there is no significant difference in the protective effect of different concentrations of L-cysteine. This may be due to strictly anaerobic environmental control. Thus, the addition of L-cysteine has no effective contribution to increase the viability ofBifidobacteriumduring freeze-drying.
3.2 Optimization of complex protectants
The single protectant, although can enhance the viability ofBifidobacteriumto a certain degree, fails to guarantee a desirable protective effect especially in comparison to those mixed protective agents. RSM is used to identify the optimal formula for each compound contained in the complex protective agents.
3.2.1Planckett-Burman design
The independent variablesx1(the concentration of HLC),x2(the concentration of trehalose),x3(the concentration of L-cysteine) andx4(the concentration of glycerol) are investigated to screen out the significant factors. Combined with single factor experimental results, each variable is represented at two levels:“-1” for the low level and “+1” for the high one. The regression analysis of PB design is shown in Table 2.Significance level is defined as prominent whenp-value is below 0.05. After analyzing,x1,x2andx4are confirmed as significant factors. They are theconcentrations of HLC, trehalose and glycerol. However, the concentration of L-cysteine shows no significant effect on cell viability. The reason is probably that anaerobic conditions in this research are under well control. The fitting equation of the unary linear is presented as below:
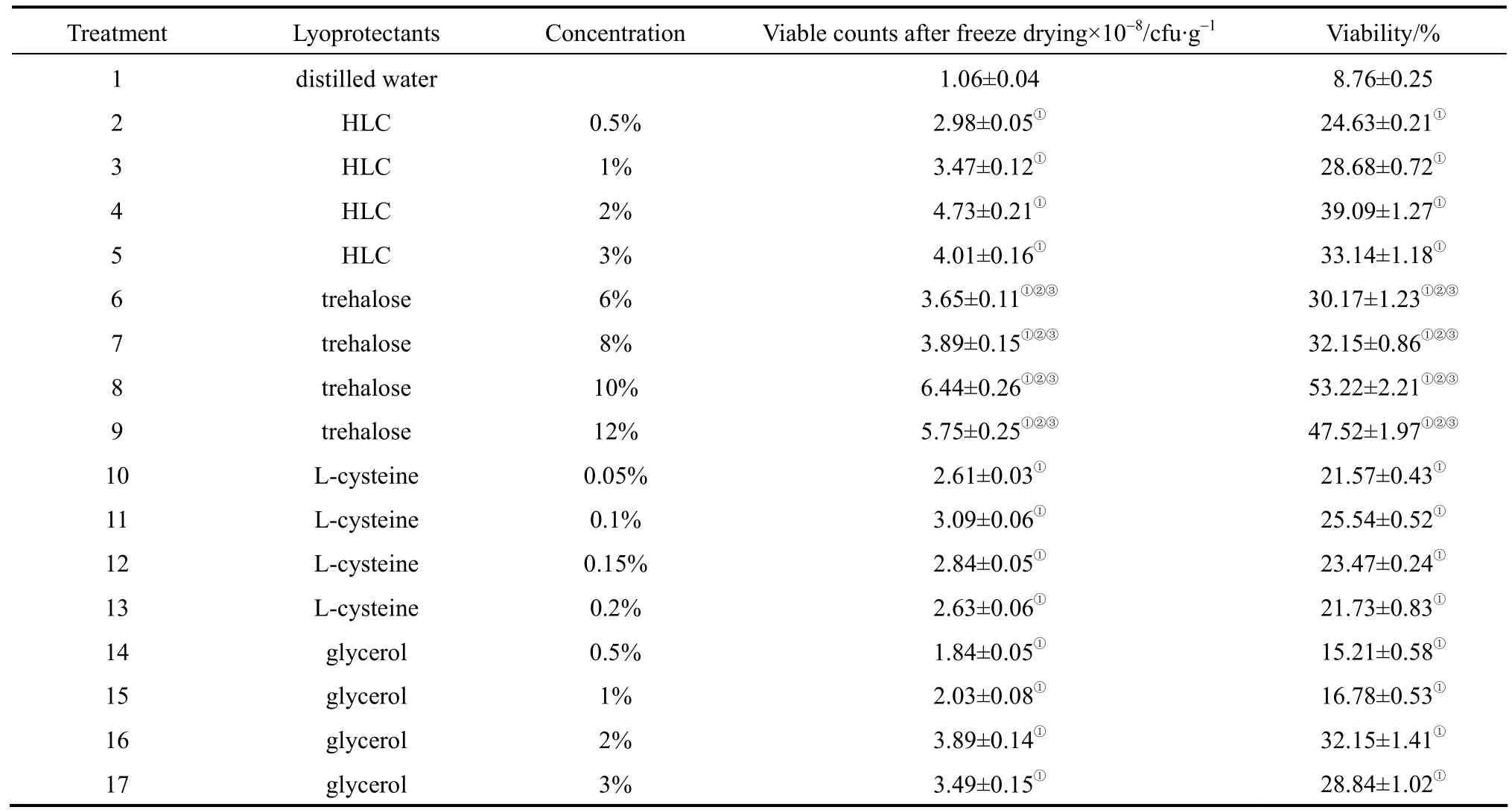
Table 1 Effects of different lyoprotectants on the survival of Bifidobacterium
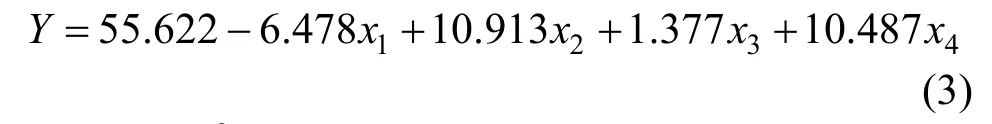
TheR2value of the fitting analysis is 91.13%,which indicates that the fitting equation can describe the actual survival ofBifidobacteriumwell.
3.2.2The steepest ascent design
According tot-value of the Planckett-Burman design, the ascent orientations ofx2andx4are ascending, while that ofx1is descending. Based on the absolute value oft, the step changes ofx1,x2andx4are set as 0.6%, 1% and 1%. The design and analysis results are given in Table 3. The Run 4 is the most close to the optimal region and is chosen for further optimization by using BBD.
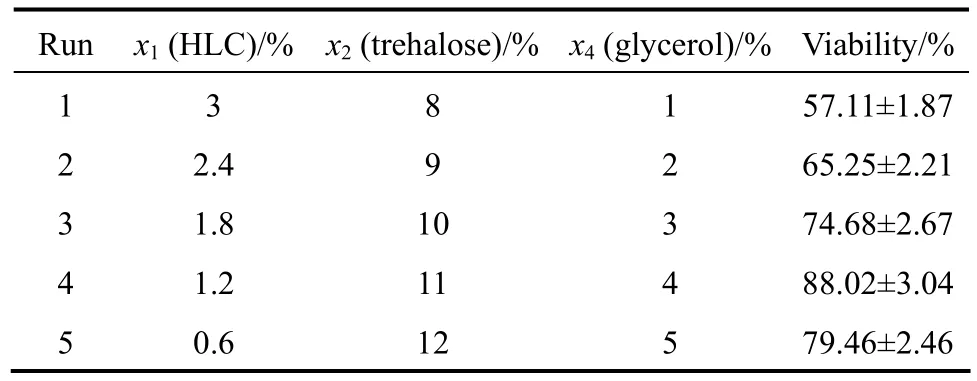
Table 3 Path of steepest ascent and results of the steepest ascent design
3.2.3Box-Behnken design
Significant factors ofx1,x2andx4are selected as variables which are subjected to BBD. The corresponding design and results with BBD are presented in Tables 4 and 5. The variance analysis of the second-order response surface model is listed in Table 6.With Minitab software, a quadratic polynomial equation is obtained as follows:
whereYrepresents the viability ofBifidobacterium.The fitting degree of this model indicated byR2value is 95.67%, denoting a preferable fitting between theactual value and theoretical response value.
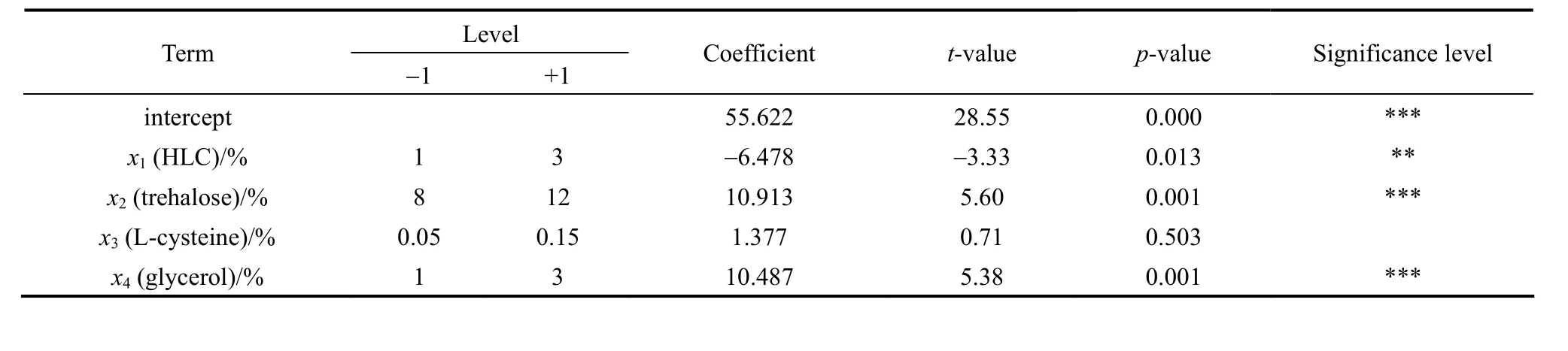
Table 2 The results of regression analysis of the Planckett-Burman design
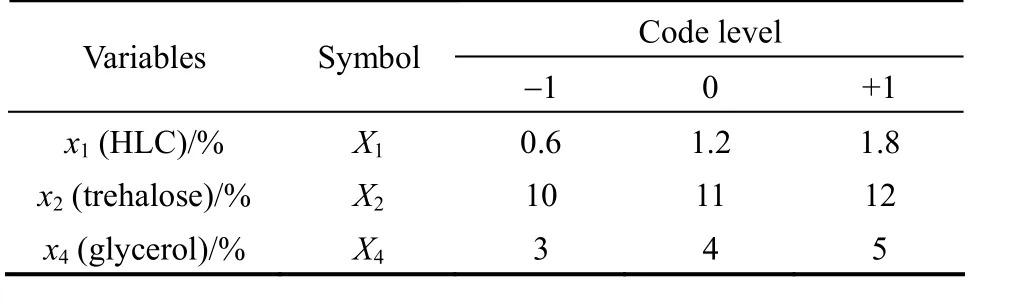
Table 4 Experimental factor levels of Box-Behnken design
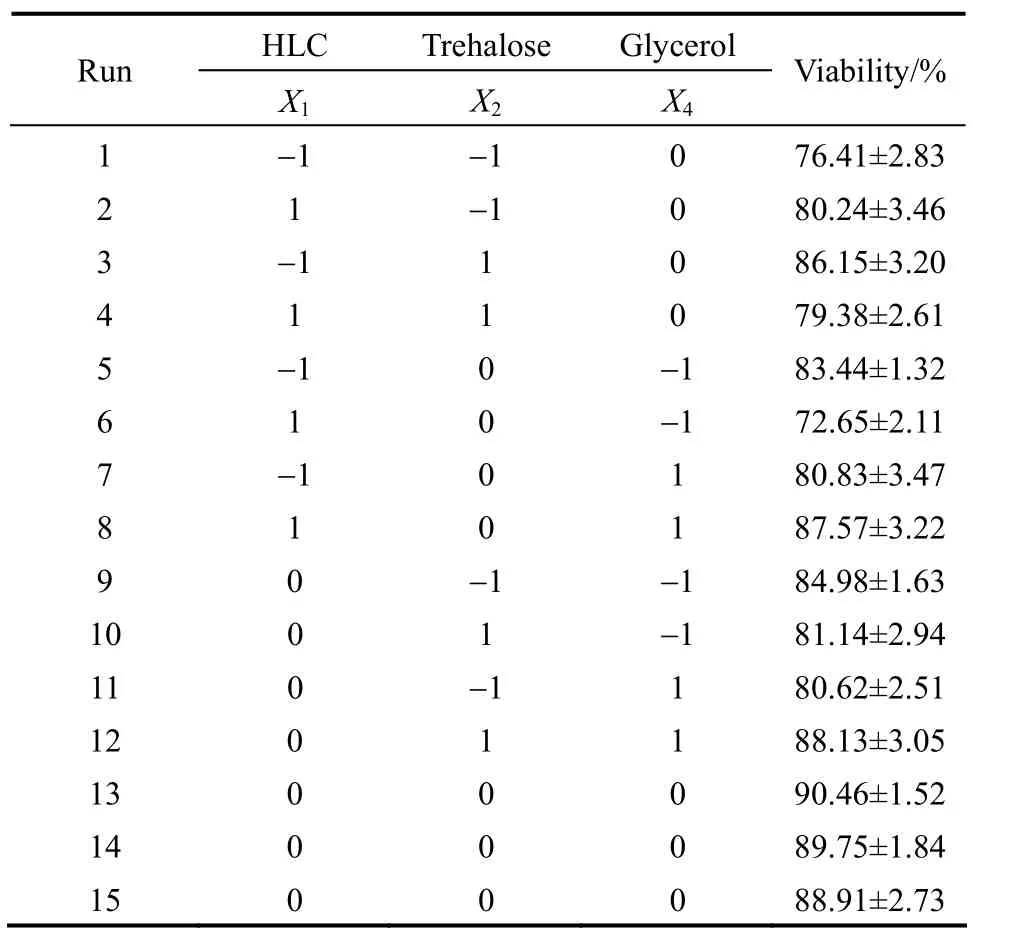
Table 5 Design and results of Box-Behnken design (N=15)
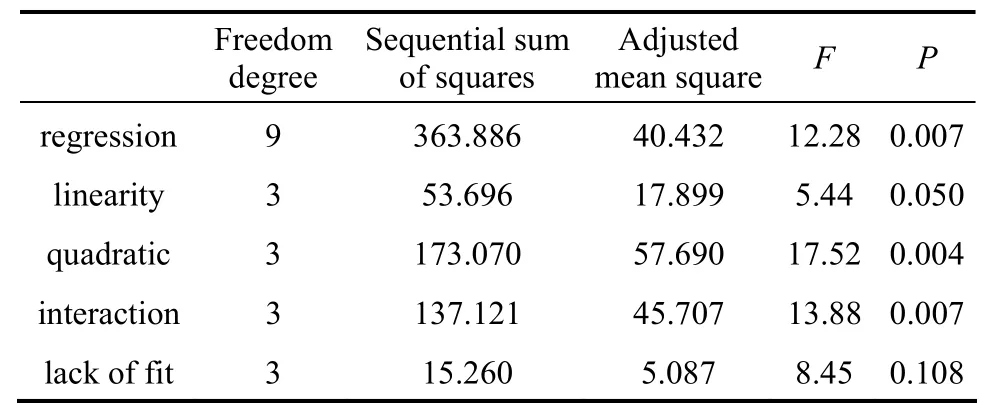
Table 6 Variance analysis results of Box-Behnken design
As shown in Table 6, the model is regarded as significant with a low probability value below 0.05,and the lack of fitting value is not significant asP-value is more than 0.05. ThroughF-test, linearity,quadratic and interaction all show significance on the response value. The 3-D response surface and contour plots are drawn in Figs. 1-3 in order to visually reflect interactions between each of these three factors.
According to Figs. 1-3, the three variables are prominent to cell viability and the interactions between each other are significant. When glycerol is applied to microorganisms, several changes will occur in the freeze drying process: (1) the solute concentration drops and the salts intaken by the cell decreases; (2)the absorption of glycerol changes the supercooled state of intra-cellular, balancing the osmotic pressures and thus reducing the dehydration; (3) protectants relieve the damage caused by permeable swell during rehydration. In addition, trehalose forms a glass state with high viscosity, and thus lowers diffusion coefficient, which bring the protective effect by blocking the macromolecules movement and reducing their permeability. HLC provides an additional protective coating for the cells. Therefore, the trehalose, glycerol and HLC have complementary effect on the survival ofBifidobacteriumand the combination of them brings a higher survival rate.
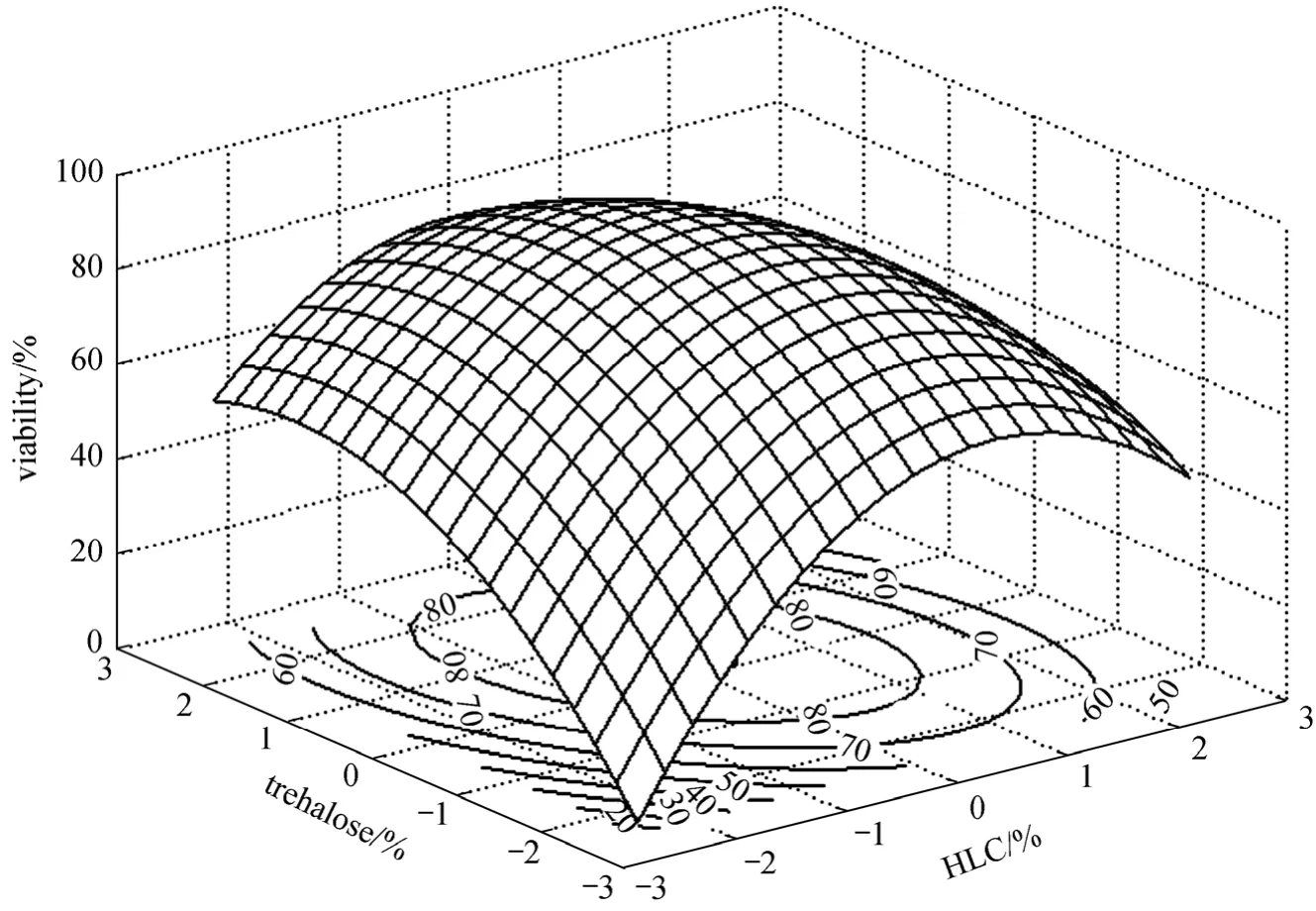
Figure 1 Response surface and contour plots of trehalose vs. HLC on cell viability
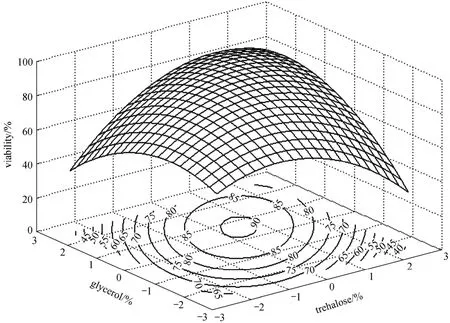
Figure 2 Response surface and contour plots of trehalose vs. glycerol on cell viability
By solving the equation and analyzing the response surface plots, the partial derivatives are deduced. The optimal values of the variables arex1=0.057,x2=0.498,x4=0.652, and the corresponding concentrations of HLC, trehalose and glycerol are 1.23%, 11.50%and 4.65%. Based on this formula, the experiment is conducted accordingly. The statistic analysis is given in Fig. 4. It is concluded that the freeze drying performs well as the high survival rate is 88.23%, close to the estimated value of 90.68% with no significant differences (P>0.05). In comparison to the control, the viability is increased by 39.67% and the viable count is 1.07×109cfu·g-1. Hence, the optimized formula was recommended for this strain.
4 CONCLUSIONS
High-viable-count is an important feature of the new probiotic product depending mainly on the added lyoprotectants [24]. Unfortunately, there are limited reports on the survival ofBifidobacteriumduring the operation of freeze drying [25-30]. Saxelinet al. [25]reported the survival ofB.animalisssp.lactisBb-12 but his paper leaked details of lyoprotectants. Champagneet al. [26] showed a low viability ofBifidobacterium longumRO23 when employed a mixture of ascorbic acid-skim milk-sucrose as its cryoprotectant.Bifidobacteriuminfantis CCRC 14633 andB.longumB6 were freeze-dried by Wanget al. [27], and then gave a 43%-52% survival rate in fermented soymilk.Capelaet al. [28] reported that an 80% improvement was realized in the survival ofB.longum536 when taking UnipectineTM RS 150 as a cryoprotectant. In the study of Rosburget al. [29], the results showed that beta-glucan (oat fiber) had a protective effect onbifidobacteriain yogurt when subjected to freeze drying. The recent research was conducted by Wonget al.[30], who evaluated the viability ofBifidobacterium PesudocatenulatumG4 after freeze drying, which led to a survival of 71.65%-82.07% by using 10% skim milk alone as the protectant. Comparing to these previous studies, the research has provided the diverse protectants on the survival ofBifidobacteriumthrough freeze-drying.
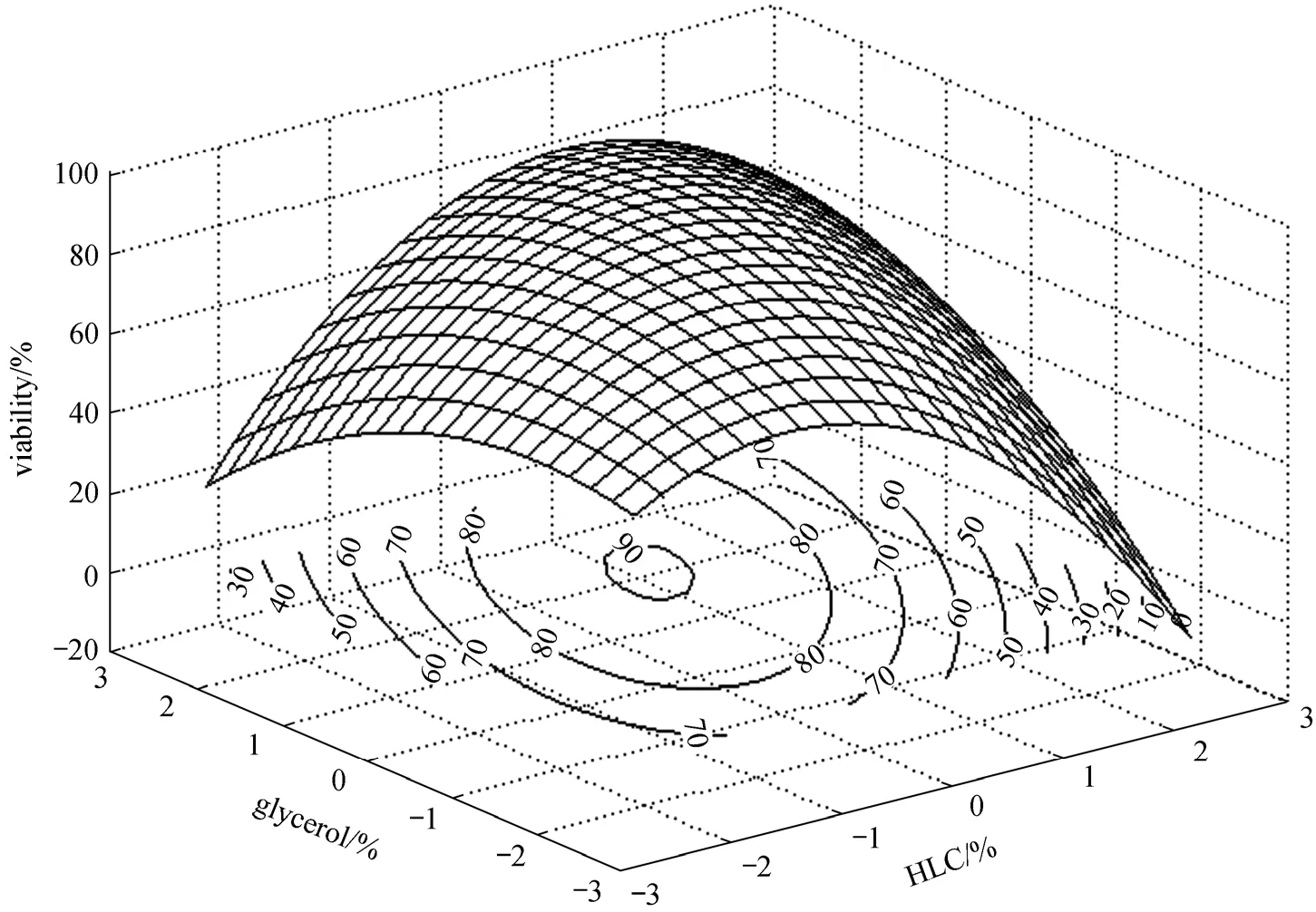
Figure 3 Response surface and contour plots of glycerol vs. HLC on cell viability
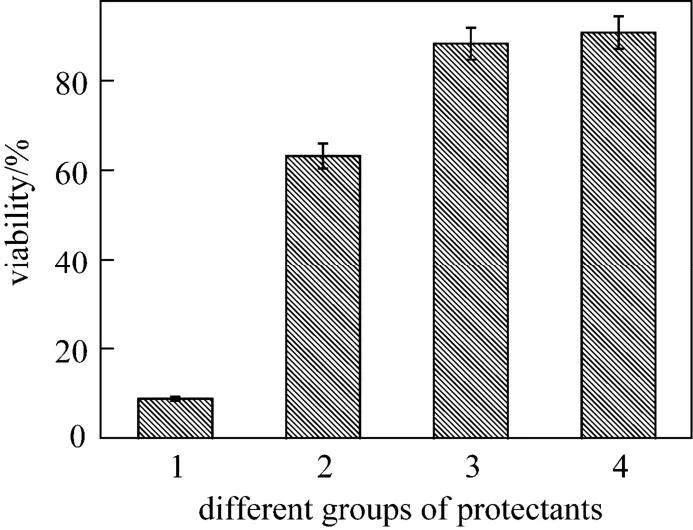
Figure 4 Viabilities of Bifidobacterium by different protectants [The error bars represent standard deviation of means(n=3)]1—distilled water group; 2—control group; 3—experimental group; 4—the predicted value of experimental group
By analysis of single protectant forBifidobacterium longumBIOMA 5920 freeze drying, the optimal concentrations of HLC, trehalose, L-cysteine and glycerol are 2%, 10%, 0.1% and 2%, respectively. The maximum survival rate is 53.22% and the viable count is 6.44×108cfu·g-1. Through Planckett-Burman design,steepest ascent design and Box-Behnken design, the optimal formula of complex protective agents is determined: HLC 1.23%, trehalose 11.50% and glycerol 4.65%, by which the viable count is 1.07×109cfu·g-1and the survival rate is up to 88.23%, 39.67% higher in comparison to the control. The viable count exceeds obviously the minimum viable count requirement (106cfu·g-1) at the end of the shelf life of the probiotic products. Meanwhile, the actual survival rate is close to its theoretical value as 90.68% provided by the RSM,which further validate the effectiveness of RSM with its extended application in this work and hence rekindle more of our interests on its further application.
1 Benno, Y., Mitsuoka, T., “Impact ofBifidobacterium longumon human fecal microflora”,Microbiol.Immunol., 36, 683-694 (1992).
2 Kailasapathy, K., Chin, J., “Survival and therapeutic potential of probiotic organisms with reference toLactobacillus acidophilusandBifidobacteriumspp”,Immunol.Cell.Biol., 78, 80-88 (2000).
3 Shortt, C., “Living it up for dinner”,Chem.Ind., 8, 300-303 (1998).
4 Ross, R.P., Desmond, C., Fitzgerald, G.F., Stanton, C., “Overcoming the technological hurdles in the development of probiotic foods”,J.Appl.Microbiol., 98, 1410-1417 (2005).
5 Gomes, A.M.P., Malcata, F.X., “Bifidobacteriumspp. andLactobacillus acidophilus: Biological, biochemical, technological and therapeutical properties relevant for use as probiotics”,Trend.Food.Sci.Tech., 10, 139-157 (1999).
6 Vinderola, C.G., Bailo, N., Reinheimer, J.A., “Survival of probiotic microflora inArgentinian yoghurtsduring refrigerated storage”,Food.Res.Int., 33, 97-102 (2000).
7 Samona, A., Robinson, R.K., “Effect of yogurt cultures on the survival of bifidobacteria in fermented milks”,Int.J.Dairy.Technol.,47, 58-60 (1994).
8 Lodato, P., Segovia de Huergo, M., Buera, M.P., “Viability and thermal stability of a strain ofSaccharomyces cerevisiaefreeze-dried in different sugar and polymer matrices”,Appl.Microbiol.Biot., 52,215-220 (1999).
9 Abadias, M., Benabarre, A., Teixidó, N., Usall, J., Vinas, I., “Effect of freeze drying and protectants on viability of the biocontrol yeast candida sake”,Int.J.Food.Microbiol., 65, 173-182 (2001).
10 Conrad, P.B., Miller, D.P., Cielenski, P.R., de Pablo, J.J., “Stabilization and preservation ofLactobacillus acidophilusin saccharide matrices”,Cryobiology, 41, 17-24 (2000).
11 Champagne, C.P., Detournay, H., Hardy, M.J., “Effect of medium on growth and subsequent survival ofLactobacillus delbrueckiisubsp.bulgaricus to freeze-drying”,J.Ind.Microbiol., 7, 147-152 (1991).
12 Hubalek, Z., “Protectants used in the cryopreservation of microorganisms”,Cryobiology, 46, 205-229 (2003).
13 Wang, W., “Lyophilization and development of solid protein pharmaceuticals”,Int.J.Pharm., 203, 1-60 (2000).
14 Luo, Y.E., Fan, D.D., Ma, X.X., Wang, D.W., Mi, Y., Hua, X.F., Li,W.H., “Process control for production of human-like collagen in fed-batch culture ofEscherichia coliBL 21”,Chin.J.Chem.Eng.,13, 276-279 (2005).
15 Patist, A., Zoerb, H., “Preservation mechanisms of trehalose in food and biosystems”,Colloids.Surf.,B:Biointerfaces., 40, 107-113(2005).
16 Zayed, G., Roos, Y.H., “Influence of trehalose and moisture content on survival ofLactobacillus salivariussubjected to freeze-drying and storage”,Process.Bioche., 39, 1081-1086 (2004).
17 Fan, D.D., Luo, Y.E., Mi, Y., Ma, X.X., Shang, L.A., “Characteristics of fed-batch cultures of recombinantEscherichia colicontaining human-like collagen cDNA at different specific growth rates”,Biotechnol.Lett., 27, 865-870 (2005).
18 Mi, Y., Xi, J.F., Fan, D.D., Liu, H.L., “The biocompatibility of human-like collagen”,JNWU(Nat.Sci.Ed.), 34, 66-72 (2004).
19 Cao, Y.M., Zhang, H., Xu, S.H., “Effects of cryoprotectant on freeze-dried bifidobacteria”,Food and Fermentation Industries, 26,40-45 (1999).
20 Pu, L.L., Liu, N., Zhang, Y.H., Huo, G.C., “The research development of cryoprotector of lactic acid bacteria and its protective mechanism”,Mod.Food.Sci.Technol., 21, 147-149 (2004).
21 Trsic-Milanovic, N., Kodzic, A., Baras, J., Dimitrijevic-Brankovic,S., “The influence of a cryoprotective medium containing glycerol on the lyophilization of lactic acid bacteria”,J.Chem.Soc., 66,435-442 (2001).
22 Ji, Z.J., Guo, D.J., “Optimization of formula of protective additive of bifidobacterium by response surface method”,Chin.J.Biologicals,21, 813-816 (2008).
23 Yuan, Y.H., Yue, T.L., Gao, Z.P., Wang, L.W., “Research on of production quality and quality ofLactobacillus acidophiluspowder”,Journal of Northwest A & F University(Nat.Sci.Ed.), 31, 139-142(2003).
24 De Valdéz, G.F., De Giori, G.S., de Ruiz Holgado, A.A.P., Oliver, G.,“Protective effect of adonitol on lactic acid bacteria subjected to freeze-drying”,Appl.Environ.Microb., 45, 302-304 (1983).
25 Saxelin, M., Grenov, B., Svensson, U., Fonden, R., Reniero, R.,Mattila-Sandholm, T., “The technology of probiotics”,Trend.Food.Sci.Tech., 10, 387-392 (1999).
26 Champagne, C.P., Mondou, F., Raymond, Y., Roy, D., “Effect of polymers and storage temperature on the stability of freeze-dried lactic acid bacteria”,Food.Res.Int., 29, 555-562 (1996).
27 Wang, Y.C., Yu, R.C., Chou, C.C., “Viability of lactic acid bacteria and bifidobacteria in fermented soymilk after drying, subsequent rehydration and storage”,Int.J.Food.Microbiol., 93, 209-217(2004).
28 Capela. P., Hay, T.K.C., Shah, N.P., “Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt”,Food.Res.Int., 39, 203-211(2006).
29 Rosburg, V., Boylston, T., White, P., “Viability ofBifidobacteriastrains in yogurt with added oat beta-glucan and corn starch during cold storage”,J.Food.Sci., 75, C439-C444 (2010).
30 Wong, S., Kabeir, B.M., Mustafa, S., Mohamad, R., Manap, M.Y.,“Viability ofBifidobacteriumPseudocatenulatumG4 after spray-drying and freeze-drying”,Microbiology Insights, 3, 37-43 (2010).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- The Short-term Effects of Temperature and Free Ammonia onAmmonium Oxidization in Granular and Floccular Nitrifying System*
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Ammoximation of Cyclohexanone to Cyclohexanone Oxime Catalyzed by Titanium Silicalite-1 Zeolite in Three-phase System*
- Turbulent Characteristic of Liquid Around a Chain of Bubbles in Non-Newtonian Fluid*
- Recovery of Tungsten (VI) from Aqueous Solutions by Complexationultrafiltration Process with the Help of Polyquaternium*
- Solubilities of Nizatidine in Methanol + Water, Ethanol + Water and i-Propanol + Water from 273.15 to 303.15 K*
