Ammoximation of Cyclohexanone to Cyclohexanone Oxime Catalyzed by Titanium Silicalite-1 Zeolite in Three-phase System*
LIU Guoqing (刘国清), WU Jian (吴剑) and LUO He’an (罗和安),**
1 School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China 2 School of Chemical Engineering, Xiangtan University, Xiangtan 411105, China
1 INTRODUCTION
Cyclohexanone oxime is an important intermediate in chemical industry, particularly as a key precursor of ε-caprolactam for nylon production [1]. A practical technology for production of ε-caprolactam involves the conversion of cyclohexanone with hydroxylamine into cyclohexanone oxime, followed by Beckmann rearrangement in oleum and then treatment with ammonia giving caprolactam [2].
In 1988, Enichem developed a new process for producing cyclohexanone oxime from ammoximation of cyclohexanone catalyzed by TS-1. Ammonia and hydrogen peroxide were used as the raw materials.t-butanol was chosed as the solvent because both cyclohexanone and cyclohexanone oxime are well soluble in the solution oft-butanol-water [3, 4]. The new process allows obtaining high conversion of cyclohexanone and selectivity to oxime with less byproducts,lower cost of energy and hydrogen compared with the method through hydroxylamine [5]. Hence, it is more efficient and friendly to environment. However, there are still some drawbacks for the process such as high cost of catalyst TS-1, trouble to separate the catalyst from the reaction mixture, additional cost of energy due to evaporating used solventt-butanol in ammoximation and transporting the oxime melt with strict heat insulation to the next unit operation of Beckmann rearrangement. This process is sketched in Fig. 1.
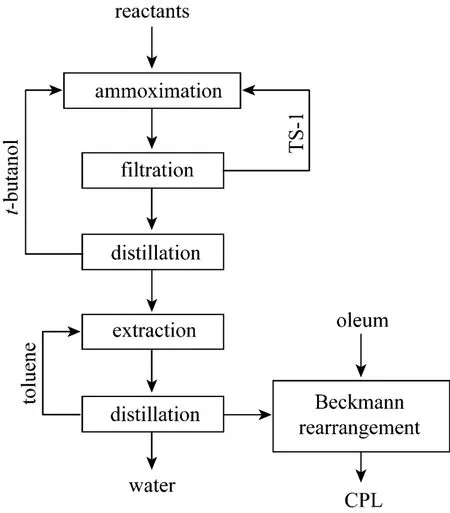
Figure 1 Enichem’s ammoximation process of cyclohexanone oxime
This work explores the possibility of ammoximation of cyclohexanone with ammonia and hydrogen peroxide in a three-phase (organic-aqueous-solid) reaction system. Cyclohexane, which is soluble with cyclohexanone and cyclohexanone oxime but immiscible with the aqueous solution of hydrogen peroxide and ammonia, is used as the solvent instead of the aqueoust-butanol solution. The three-phase system has some advantages over the two-phase (aqueoust-butanol solution-solid) system. Firstly, cyclohexane is easily obtained and much cheaper thant-butanol.Secondly, TS-1 is easier to separate from the aqueous phase than from thet-butanol-water solution, which makes the separation and recovery of the catalyst simpler in three-phase system [6, 7]. Thirdly, the organic phase exiting from the three-phase ammoximation mainly contains cyclohexane and cyclohexanone oxime,which can directly be transported to Beckmann rearrangement with oleum since cyclohexane is inert in oleum [8]. Thus, ammoximation of cyclohexanone and Beckmann rearrangement of oxime can be integrated effectively to produce caprolactam so that there might be less intermediate steps. Moreover, compared to oxime melt in industrial process, transporting the solution of cyclohexanone oxime in cyclohexane is simpler and less costly. This new process is sketched as Fig. 2.
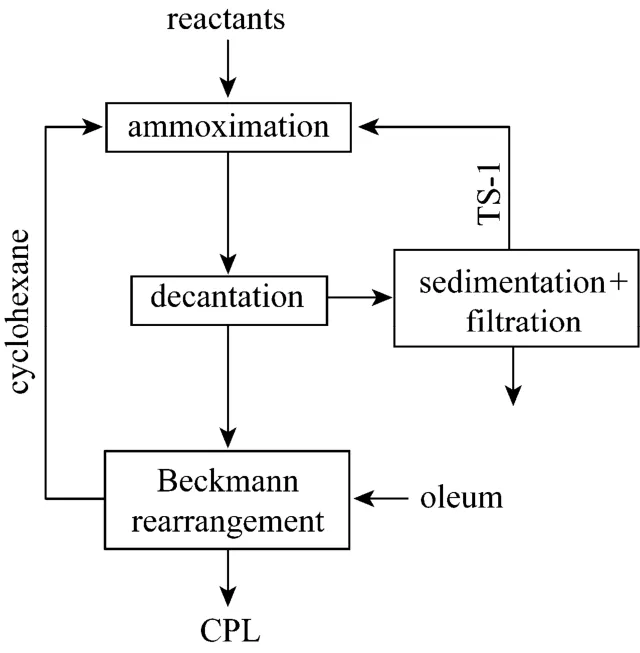
Figure 2 Integration of ammoximation and Beckmann rearrangement
The aim of this work is to investigate the optimal technological parameters for the TS-1-catalyzed ammoximation of cyclohexanone with ammonia and hydrogen peroxide in three-phase system. The influences of the following parameters on the conversion of cyclohexanone and selectivity to cyclohexanone oxime are observed: the amount of cyclohexane used, reaction temperature, reaction time, concentration of catalyst TS-1 and H2O2/NH3/cyclohexanone molar ratio. We also comment on the stability of TS-1 in the three-phase system from a practical view.
2 EXPERIMENTAL
2.1 Preparation of TS-1
TS-1 zeolite was prepared by the method reported by Thangarajet al[9]. Tetraethyl orthosilicate and butyl titanate were used as raw materials with tetrapropylammonium hydroxide as the template. The gelcrystallization was carried out under static condition at 170 °C for 72 h. The crystals obtained were dried at 120 °C for 12 h and subsequently calcinated at 550 °C for 24 h. The calcinated catalyst was activated by washing with a mixture of diluted hydrogen peroxide and sulfuric acid, then re-calcinated at 550 °C for 24 h.TS-1 samples with Si/Ti=31 (atom ratio) were obtained.Results of XRD (X-ray diffraction), FTIR (Fourier transform-infrared spectroscopy) and SEM (scanning electron micrograph) showed that they have perfect MFI (mordenite framework inverted) framework structure, high crystallization and uniform particle size.
2.2 Ammoximation procedure, apparatus and analytical methods
The ammoximation reaction was carried out in a three-necked glass bottle fitted with a magnetic stirrer,a condenser and a thermometer pocket. In a typical run, 2.5 g of catalyst TS-1, 15.0 g of cyclohexanone,40.0 g of cyclohexane were taken into the bottle and the mixture was heated to desired temperature. The reaction was initiated by adding 19.1 g of hydrogen peroxide (30%, by mass) and 22.9 g of ammonia aqueous solution (25%, by mass) at constant rate with a peristaltic pump in 2 h. After the addition, the mixture was stirred for another 3 h. Catalyst TS-1 was separated from the reaction mixture using a centrifuge.The clear liquid left was subject to analysis by GC(gas chromatography) equipped with FID (flame ionization detector), using toluene as an internal standard.The conversion of cyclohexanone and the selectivity to cyclohexanone oxime were calculated as follows:

3 RESULTS AND DISCUSSION
3.1 Effect of the amount of cyclohexane
In order to dissolve the reactant (cyclohexanone)and the product (cyclohexanone oxime) because of their low solubility in water, organic solvent is needed in the ammoximation reaction system. The nature of the solvent selected is of great importance to titanium-silicalite-catalyzed ammoximation. A considerable amount of research has been done on several kinds of solvent during the last decade [10-12]. With methanol or toluene as the solvent, it gives too low conversion of cyclohexanone and selectivity to oxime.Although the ammoximation reaction proceeds effectively with water as the solvent, it is unrealistic to be used in industry for the low solubility of cyclohexanone and corresponding oxime. The conversion of cyclohexanone and selectivity to oxime both can reach 99% in the mixed solvent of aqueoust-butanol solution. However, as mentioned above, one of the main drawbacks for the mixed solvent is requirement of additional energy in the subsequent refining step,which disfavors seriously the economic efficiency of ammoximation process.
Different from the above research, cyclohexane is chosen as the solvent in our study. The new selection introduces a three-phase (organic-aqueous-solid)reaction system since cyclohexane is immiscible with the aqueous solution of hydrogen peroxide and ammonia. Thus, its effects on the conversion of cyclohexanone and selectivity to oxime are focused in this work. To ensure enough solvent to dissolve cyclohexanone and corresponding oxime formed, the influence of the amount of cyclohexane loaded is investigated in 1-5 molar ratio of cyclohexane to cyclohexanone by keeping other technological parameters constant. As shown in Fig. 3, both the conversion and selectivity can reach 99% in the range investigated. This result suggests that the amount of cyclohexane used has little effect on the conversion and selectivity in a certain range. In addition, a relatively small amount of cyclohexane is sufficient and catalyst TS-1 still shows good catalytic activity in the three-phase reaction system. It can be inferred that the design and operation for the three-phase ammoximation unit may be simple, which shows additional potential advantage over the two-phase system.
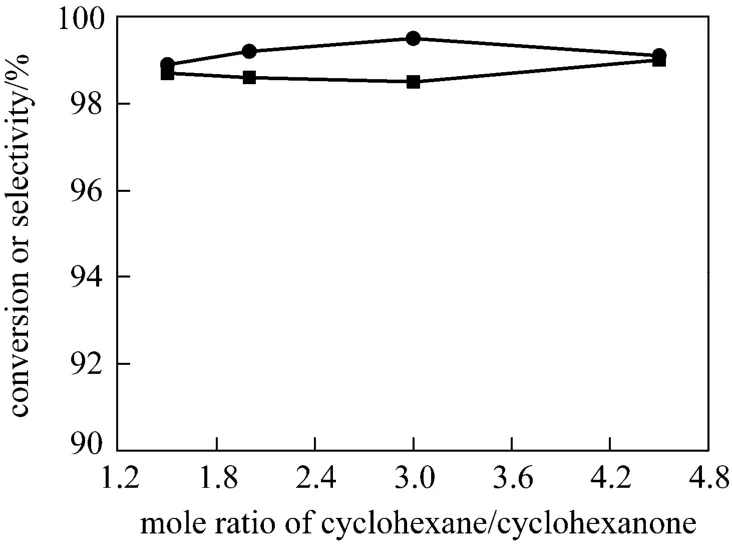
Figure 3 Effect of solvent amount on conversion and selectivity at 343 K for 3-phase system (Other conditions see Section 2.2)■ conversion in 3-phase system; ● selectivity in 3-phase system
3.2 Effect of the reaction temperature
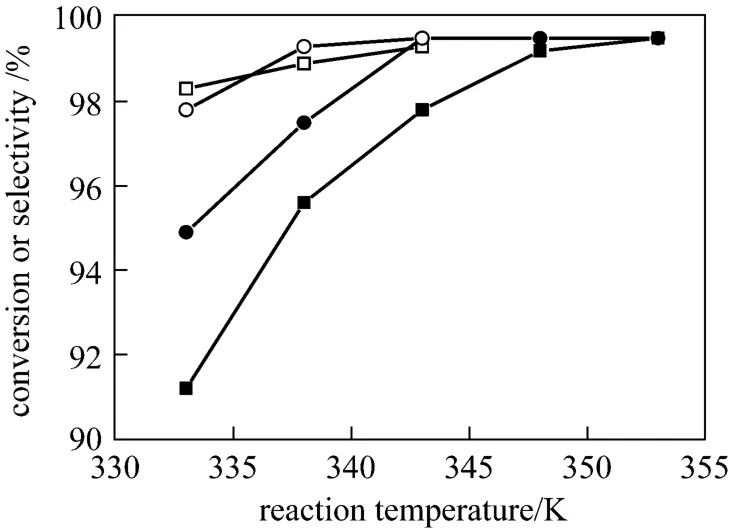
Figure 4 Effect of reaction temperature on conversion and selectivity (Reaction conditions see Section 2.2)■ conversion in 2-phase system; ● selectivity in 2-phase system;□ conversion in 3-phase system; ○ selectivity in 3-phase system
TS-1-catalyzed ammoximation of cyclohexanone depends greatly on the reaction temperature and will result in much more byproducts if it is above or below a suitable temperature in the two-phase system [5, 13].Considering the azeotropic points of water with the solvent used, the effects of reaction temperature on ammoximation were investigated in the ranges of 333-353 K for the two-phase system and 333-343 K for the three-phase system, as shown in Fig. 4. For the two-phase system, it can be seen that both the conversion of cyclohexanone and the selectivity to oxime increase to above 99% dramatically with temperature until 348 K and 343 K, respectively. This is in agreement with the results of some earlier works [14, 15]. It is amazing to notice that both the conversion and selectivity can reach 98% at lower temperature (333 K) and then slightly increase to above 99% in the three-phase system. This shows that a preferable reaction temperature is the azeotropic point of 343 K for the three-phase system under atmosphere pressure, lower than that for the two-phase system [8]. Moreover, it is found that the mixture at end of reaction is colorless from the three-phase system while it is straw yellow to crimson in the two-phase system, which means there may be less heavy volatile byproducts or NOxin the mixture from the former system.
In the three-phase system, it is known that cyclohexanone and cyclohexanone oxime exist mainly in organic phase, while H2O2, NH3and TS-1 are in aqueous phase. The processes of ammoximation in the system may be as follows: cyclohexanone transfers from the organic phase to the aqueous phase where it reacts with ammonia and hydrogen peroxide under catalysis of TS-1 to generate cyclohexanone oxime,which then transfers back to the organic phase. Thus,the reactant (ketone) and the product (oxime) exist in organic phase and seem to be isolated from H2O2and NH3in aqueous phase. Hence, there may be less side reactions such as cyclohexanone oxime oxidized to cyclohexenone oxime by H2O2, or hydrolyzed back to cyclohexanoneet al[16]. It leads to higher conversion and selectivity than those in a single liquid phase under lower reaction temperature. As well known, the molecular sieve jammed by the organic byproducts can cause the deactivation of TS-1 in ammoximation process [17, 18]. With higher cyclohexanone conversion and less byproducts, the life-span of TS-1 may be prolonged in the three-phase system.
3.3 Effect of the reaction time
In order to decrease the loss of H2O2and NH3in reaction, the aqueous solution of hydrogen peroxide and ammonia should be added dropwise into the cyclohexanone-cyclohexane mixture within a period of time. It is worth mentioning that vigorous agitation is necessary for lower concentration of reactants. Later the mixture should be continuously stirred for another several hours to achieve high conversion and selectivity. Fig. 5 reports the conversion and the selectivity against the reaction time during 3-6 h for the two reaction systems. In the time span of 3-6 h, reaction time has little effect on the selectivity for both systems,but approaching such high conversion of about 99%needs much more time for the three-phase system than for the two-phase system where 3 h may be enough. It is evident that ammoximation of cyclohexanone proceeds faster in the two-phase system than in the three-phase system. This is mainly because the solubility of cyclohexanone in the aqueous phase is much smaller than that in the organic phase, which results in a quite low concentration of ketone and then a lowered rate of its conversion to oxime. In other words, the three-phase ammoximation is a so-called diffusioncontrolled reaction,i.e. the overall reaction rate is limited by the transfer of cyclohexanone from the organic phase to the aqueous phase. As seen in Fig. 5, a minimum reaction time of 5 h is needed for the three-phase system in lab under atmosphere pressure. With raised reaction temperature under higher pressure and vigorous agitation to ensure little mass transfer resistance,higher cyclohexanone conversion may be obtained with less reaction time in industry. In addition, more decomposition of H2O2in the three-phase system may also lead to low conversion of cyclohexanone, which is discussed in detail later in the paper.
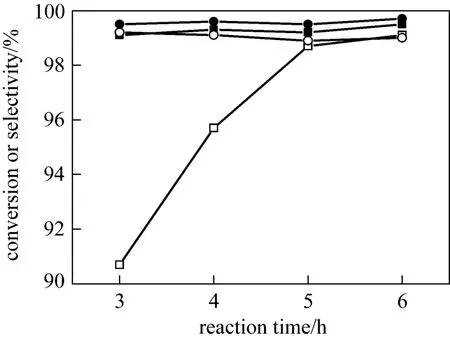
Figure 5 Effect of reaction time on conversion and selectivity at the azeotropic temperatures (Other conditions see Section 2.2)■ conversion in 2-phase system; ● selectivity in 2-phase system;□ conversion in 3-phase system; ○ selectivity in 3-phase system
3.4 Effect of the amount of catalyst TS-1
It is known that catalyst concentration of 2%-3%(by mass) is applied in ammoximation for the two-phase system in industry [13]. Thus the effect of catalyst concentration is studied within 1.5%-3% (by mass) in this work. As shown in Fig. 6, the catalyst shows good catalytic activity and no changes are seen as long as the mass concentration of TS-1 keeps over 2% for the two-phase system. However, the minimum mass concentration is about 2.5% for the three-phase system. It means that ammoximation in the three-phase system usually needs a little more catalyst TS-1 to approach the same conversion and selectivity as in the two-phase system. It may be attributed to the competition between the catalytic main reaction and decomposition of H2O2in the three-phase system. On the one hand, a much higher concentration of hydrogen peroxide in aqueous phase than that in the two-phase system,caused by the absence of H2O2in organic phase, can accelerate the main non-catalytic side reaction decomposition of H2O2while other reaction conditions keeps constant. On the other hand, the diffusioncontrolled ammoximation of cyclohexanone proceeds much slower in the three-phase system. Thus, it is more sensitive to the concentration of TS-1. More catalyst is required for the predominance of the catalytic main reaction compared to the two-phase system.
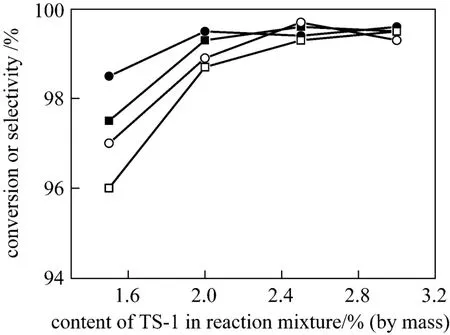
Figure 6 Effect of TS-1 amount on conversion and selectivity at the azeotropic temperatures (Other conditions see Section 2.2)■ conversion in 2-phase system; ● selectivity in 2-phase system;□ conversion in 3-phase system; ○ selectivity in 3-phase system
3.5 Effect of H2O2/ketone molar ratio
Considering the inevitable decomposition of H2O2, a little more hydrogen peroxide than in theory is essential for high conversion of cyclohexanone in ammoximation. Fig. 7 shows the effect of H2O2/ketone molar ratio on the catalytic performance of TS-1. The ratio has little effect on the selectivity to oxime (>98%)for both systems within the range of 1.0-1.2. However,more hydrogen peroxide is needed to obtain relatively high conversion of cyclohexanone (>99%) for the three-phase system compared to the two-phase system in which 1.05 is appropriate. The likely reason is that the concentration of H2O2is much higher and the ammoximation reaction proceeds slower in three-phase system, which results in more decomposition of H2O2and subsequently more cyclohexanone are left unreacted.As seen in Fig. 7, 1.10 is the appropriate H2O2/ketone molar ratio for the three-phase system. As an important economic evaluation index for the process, the utilization efficiency of hydrogen peroxide seems to be a little lower in the three-phase system. However, with vigorous agitation and higher reaction temperature(<358 K) in a closed system, the ammoximation main reaction can be accelerated [19]. Higher conversion of cyclohexanone may be achieved with less H2O2.
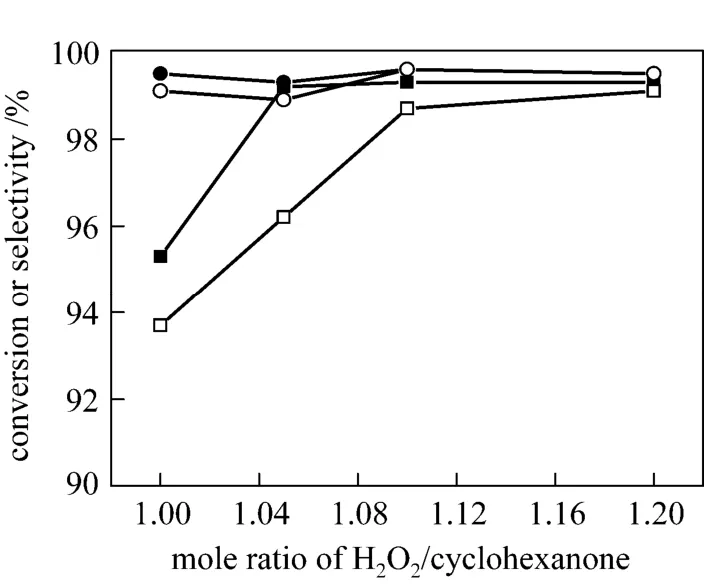
Figure 7 Effect of H2O2on conversion and selectivity at the azeotropic temperatures (Other conditions see Section 2.2)■ conversion in 2-phase system; ● selectivity in 2-phase system;□ conversion in 3-phase system; ○ selectivity in 3-phase system
3.6 Effect of NH3/ketone molar ratio
It is known that much more NH3than in theory is required for a suitable basic environment which is favor of the ammoximation main reaction and decreases the loss caused by vaporization of NH3. The NH3/ketone molar ratio of 1.7-2.7 is appropriate for the two-phase system [13]. Thus, the effect of the ratio on ammoximation in the three-phase system is studied within the range. Fig. 8 shows relatively high conversion of cyclohexanone and selectivity to oxime (both>99%)can be achieved at the ratio of 2.2, which is a little higher than that of the two-phase system. Regarding the relatively slower main reaction and the absence of ammonia in organic phase, less NH3can be dissolved in the aqueous phase and then take part in the ammoximation reaction in the three-phase system. A considerable amount of NH3is lost by vaporization and thus more NH3is required in an open system. However, as to an industry process, the excess NH3can be separated and fed back into the ammoximation reactor. In addition, due to the different polarity of the new solvent,the titanium leaching and the silica dissolution, which is one of the most important reasons for catalyst deactivation [20], may be not the same as that in the two-phase system, which would be investigated later in another work.
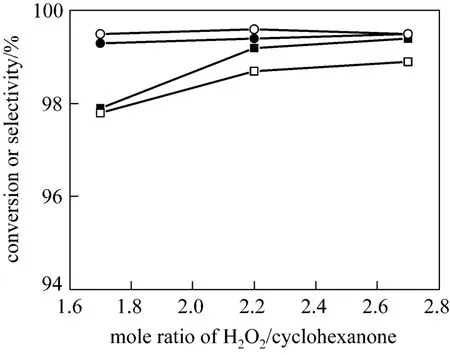
Figure 8 Effect of NH3 amount on conversion and selectivity at the azeotropic temperature (Other conditions see Section 2.2)■ conversion in 2-phase system; ● selectivity in 2-phase system;□ conversion in 3-phase system; ○ selectivity in 3-phase system
3.7 The stability of TS-1
The stability of TS-1 in the three-phase ammoximation process is investigated (Table 1). The catalyst is separated from the reaction mixture by centrifugationand then subjected to repeated three-phase ammoximation under the same reaction conditions. The result showed that after ten recycles of separation and ammoximation, TS-1 maintained its good catalytic activity with relative high conversion of ketone and selectivity to oxime (both>99%). Moreover, it is observed that the mixture after ten recycles remains colorless,which means little byproduct or NOx.
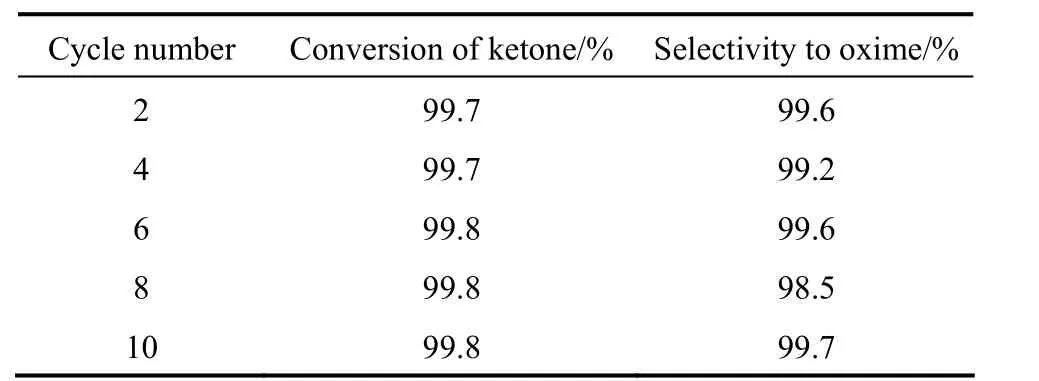
Table 1 The stability of TS-1 in three-phase system①
4 CONCLUSIONS
This work has demonstrated that ammoximation of cyclohexanone with ammonia and hydrogen peroxide can occur very well in the three-phase reaction system. Different from the industrial two-phase system,the mixture of cyclohexane and water is used as the solvent. Both the conversion of cyclohexanone and the selectivity to oxime can be reached 99% at lower temperature under following conditions: a catalyst content of 2.5%, H2O2/cyclohexanone molar ratio of 1.10, an NH3/cyclohexanone molar ratio of 2.20, reaction temperature of 343 K and reaction time of 5 h.
1 Roffia, P., Leofanti, G., Cesana, A., Mantegazza, M., Padovan, M.,Petrini, G., Tonti, S., Gervasutti, P., “Cyclohexanone ammoximation:A break through in the ε-caprolactam production process”,Stud.Surf.Sci.Catal., 55, 43-52 (1990).
2 You, K.Y., Mao, L.Q., Chen, L., Yin, D.L., Liu, P.L., Luo, H.A.,“One-step synthesis of ε-caprolactam from cyclohexane and nitrosyl sulfuric acid catalyzed by VPO supported transition metal composites”,Catal.Commun., 9, 2136-2139 (2008).
3 Roffia, P., Padovan, M., Leofanti, G., Mantegazza, M., de Alberti, G.,Tauszik, G.R., “Catalytic process for the manufacture of oximes”,US Pat., 4794198 (1988).
4 Taramasso, M., Perego, G., Notari, B., “Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides”,US Pat., 4410501 (1983).
5 Thangaraj, A., Sivasanker, S., Ratnasamy, P., “Catalytic properties of crystalline titanium-silicalite Ⅲ Ammoximation of cyclohexanone”,J.Catal., 131, 394-400 (1991).
6 Bengoa, J.F., Gallegos, N.G., Marchetti, S.G., Alvarez, A.M., Cagnoli,M.V., Yeramian, A.A., “Influence of TS-1 structural properties and operation conditions on benzene catalytic oxidation with H2O2”,Microporous.Mesoporous.Mater., 24, 163-172 (1998).
7 Luo, H.A., Wu, J., Liu, G.Q., “Separating production of ketoxime by three-phase amino-oximate reaction”, CN Pat., 1939897 (2007). (in Chinese)
8 Cheng, N.L., Solvents Handbook, Chemical Industry Press, Beijing,122-126 (2002). (in Chinese)
9 Thangaraj, A., Kumar, R., Ratnasamy, P., “Direct catalytic hydroxylation of benzene with hydrogen peroxide over titanium-silicate zeolites”, Appl. Catal., 57, 1-3 (1990).
10 Roffia, P., Padovan, M., Moretti, E., de Alberti, G., “Catalytic process for preparing cyclohexanone-oxime”, Eur. Pat., 0208311 (1986).
11 Padovan, M., Genoni, F., Leofani, G.., Petrini, G., Roffia, P., Cesana,A., “Catalytic process for the manufacture of oximes”, US Pat.,4968842 (1990).
12 Wu, C.T., Wang, Y.Q., Mi, M.T., Li, X., Wu, W., Min, E.Z., Han, S.,He, F., Fu, S.B., “Effects of organic solvents on the structure stability of TS-1 for the ammoximation of cyclohexanone”, React. Kinet.Catal. Lett., 77, 73-81 (2002). (in Chinese)
13 Sun, B., Zhu, L., “Study on ammoximation of cyclohexanone to cyclohexanone oxime catalyzed by titanium-silicalite-1 zeolite”, Pet.Process. Petrochem., 32, 22-24 (2001). (in Chinese)
14 Gao, H.X., Shu, Z.B., Cao, J., Zhang, Y.X., Lu, W.K., Chen, Q.L.,“Study on the ammoximation of cyclohexanone to cyclohexanone oxime catalyzed by titanium silicalite-1”, Chinese J. Catal., 19,329-333 (1998). (in Chinese)
15 Li, P., Lu, G.Z., Luo, Y., Dai, Y.N., “Study on catalytic oxidation activity of TS zeolite (V) Ammoximation of cyclohexanone”, Acta Chim. Sinica, 58, 204-208 (2000). (in Chinese)
16 Li, Y.X., Wu, W., Min, E.Z, “Kinetics of cyclohexanone ammoximation over titanium silicate molecular sieves”, Chin. J. Chem. Eng.,13, 32-36 (2005).
17 Cesana, A., Mantegazza, M.A., Pastori, M., “A study of the organic by-products in the cyclohexanone ammoximation”, J. Mol. Catal.,117, 367-373 (1997).
18 Petrini, G., Cesana, A., de Alberti, G., Genoni, F., Leofanti, G., Padovan, M., Paparatto, G., Roffia, P., “Deactivation phenomena on Ti-silicalite”, Stud. Surf. Sci. Catal., 68, 761-766 (1991).
19 Li, Y.X., Wu, W., Min, E.Z., “Ammoximation of cyclohexanone to cyclohexanone oxime over titanium silicalite molecular sieve”, Pet.Process. Petrochem., 41, 1-6 (2010). (in Chinese)
20 Liu, Y.Q., Li, Y.X., Wu, W., Min, E.Z., “Study on deactivation behavior of TS-1 molecular sieve in cyclohexanone ammoximation”,Pet. Process. Petrochem., 33, 41-45 (2002). (in Chinese)
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- The Short-term Effects of Temperature and Free Ammonia onAmmonium Oxidization in Granular and Floccular Nitrifying System*
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Turbulent Characteristic of Liquid Around a Chain of Bubbles in Non-Newtonian Fluid*
- Isolation and Characterization of Heterotrophic Nitrifying Strain W1*
- Recovery of Tungsten (VI) from Aqueous Solutions by Complexationultrafiltration Process with the Help of Polyquaternium*
- Optimizing the Chemical Compositions of Protective Agents for Freeze-drying Bifidobacterium longum BIOMA 5920*
