Hydrophobic TixOy-CmHnNanoparticle Film Prepared by Plasma Enhanced Chemical Vapor Deposition
WANG De-xin(王德信),XU Jin-zhou(徐金洲),LIU Wei(刘 伟),GUO Ying(郭 颖),YANG Qinyu(杨沁玉),DING Ke(丁 可),SHI Jian-jun(石建军),ZHANG Jing(张 菁)*
1 College of Material Science and Engineering,State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,Donghua University,Shanghai 201620,China
2 College of Science,Donghua University,Shanghai 201620,China
Introduction
The control of surface wettability is important for many biological processes and industrial applications[1-8].In recent years,hydrophobic surfaces,with a water contact angle(WCA)greater than 90°,have attracted considerable interests in their fundamental research and practical applications[9-14].Generally,there are two hydrophobic surfacesin nature corresponding with Wenzel's state and Cassie's state.The former is the wet-contact mode of water and rough surface,where water droplets sit suspended on the surface and can not roll off even with a 180°tilt while the later represents a nonwetcontact mode and water droplets can roll off easily owing to the low contact angle hysteresis[15].
TiO2,which exhibits high chemical stability,avirulence,and strong photo induced oxidation,has been studied intensively due to their applications in optical coatings,gas sensors,smart windows and photocatalysts[16],biomedical materials,and dyesensitized solar cells[17,18].Many efforts have been done to fabricate hydrophobic TiO2films.They are especially useful as hydrophobic ultraviolet absorbing coating on protection cover,electrochromic glass or as photocatalyst for diluted hydrophobic organic compounds in water[19,20].It is expected that the TiO2film could be unique self-cleaning so that the surface coated with such film can be maintained clean under circumstance of sunlight,rainfall,and other stains[21-23].Various methods have been developed so far for the fabrication of TiO2films with selfcleaning performance,such as so-gel[22,24],electrochemical anodization[25],hydrothermal treatment[26],and ion assisted deposition[27].In most of the cases,however,the surface of the film is hydrophilic.Here,we report the preparation of hydrophobic TixOy-CmHnnanoparticle film via RF plasma enhanced chemical vapor deposition(PECVD)from the mixture of the titanium isopropoxide(TTIP)and oxygen through one deposition cycle for short time.This hydrophobic TixOy-CmHnfilm is composed of micropapillae with average size 1-3 μm which are aggregated by inorganic and organic phases of nanoparticles with size of 50-200 nm.The organic CmHnis incorporated in the film in the deposition process.This makes the film present hydrophobicity with WCA exceeding 160°,and ultraviolet absorption and X-Ray diffraction(XRD)spectrum are similar with TiO2nanoparticle film.
1 Experimental
The apparatus used for the film deposition is shown in Fig.1.The deposition was carried out in a glass cylindrical reactor with a diameter of 8 cm.Two arc copper sheets with the size of 6 cm2×8 cm2were used as electrode and located oppositely outside glass reactor.The substrates were placed on a glass platform in the discharge zone apart from the electrodes.The RF power with frequency of 13.56 MHz(American Advance Energy Inc(PF-10S System))was delivered to the system through an automatic matching network.The monomer was TTIP with a purity of 98%.The substrates of glass slides(2 cm2× 2 cm2)were ultrasonically cleaned in acetone,ethyl,ethanol,and deionized water for 20 min,respectively,to remove the surface containment before being put into the reactor.
The system was pumped to a base pressure of 8.5 ×10-1Pa before igniting the discharge.The substrate was exposed to argon discharge plasma for about 10 min to provide an additional surface cleaning.Then the precursor heated at 60℃constant temperature by a water bath was introduced to the discharge zone through oxygen carrier gas.The flow rate of the oxygen was 10 sccm controlled by a mass flow controller.The system pressure was stabilized by continuous flow of the monomer vapor and kept at(35±2)Pa.The RF discharge powers were varied in the deposition process from 40 W to 150 W.The deposition time was about 30 min.

Fig.1 Schematic of the set-up used for film deposition
The chemical content of film was analyzed by elemental analyzer(EA,VarioEL,Elementar,Germany). The morphology and crystallization structure analysis of the film were done using scanning electron microscope(SEM,JSM-5600 LV,JEOL,Japan),transmission electron microscope(TEM,JEM-2100 F,JEOL,Japan),and XRD(D/Max-2550 PC,RIGAKU,Japan),respectively.Further investigations about the chemical structure were carried out by Fourier transform infrared spectrometer(FTIR,NEXUS-670,Nicolet,America).The double-beam UV-Vis spectrometer(Lambda 35,Perkin Elmer,America)was used to study the optical properties of the deposited films.The WCA measurement was carried out in a computer-video-processed goniometer(JC2000A,Powereach,Shanghai,China).
2 Results and Discussion
2.1 Surface hydrophobicity
The changes ofWCA with the discharge power are displayed in Fig.2.It's obvious that all deposited film surfaces are hydrophobic with WCA varying from 130°to 160°when the discharge power changes from 40 W to 180 W.The WCA increases with increasing the RF discharge power and reaches the maximum value when the RF discharge power is 150 W.Then the WCA decreases if the RF discharge power increases continuously.

Fig.2 The WCA of the films varied with discharge powers According to Wenzel equation

where θris the contact angle on the rough surface,θsis the contact angle on the smooth surface,and r is the roughness correction factor.r equals the ratio of the surface actual area to the apparent project area.When θsis less than π/2,cos θsis greater than 0 and less than 1,and θrdecreases as r increases.When θsis greater than π/2,cos θsis less than 0,and θrincreases as r increases.Because all WCAs for the deposited films were greater than π /2 and increased with power,it's estimated that r increased along with the discharge power and the roughnessofthe deposited filmscontributed to the hydrophobicity of the films.
2.2 Surface morphology
Figure 3 shows the SEM images of as-deposited films with different RF powers,and Figs.3(d),(e),and(f)are the magnified SEM images corresponding to Figs.3(a),(b),and(c).The surface of the films is covered with average size 1-3 μm micropapillae aggregated by small nanoparticles. The relativelymagnified SEM imagesclearly revealthatthe nanoparticle size ranges from 50 nm to 200 nm.From Figs.3(d),(e),and(f),the size of the nanoparticles increases with the increase of RF discharge power.The surface of the film exhibits loose and rough structure rather than dense aggregating.There are also many pores among the micropapillae.These pores are believed to do favor to the surface hydrophobicity:the air is trapped within the pores so that the droplet can not penetrate into the pores and sits suspended on the peaks of the micropapillae while the surface remains dry below[28].According to the Wenzel equation,this kind of loose and rough structure contributes to the hydrophobicity of the film.

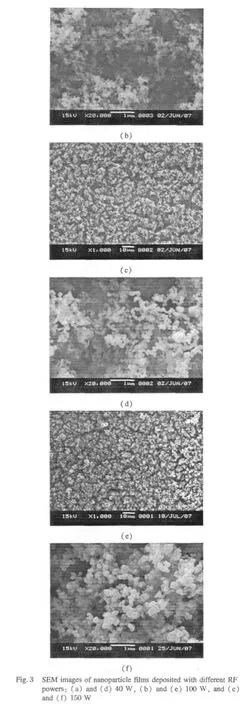
2.3 Chemical composition and structure
The infrared spectra of the films deposited from the mixture gases of TTIP and O2are shown in Fig.4.

Fig.4 Infrared spectra of the films deposited with different RF powers:(a)40 W,(b)100 W,and(c)150 W
The main vibration peaks and its vibration modes are listed in Table 1.As shown in Fig.4,all IR spectra of the deposited films with different RF discharge powers exhibit a strong absorption band at 3 150 cm-1attributed to the stretching vibration of—OH,indicating the combination of O and H from the precursor molecules or the unassociated—OH groups strongly absorbed in the deposited films[29,30].Absorption peaks at 2 930 and 2 840 cm-1are thought to be the C—H stretching vibration bands[31].The C=O stretching vibration is found at 1 660 cm-1.The bending vibration of C—H is shown at 1 388 cm-1.The C—H bending and stretching vibration bands mentioned above strongly confirm the existence of the C—H bonds[32].Furthermore,the peak at 1 080 cm-1has been identified to be Ti—O—C stretching vibration in the deposited film[33].The observation of Ti—O—C,C =O,—OH,and C—H bonds in the FTIR spectra of the deposited film indicates the introduction of the organic groups in the film deposition process[34].The broad absorption band from 800 cm-1to 400 cm-1obviously belongs to the Ti—O—Ti groups similar to the bulk TiO2frequency region,while the FTIR of the TTIP precursor only showsa narrow absorption band at616 cm-1[35,36].
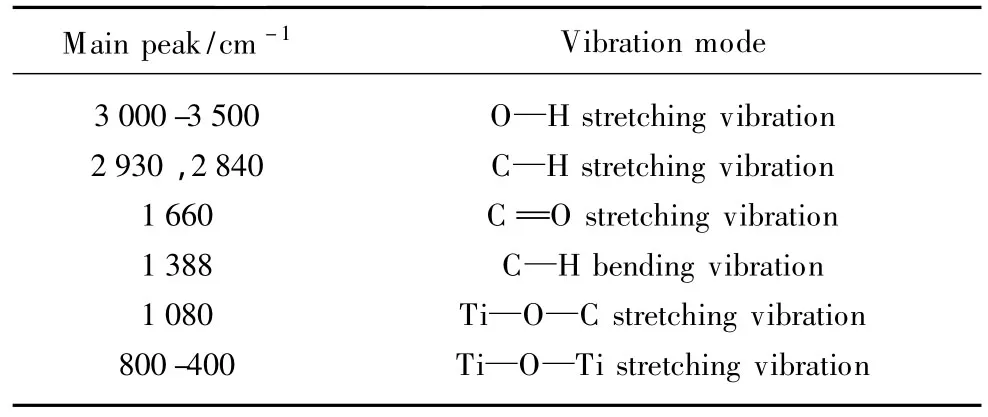
Table 1 Main vibration peaks and vibration modes in FTIR spectra of the deposited films
The film is scraped from the glass substrate to be further investigated by TEM and XRD.The results are displayed in Figs.5 and 6.From Fig.5,it can be seen that the average particle diameter of the film deposited at 100 W is about 80 nm.When the RF power is increased to 150 W,the average diameter of the film particles increases to be about 200 nm.The average size of the particles deposited at 150 W is more uniform than at 100 W.According to our deposition experiment,the plasma discharge can fill the whole reactor tube but is not as uniform at low power as that at high power.Therefore the size of the particles we get at low power is not as uniform as that we get at high power.Because the substrates were put just close to the electrodes,the larger diameter of some particles deposited at 150 W could be caused by the not so uniform discharge and deposition close to the electrodes.
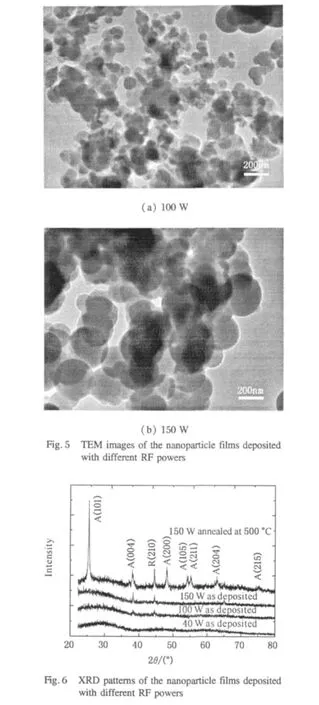
Figure 6 shows the XRD spectra of the films deposited at glass substrates.The crystallinity is different when the discharge power changes.The film is entirely amorphous when the RF power is 40 W.When the RF power is increased to 100 W,two weak peaks at 38.1°and 44.5°which belong to anatase phase A(004)and rutile phase R(210)of TiO2begin to appear(PCPDF Card,No.78-2486,No.82-0514),while keeping the broad diffraction peak of amorphous TiO2in the low angle range.This illustrates that the structure of the deposited film changes from amorphous TiO2to the structure with anatase and rutile TiO2.Combining with TEM observation,it is indicated that there is crystal particles embedded in the film matrix.It is also found that the two peaks become stronger when the RF power is 150 W,indicating that the crystallization become stronger with increasing the discharge power.It is believed that the substrate temperature and the dissociation of the monomer affected by the discharge power should be responsible for the structure transition of the film.The substrate temperature increases when the RF power increases because of the selfheating effect of the plasma,and the dissociation of the monomer becomes intense,making the deposited film changes from amorphism to crystallization with anatase and rutile phase.
In order to make further comparison analyses,we carefully studied the XRD patterns of the film deposited at 150 W and then annealed at 500℃ at atmosphere.We found that more diffraction peaks appear.These peaks can be well indexed to TiO2anatase structures with(h,k,l)miller indices of(101),(004),(200),(105),(211),(204),and(215),except for the peak at 2θ=44.5°which belongs to the rutile structure with(h,k,l)miller indices of(210).This illustrates that the crystallization ofthe annealed film is morecompleteas compared with that of deposited film at 150 W.We have a further investigation of the film composition deposited at 150 W by elemental analyzer(EA).The result reveals that the deposited films from plasma enhanced chemical vapor deposition are mainly composed of Ti,C,and O as shown in Table 2.

Table 2 Mass percentage of the elements in the deposited film at 150 W
There is also H incorporated into the films.The all four elements found in EA are well corresponding with results observed in the FTIR spectrum.According to the analysis results,we can give the film structure formula as Ti0.3O2-C1.5H3.Because of the obvious TiO2crystal phase in XRD and inorganic nanoparticle as shown in TEM,the structure formula of the film deposited at 150 W could also be expressed as TiO2-C5H10O4.7.
2.4 UV-Vis absorption
The UV-Vis absorption spectra of the films are shown in Fig.7.The thickness of the nanoparticle films is 750,850,and 900 nm corresponding to the RF power 40,100,and 150 W,respectively.All films deposited at different RF discharge powers have strong absorption in the ultraviolet band below 320 nm,which is similar to that of TiO2film[37,38].Because the absorption in the UV area of the deposited film is so strong and exceeds the upper integrating limit of the UV-Vis instrument,all the absorbing signals above 6 are leveled to 6.But it displays that all the absorption in the UV area of the deposited film is very strong.With the film thickness decreases and the RF discharge power decreases,the absorption edge shows obvious blue shiftofabout 10 nm to 20 nm respectively.As corresponding with SEM and TEM results above,the size of the nanoparticles decreases from about 200 nm to 50 nm with the decrease of RF discharge power from 150 W to 40 W.Through the estimation of XRD spectrum by Debye-Scherrer equation,the size of anatase TiO2particle is about 25.9 nm at 100 W and 43.7 nm at 150 W.Therefore,it is believed that the quantum size effect caused by particle size is attributed to the blue shift of the UV-Vis absorption spectrum during decreasing the RF discharge power[39].

Fig.7 UV-Vis absorption spectra of the nanoparticle films deposited with different RF powers:(a)40 W,(b)100 W,and(c)150 W
3 Conclusions
A kind of TixOy-CmHnnanoparticle films with WCA as high as(160±1)°were successfully fabricated from the mixture of TTIP and oxygen via the PECVD through one deposition cycle for 30 min.Surface morphology,hydrophobicity,chemicalstructure,chemicalcomposition,crystallity,and UV-Vis absorption were studied in dependence on discharge power to investigate the influence of discharge power on the structure and property of the deposited films through SEM,contact angle measurement,FTIR,EA TEM,XRD,and UV-Vis SEM and TEM.Results show that the surface of the film is characterized with 1-3 μm microsized papillae aggregated by nanoparticles with size from 50 nm to 200 nm,leading to the roughness of the surface.FTIR analysis indicated the existence of the Ti—O—Ti,Ti—O—C,C =O,—OH,and C—H bonds in all deposited films through their characteristic absorption bands from 800 cm-1to 400 cm-1,1 660 cm-1,3 000 cm-1to 3 500 cm-1and 1 388 cm-1,2 840 cm-1and 2 930 cm-1respectively.The XRD results confirmed the existence of anatase and rutile phase of TiO2with crystal size of about 25.9 nm in the film when the discharge power increased to 100 W.UV-Vis absorption spectrum displayed that all the deposited films showed similar strong absorption in the ultraviolet band below 320 nm to that of TiO2film.According to EA analysis,the chemical formula of the deposited film at 150 W could be expressed as Ti0.3O2-C1.5H3.Therefore it is believed that the film structure is piled together by rough micropapillae aggregated by complex inorganic and organic phase of nanoparticles with TiO2nanocrystals size of 43.7 nm.It is believed that the combination of the loose and rough micro/nano structure of the film and the introduction of organic residues lead to the film superhydrophobicity.And the TiO2nanocrystals in the complex film lead to the strong absorption in the ultraviolet band below 320 nm.
[1]Schmidt-Stein F,Gnichwitz J F, Salonen J, et al.Electrochemical Wettability Control on Conductive TiO2Nanotube Surfaces Modified with a Ferrocene Redox System [J].Electrochemistry Communications,2009,11(10):2000-2003.
[2]Nakata K,Udagawa K,Ochiai T,et al.Rapid Erasing of Wettability Patterns Based on TiO2-PDMS Composite Films[J].Materials Chemistry and Physics,2011,126(3):484-487.
[3]Shirtcliffe N J,McHaleG,Newton M I,etal. Plastron Properties of a Superhydrophobic Surface[J].Applied Physics Letters,2006,89(10):104106-104107.
[4]Song Y Y,Roy P,Paramasivam I,et al. Voltage-Induced Payload Release and Wettability Control on TiO2and TiO2Nanotubes[J].Angewandte Chemie,2010,122(2):361-364.
[5]Shin D H,Shokuhfar T,Choi C K,et al.Wettability Changes of TiO2Nanotube Surfaces[J].Nanotechnology,2011,22(31):315704-315709.
[6]Feng X J,Jiang L.Design and Creation of Super-Wetting/Dewetting Surfaces[J].Advanced Materials,2006,18(23):3063-3078.
[7]Niu F,Zhang L S,Chen C Q,et al.Hydrophilic TiO2Porous Spheres Anchored on Hydrophobic Polypropylene Membrane for Wettability Induced High Photodegrading Activities [J].Nanoscale,2010,2(8):1480-1484.
[8]Tang X H,Li D Y.Evaluation of Asphaltene Degradation on Highly Ordered TiO2NanotubularArraysviaVariationsin Wettability[J].Langmuir,2011,27(3):1218-1223.
[9]Liu B,Aydil E S.Growth of Oriented Single-Crystalline Rutile TiO2Nanorods on Transparent Conducting Substrates for Dye-Sensitized Solar Cells[J].Journal of the American Chemical Society,2009,131(11):3985-3990.
[10]Wang S T,Song Y L,Jiang L.Photoresponsive Surfaces with Controllable Wettability[J].Journal of Photochemistry and Photobiology C:Photochemistry Reviews,2007,8(1):18-29.
[11]Lee C Y,Hupp JT. Dye Sensitized SolarCells:TiO2Sensitization with a Bodipy-Porphyrin Antenna System [J].Langmuir,2010,26(5):3760-3765.
[12]Satyaprasad A,Jain V, Nema S K.Deposition of Superhydrophobic Nanostructured Teflon-Like Coating Using Expanding Plasma Arc[J].Applied Surface Science,2007,253(12):5462-5466.
[13]Wolcott A,Smith W A,Kuykendall T R,et al.Photoelectrochemical Water Splitting Using Dense and Aligned TiO2Nanorod Arrays[J].Small,2009,5(1):104-111.
[14]Chen D H,Huang F Z,Cheng Y B,et al.Mesoporous Anatase TiO2Beads with High Surface Areas and Controllable Pore Sizes:a Superior Candidate for High-Performance Dye-Sensitized Solar Cells[J].Advanced Materials,2009,21(21):2206-2210.
[15]Feng L,ZhangY N,XiJM,etal. PetalEffect:a Superhydrophobic State with High Adhesive Force [J].Langmuir,2008,24(8):4114-4119.
[16]Gilma G O,Carlos A P M,Fernando M O,et al.Photocatalytic Degradation of Phenol on TiO2and TiO2/Pt Sensitized with Metallophthalocyanines[J].Catalysis Today,2005,107/108:589-594.
[17]Yerokhin A L,Nie X,Leyland A,et al.Review:Plasma Electrolysis for Surface Engineering[J].Surface and Coatings Technology,1999,122(2/3):73-93.
[18]O'Regan B,Gratzel M.A Low-Cost,High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2Films [J].Nature,1991,353(6346):737-740.
[19]Parkin I P,Palgrave R G.Self-cleaning Coatings[J].Journal of Materials Chemistry,2005,15(17):1689-1695.
[20]Wang C M,Lin S Y.Electrochromic Properties of Sputtered TiO2Thin Films[J].Journal of Solid State Electrochemistry,2006,10(4):255-259.
[21]Ohko Y,Saitoh S,Tatsuma T,et al.Photoelectrochemical Anticorrosion and Self-cleaning Effects of a TiO2Coating for Type 304 Stainless Steel[J].Journal of the Electrochemical Society,2001,148(1):B24-B28.
[22]Silva C G,Faria J L.Effect of Key Operational Parameters on the Photocatalytic Oxidation of Phenol by Nanocrystalline Sol-Gel TiO2under UV Irradiation[J].Journal of Molecular Catalysis A,2009,305(1/2):147-154.
[23]Guan K S. Relationship between Photocatalytic Activity,Hydrophilicity and Self-cleaning Effect of TiO2/SiO2Films[J].Surface and Coatings Technology,2005,191(2/3):155-160.
[24]Shi P,Ng W F,Wong M H,et al.Improvement of Corrosion Resistance of Pure Magnesium in Hanks'Solution by Microarc Oxidation with Sol-Gel TiO2Sealing[J].Journal of Alloys and Compounds,2009,469(1/2):286-292.
[25]Balaur E,Macak J M,Taveira L,et al.Tailoring the Wettability of TiO2Nanotube Layers[J].Electrochemistry Communications,2005,7(10):1066-1070.
[26]Feng X J,Zhai J,Jiang L.The Fabrication and Switchable Superhydrophobicity of TiO2Nanorod Films[J].Angewandte Chemie International Edition,2005,44(32):5115-5118.
[27]Yamashita H,Nakao H,Takeuchi M,et al.Coating of TiO2Photocatalysts on Super-hydrophobic Porous Teflon Membrane by an Ion Assisted Deposition Method and Their Self-cleaning Performance[J].Nuclear Instruments and Methods in Physics Research Section B:Beam Interactions with Materials and Atoms,2003,206:898-901.
[28]Cassie A B D,Baxter S.Wettability of Porous Surfaces[J].Transactions of the Faraday Society,1944,40:546-551.
[29]Kumar P M,Badrinarayanan S,Sastry M.Nanocrystalline TiO2Studied by Optical,FTIR and X-Ray Photoelectron Spectroscopy:Correlation to Presence of Surface States[J].Thin Solid Films,2000,358(1/2):122-130.
[30]Socrates G.Infrared Characteristic Group Frequencies[M].2nd ed.USA:John Willey and Sons,1994:62.
[31]Ivanova T,Harizanova A,Surtchev M. Formation and Investigation of Sol-Gel TiO2-V2O5System [J].Materials Letters,2002,55(5):327-333.
[32]López T,Moreno J A,Gómez R,et al.Characterization of Iron-Doped Titania Sol-Gel Materials[J].Journal of Materials Chemistry,2002,12(3):714-718.
[33]Harris M T,Singhal A,Look J L,et al.FTIR Spectroscopy,SAXS and Electrical Conductivity Studies of the Hydrolysis and Condensation of Zirconium and Titanium Alkoxides[J].Journal of Sol-Gel Science and Technology,1997,8(1/2/3):41-47.
[34]Jensen H,Soloviev A,LiZ S,etal. XPS and FTIR Investigation of the Surface Properties of Different Prepared Titania Nano-powders[J].Applied Surface Science,2005,246(1/2/3):39-249.
[35]Kalache B,Kosarev A I,Vanderhaghen R,etal. Ion Bombardment Effects on Microcrystalline Silicon Growth Mechanisms and on the Film Properties[J].Journal of Applied Physics,2003,93(2):1262-1274.
[36]Ahn K H,Park Y B,Park D W.Kinetic and Mechanistic Study on the Chemical Vapor Deposition of Titanium Dioxide Thin Films by in situ FT-IR Using TTIP[J].Surface and Coatings Technology,2003,171(1/2/3):198-204.
[37]Christy P D,Jothi N S N,Melikechi N,et al.Synthesis,Structural and Optical Properties of Well Dispersed Anatase TiO2Nanoparticles by Non-hydrothermalMethod [J]. Crystal Research and Technology,2009,44(5):484-488.
[38]Yu J G,WangW G,ChengB,etal. Enhancementof Photocatalytic Activity of Mesoporous TiO2Powders by Hydrothermal Surface Fluorination Treatment[J].Journal of Physical Chemistry C,2009,113(16):6743-6750.
[39]Ding Z,Hu X J,Lu G Q,et al.Novel Silica Gel Supported TiO2Photocatalyst Synthesised by CVD Method [J].Langmuir,2000,16(15):6216-6222.
 Journal of Donghua University(English Edition)2012年3期
Journal of Donghua University(English Edition)2012年3期
- Journal of Donghua University(English Edition)的其它文章
- Frost Resistance and Damage Velocity of Compressively Preloaded Concrete
- Vehicle OHT DispatchingPerformance Analysis ofan AMHS in 300mm Semiconductor FABs
- Research on Cooperation Mechanism ofChina'sFinancialRegulation and Supervision System
- Determination of the Dose of PAC in Ultrafiltration System for Drinking Water Treatment
- Comparison and Analysis of Fabric Deodorization Test Methods
- Sorption Kinetics and Capacity of Composite Materials Made up of Polymeric Fabric and Expanded Perlite for Oil in Water
