Onsite-effects of dual-hemisphere versus conventional single-hemisphere transcranial direct current stimulation A functional MRI study*☆●
Yong Hyun Kwon, Sung Ho Jang
1 Department of Physical Therapy, Yeungnam College of Science & Technology, Daegu 705-703, Republic of Korea
2 Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu 705-717, Republic of Korea
Onsite-effects of dual-hemisphereversusconventional single-hemisphere transcranial direct current stimulationA functional MRI study*☆●
Yong Hyun Kwon1, Sung Ho Jang2
1Department of Physical Therapy, Yeungnam College of Science & Technology, Daegu 705-703, Republic of Korea
2Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu 705-717, Republic of Korea
We performed functional MRI examinations in six right-handed healthy subjects. During functional MRI scanning, transcranial direct current stimulation was delivered with the anode over the right primary sensorimotor cortex and the cathode over the left primary sensorimotor cortex using dual-hemispheric transcranial direct current stimulation. This was compared to a cathode over the left supraorbital area using conventional single-hemispheric transcranial direct current stimulation. Voxel counts and blood oxygenation level-dependent signal intensities in the right primary sensorimotor cortex regions were estimated and compared between the two transcranial direct current stimulation conditions. Our results showed that dual-hemispheric transcranial direct current stimulation induced greater cortical activities than single-hemispheric transcranial direct current stimulation. These findings suggest that dual-hemispheric transcranial direct current stimulation may provide more effective cortical stimulation than single-hemispheric transcranial direct current stimulation.
transcranial direct current stimulation; dual-hemispheric stimulation; cortical activation; functional MRI; primary sensorimotor cortex; neuroimaging; regeneration; neural regeneration
Research Highlights
(1) We compared cortical activation during dual-hemispheric transcranial direct current stimulation and single-hemispheric transcranial direct current stimulation.
(2) During functional MRI scanning, for the dual-hemispheric transcranial direct current stimulation, the anode was placed over the right primary sensorimotor cortex, and the cathode was placed over the left primary sensorimotor cortex, whereas the cathode was placed over the right supraorbital area for conventional single-hemispheric transcranial direct current stimulation.
(3) Dual-hemispheric transcranial direct current stimulation more stongly enhanced higher cortical activity than conventional single-hemispheric transcranial direct current stimulation, as indexed by higher voxel counts and blood oxygenation level-dependent signal intensities.
(4) Dual-hemispheric transcranial direct current stimulation may be more effective for cortical stimulation than single-hemispheric transcranial direct current stimulation.
Abbreviations
SM1, Primary sensorimotor cortex; BOLD, blood oxygenation level-dependent
INTRODUCTION
Transcranial direct current stimulation can alter human brain functions noninvasively by modulating the excitability of targeted cortical neurons. This functional modulation could be mediated by the alteration of neural membrane potentials and N-methyl-D-aspartate receptor potential efficacy[1-3]. During transcranial direct current stimulation, a weak direct current is continuously applied over the scalp through an anodal and a cathodal electrode[4-5]. It has been well established that the two polarities have different neurophysiologic properties[1,5-6].
For example, anodal currents increase cortical excitability, whereas cathodal currents decrease cortical excitability.
Numerous previous studies have demonstrated that transcranial direct current stimulation can modulate cognitive and motor functions associated with targeted brain areas in normal subjects and patients with a brain lesion[7-11].
In transcranial direct current stimulation electrode applications, the anode is used as the active electrode to facilitate the targeted cortex[5,7], whereas the cathode is applied on the supraorbital area of the opposite hemisphere as a reference electrode, without affecting the target cortex. Although the neurophysiologic properties of each of the two polarities are different, both the anodal and cathodal current can facilitate motor and cognitive functions of homologous cortical areas in the non-dominant hemisphere[12-14]. In this regard, in a recent study, the two currents were applied simultaneously to enhance dominant cortical function using the inter-hemispheric connectivity-driven effect of transcranial direct current stimulation. Specifically, anodal transcranial direct current stimulation was applied on the dominant cortical area and cathodal transcranial direct current stimulation on the non-dominant homologous cortical area (dual-hemispheric transcranial direct current stimulation)[15].
Recently, the effects of transcranial direct current stimulation on various brain functions have been demonstrated in several studies using functional MRI and positron emission tomography[16-20]. Therefore, the fact that anodal current in single-hemispheric transcranial direct current stimulation can induce activation of the underlying targeted cortex has been well established[16-18]. However, no functional neuroimaging study has been conducted to investigate cortical activation by dual-hemispheric transcranial direct current stimulation. In the present study, we compared cortical activation during dual-hemispheric transcranial direct current stimulation and single-hemispheric transcranial direct current stimulation using functional MRI.
RESULTS
Quantitative analysis and general description of subjects
Of the nine healthy subjects recruited for this study, three subjects were excluded because cortical activation on the primary sensorimotor cortex (SM1) was not detected during dual-hemispheric transcranial direct current stimulation (one subject) or single-hemispheric transcranial direct current stimulation (two subjects).
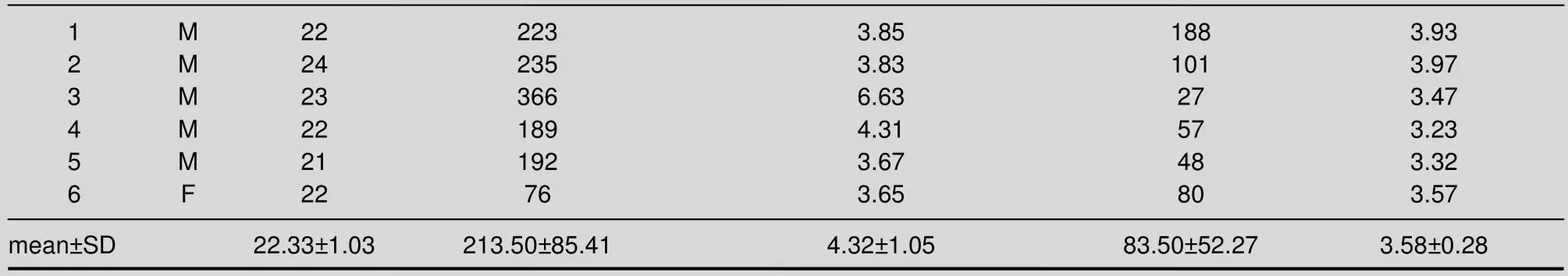
Consequently, six subjects were included in the final functional MRI analysis. The baseline data of each subject is shown in Table 1. None of the subjects complained of any adverse symptoms or signs during or after stimulation, except a slight itching sensation under the electrodes.
Voxel counts and blood oxygenation level-dependent (BOLD) signal intensity on the underlying SM1 in dual-hemispheric transcranial direct current stimulation and single-hemispheric transcranial direct current stimulation
The voxel counts and BOLD signal intensities in the right SM1 during dual-hemispheric transcranial direct current stimulation were significantly higher than those induced by single-hemispheric transcranial direct current stimulation (P< 0.05; Table 1).

Table 1 Functional MRI activation on the primary sensorimotor cortex during dual-hemispheric transcranial direct current stimulation (tDCS) and single-hemispheric tDCS in healthy subjects
DISCUSSION
In the present study, we found cortical activation in the SM1 during both dual- and single-hemispheric transcranial direct current stimulation and both voxel counts and BOLD signal intensities during dual-hemispheric transcranial direct current stimulation. These results suggest that dual-hemispheric transcranial direct current stimulation has an effect similar to conventional single-hemispheric transcranial direct current stimulation with respect to the activation of targeted cortex and that dual-hemispheric transcranial direct current stimulation is more effective than conventional single-hemispheric transcranial direct current stimulation in this respect. However, in three subjects, the cortical activation in the SM1 was not observed during dual- or single-hemispheric transcranial direct current stimulation. This is consistent with a previous study, in which a functional MRI BOLD signal changes induced by on-site transcranial direct current stimulation were not always detected[17]. This phenomenon may be due to individual anatomical differences (e.g., thickness of connective tissue over epidermis and sweat duct resistivity) or electrode parameters (e.g., hydration and resistivity)[21-23].
Several previous neuroimaging studies have demonstrated that the functional MRI BOLD signals are induced by the direct ongoing-effect of transcranial direct current stimulation[16-18]. To the best of our knowledge, the first study designed to investigate the onsite-effect of transcranial direct current stimulation during concurrent functional MRI scanning was conducted by Kwonet al[16], and it indicated that cortical activity was induced in the SM1 by anodal transcranial direct current stimulation. In their second transcranial direct current stimulation experiment using functional MRI, it was found that cortical activity in the SM1 was induced after 1 minute of direct current application to the target neurons, and that this activity was maintained with some fluctuations[17].
These prior neuroimaging studies supported our finding that functional MRI BOLD signal changes were induced by the ongoing effect of transcranial direct current stimulation. In addition to the onsite effect of transcranial direct current stimulation, the concurrent application of transcranial direct current stimulation and motor tasks caused an increase in cortical target neuron activity after transcranial direct current stimulation[20,24-25]. A possible neurophysiologic mechanism underlying these findings is that anodal stimulation can induce changes in the neural excitability of underlying target neural cells in the human brain. Accordingly, we believe that dual-hemispheric direct current stimulation is sufficient to lead to activation of cortical neurons in the resting state.
The present findings showed that dual-hemispheric transcranial direct current stimulation induced higher cortical activities than conventional single-hemispheric transcranial direct current stimulation. This outcome is supported by several prior studies that investigated improvements in behavioral functions induced by the dual-hemispheric and single-hemispheric-connectivity transcranial direct current stimulation effects during or after single-hemispheric stimulation[25-29]. Vineset al[15]reported that dual-hemispheric transcranial direct current stimulation, using the simultaneous application of cathodal transcranial direct current stimulation over the dominant motor cortex and anodal transcranial direct current stimulation over the non-dominant motor cortex, improved motor skills of the non-dominant hand in a motor sequencing task significantly more than the single-hemispheric stimulation. In addition, according to prior studies that addressed hemispheric modulation[14,30], cathodal transcranial direct current stimulation applied over a unilateral hemisphere had a facilitative effect on the ipsilateral upper limb, but an inhibitory effect on the contralateral upper limb. In contrast, anodal transcranial direct current stimulation had the opposite effect.
Moreover, Sparinget al[13]reported that the inhibitory effects of cathodal transcranial direct current stimulation over the unlesioned posterior parietal cortex and the facilitative effects of anodal transcranial direct current stimulation over the lesioned posterior parietal cortex reduced visuospatial neglect following stroke. These converging evidences may be explained by inter-hemispheric interactionsviatranscallosal inhibition. In the healthy brain, neural activity in homologous areas of both hemispheres was functionally coupled and equally balanced because of mutual inhibitory controlviatranscallosal connections[26]. Therefore, dual-hemispheric transcranial direct current stimulation may have produced greater cortical activity because the cortical excitability was decreased by cathodal transcranial direct current stimulation over the left SM1, which reduced inhibitory inter-hemispheric control of the homologous right SM1. This disinhibitory control in the left homologous area engendered cortical activity in the right SM1 induced by anodal transcranial direct current stimulation over the right SM1. Consequently, we speculate that our observations resulted from onsite transcranial direct current stimulation modulation of inhibitory inter-hemispheric projects.
In conclusion, we found that simultaneous application of anodal transcranial direct current stimulation over the target brain area with cathodal transcranial direct current stimulation over the same brain area in the opposite hemisphere provides a more effective means of activating the underlying target cortex than conventional single-hemispheric transcranial direct current stimulation.
These findings provide a means of more effectively increasing cortical excitability in the underlying motor cortex by transcranial direct current stimulation in the human brain. Nevertheless, we acknowledge that this study has limitations. For example, we focused on the effects of anodal transcranial direct current stimulation in the dominant hemisphere. The small sample size makes generalizations difficult, and no behavioral assessment was performed. Furthermore, the transcranial direct current stimulation was applied for a relatively short time because of mechanical restrictions of the MR equipment.
Accordingly, we suggest that future large-scale studies that include neurobehavioral assessments are necessary to fully ascertain the onsite effects of dual-hemispheric stimulation in dominant and non-dominant hemispheres.
SUBJECTS AND METHODS
Design
A non-randomized, concurrent controlled study.
Time and setting
This study was performed in the functional MRI room of Yeungnam University Medical Center, Yeungnam University, Republic of Korea, from August 2011 to November 2011.
Subjects
Nine healthy subjects, including five males and four females, with a mean age of 22.22 ± 0.87 years old, were included in this study. These subjects denied having an abnormal neurological or psychiatric history. All subjects were confirmed to be right-handed by the modified Edinburg Handedness Inventory[27]. None of the subjects had ever participated in a brain stimulation experiment using, for example, transcranial direct current stimulation or transcranial magnetic stimulation. All subjects understood the purpose of this study and provided written informed consent in accordance with the ethical standards of theDeclaration of Helsinki.
Methods
Application of transcranial direct current stimulation
Direct current was providedviaa battery-driven constant direct current stimulator (NeuroConn GmbH, Ilmenau, Germany) located outside the MRI room. Current was delivered to the scalp using a pair of electrodes (EL508, Biopac System INC, US) and leads (LEAD108, Biopac System INC, Goleta, CA, USA) designed for use in a magnetic field. MRI compatible electrodes were placed on a water-soaked sponge (5 cm × 7 cm), which was in direct contact with the scalp. The 10/20 international electroencephalographic system, in which M1
corresponds to C3 or C4 in both hemispheres respectively, was used for electrode placement. For the dual-hemispheric transcranial direct current stimulation, the center of anodal electrode was placed over C3 of the SM1 in the left hemisphere, whereas one of the cathodal electrodes was placed over C4 of the SM1 in the right hemisphere. For conventional single-hemispheric transcranial direct current stimulation, the center of the anodal electrode was placed over C3 of the SM1 in the left hemisphere, whereas one of the cathodal electrodes was placed over the supraorbital area in the right hemisphere (Figure 1). The C3 and C4 areas are well known as the neural representational areas of hand motor function[28].
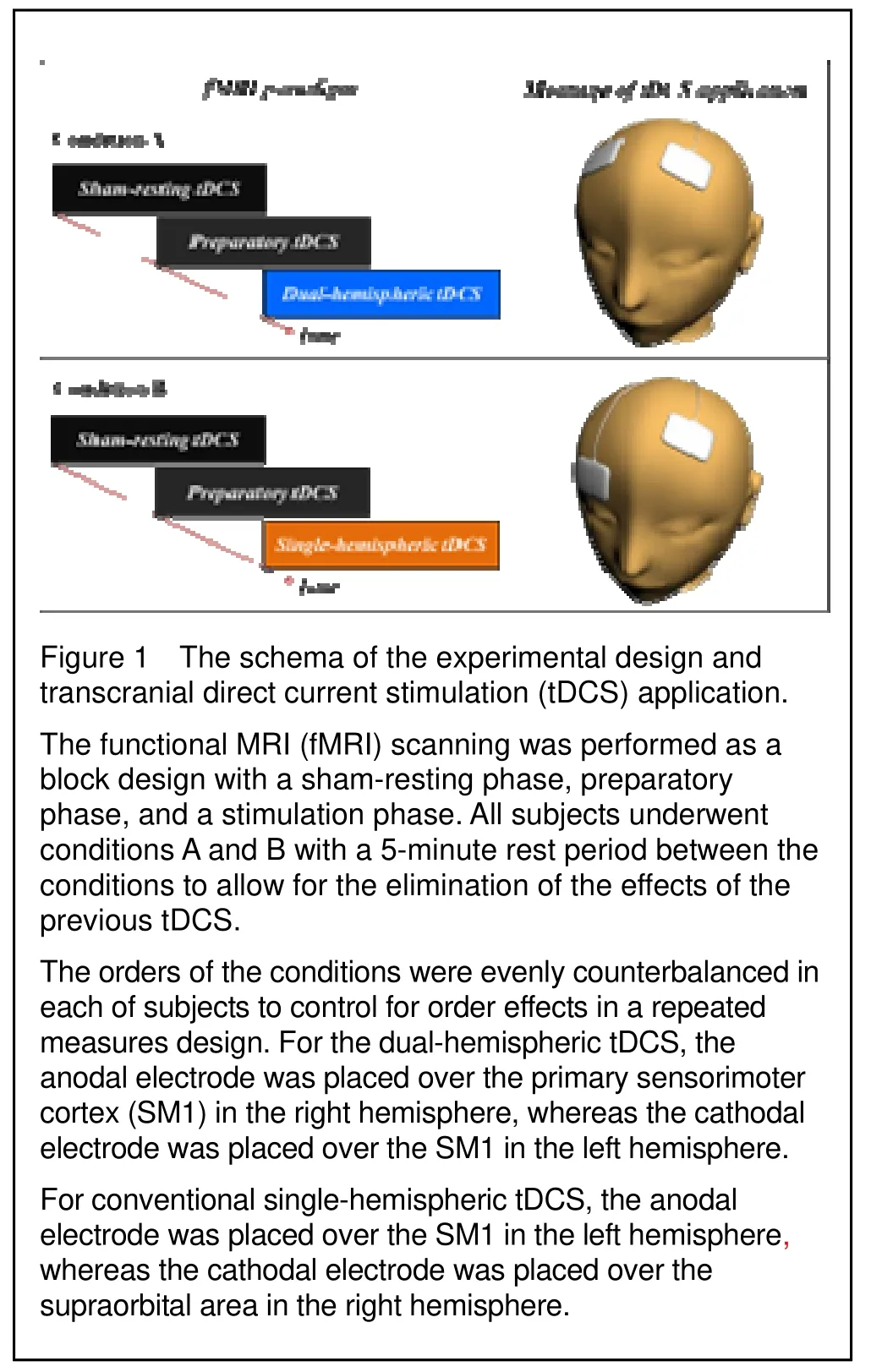
Experimental paradigm for transcranial direct current stimulation and functional MRI
The subjects were placed in a supine position with their eyes closed, and wore headphones for hearing protection. To prevent motion artifacts during functional MRI scans, movements of the head, trunk, and arms were restricted. The functional MRI paradigm consisted of two conditions (A and B) and was composed of a sham-resting phase, preparatory phase, and a transcranial direct current stimulation phase. Condition A was scanned in the following order: the sham-resting phase – the preparatory phase – the dual-hemispheric transcranial direct current stimulation phase. Condition B scanned in the following order: the sham-resting phase –the preparatory phase – the single-hemispheric transcranial direct current stimulation phase. All subjects underwent conditions A and B with a 5-minute rest period between the conditions to allow for the elimination of the effects of the previous transcranial direct current stimulation. The orders of the conditions were evenly counterbalanced in each subject to control for order effects in a repeated measure design, such as habituation and learning of the motor task, and to offset any remaining effects of the transcranial direct current stimulation. These factors have been well established in prior studies[7,29,31].
The functional MRI paradigm was conducted as a block design with a sham-resting phase, preparatory phase, and a stimulation phase. The sham-resting transcranial direct current stimulation phase lasted for 60 seconds, and served as the control phase for subtraction functional MRI analysis. The preparatory transcranial direct current stimulation cycle of 60 seconds was composed of a preparation period to reach the output of a stable direct current, and the data obtained were excluded from the final data analysis. Transcranial direct current stimulation stimulations included dual- and single-hemispheric transcranial direct current stimulation that lasted over 60 seconds each. The preparatory and stimulation phases were applied at a constant current of 1.0 mA for 2 minutes, with a ramp-up and down over the initial and final 3 seconds of the 60 second stimulation period, respectively. Finally, the sham-resting phase lasted 1 minute and included transcranial direct current stimulation at a current density of 0.029 mA/cm2for 2 minutes during the preparatory and stimulation phases. All subjects were instructed to notify our inspector when they felt any adverse effects such as headaches and nausea. In our experiments, the subjects did not complain of any adverse effects, with the exception of a mild itching sensation under the electrodes. Finally, to test region-specific effects for the stimulation phase, we subtracted the sham-rest phase from each of the transcranial direct current stimulation phases, including dual- and single-hemispheric transcranial direct current stimulation.
Functional MRI analysis
BOLD functional MRI and Echo Planar Imaging (EPI) technique were performed using a 1.5T MR scanner (Gyroscan Intera System, Phillips, Germany) with a standard head coil. For anatomic base images, 20 axial, 5-mm thick, T1-weighted, spin echo images were obtained with a matrix size of 256 × 205 and a field of view of 210 mm parallel to the bicommissural line of the anterior commissure-posterior commissure. EPI-BOLD images were acquired over identical 20 axial sections and 310 images per subject (including 10 dummy images) were produced. The imaging parameters used were: repetition time/echo time, 2.0 seconds/50 ms, field of view = 210 mm, matrix size = 64 × 64, and slice thickness = 5 mm. Functional MRI data analysis was performed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) running under the MATLAB environment (The Mathworks, USA). Functional data for each participant were motion-corrected. All images were realigned and normalized, and smoothed with an 8-mm isotropic Gaussian kernel. Statistical parametric maps were obtained, and voxels were considered significant at an uncorrectedP< 0.001 or family wise errorP< 0.05, depending on the individual threshold of direct current stimulation. We adopted the optimal significancePvalue that best depicted the cortical effect of transcranial direct current stimulation, and applied the samePvalue to the dual- and single-hemispheric transcranial direct current stimulation. Activations were defined as regions of five voxels. Regions of interest were drawn around the SM1 and right frontal areas. The SM1 regions of interest included the precentral and postcentral gyri centered on the precentral knob. Voxel counts were used to estimate the amount of cortical activation in response to transcranial direct current stimulation, because they reliably reflect cortical activations and changes in cerebral blood flow[32-33].
Statistical analysis
To compare voxel counts and BOLD signal intensities between dual-hemispheric transcranial direct current stimulation and single-hemispheric transcranial direct current stimulation, we analyzed these dependent variables using the Mann-Whitney test. Statistical analysis was conducted using PASW 18.0 software (SPSS, Chicago, IL, USA), and statistical significance was accepted atPvalues of < 0.05.
Funding: This work was supported by a National Research Foundation of Korea Grant funded by the Korean Government, No. 2009-0064682.
Author contributions:Yong Hyun Kwon designed this study and analyzed the experimental data. Yong Hyun Kwon and Sung Ho Jang wrote the paper. Sung Ho Jang contributed to the paper review and revision.
Conflicts of interest:None declared.
Ethical approval:The study protocol was approved by the Institutional Review Board of Yeungnam University Hospital, Republic of Korea.
[1] Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 Pt 3:633-639.
[2] Siebner HR, Lang N, Rizzo V, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379-3385.
[3] Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899-1901.
[4] Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276.
[5] Paulus W. Transcranial direct current stimulation (tDCS). Suppl Clin Neurophysiol. 2003;56:249-254.
[6] Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257-2260.
[7] Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206-223.
[8] Boggio PS, Castro LO, Savagim EA, et al. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404:232-236.
[9] Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708-712.
[10] Sparing R, Mottaghy FM. Noninvasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/tDCS)-From insights into human memory to therapy of its dysfunction. Methods. 2008;44:329-337.
[11] Stone DB, Tesche CD. Transcranial direct current stimulation modulates shifts in global/local attention. Neuroreport. 2009;20:1115-1119.
[12] Williams JA, Pascual-Leone A, Fregni F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther. 2010;90:398-410.
[13] Sparing R, Thimm M, Hesse MD, et al. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132: 3011-3020.
[14] Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17:671-674.
[15] Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103.
[16] Kwon YH, Ko MH, Ahn SH, et al. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett. 2008;435:56-59.
[17] Kwon YH, Nam KS, Lee MY, et al. The temporal change of cortical activation induced by the ongoing effects of transcranial direct current stimulation. J Phys Ther Sci. 2011;22:65-69.
[18] Antal A, Polania R, Schmidt-Samoa C, et al. Transcranial direct current stimulation over the primary motor cortex during fMRI. NeuroImage. 2011;55:590-596.
[19] Polania R, Paulus W, Antal A, et al. Introducing graph theory to track for neuroplastic alterations in the resting human brain: a transcranial direct current stimulation study. NeuroImage. 2011;54:2287-2296.
[20] Lang N, Siebner HR, Ward NS, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22:495-504.
[21] Sha N, Kenney LP, Heller BW, et al. A finite element model to identify electrode influence on current distribution in the skin. Artif Organs. 2008;32:639-643.
[22] Doheny EP, Caulfield BM, Minogue CM, et al. The effect of subcutaneous fat thickness on the efficacy of transcutaneous electrical stimulation. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5684-5687.
[23] Birlea SI, Birlea NM, Breen PP, et al. Identifying changes in human skin electrical properties because of long-term NeuroMuscular Electrical Stimulation. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:326-329.
[24] Kwon YH, Jang SH. The enhanced cortical activation induced by transcranial direct current stimulation during hand movements. Neurosci Lett. 2011;492:105-108.
[25] Jang SH, Ahn SH, Byun WM, et al. The effect of transcranial direct current stimulation on the cortical activation by motor task in the human brain: an fMRI study. Neurosci Lett. 2009;460:117-120.
[26] Bütefisch CM, Wessling M, Netz J, et al. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22(1):4-21.
[27] Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9: 97-113.
[28] Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt 1):141-157.
[29] Loubinoux I, Carel C, Alary F, et al. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test--retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2001;21:592-607.
[30] Vines BW, Nair D, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci. 2008;28(8):1667-1673.
[31] Hsu EW, Hedlund LW, MacFall JR. Functional MRI of the rat somatosensory cortex: effects of hyperventilation. Magn Reson Med. 1998;40:421-426.
[32] Ugurbil K, Ogawa S, Kim S, et al. Imaging Brain Activity Using Nuclear Spins. Amsterdam: IOS Press. 1999.
[33] Waldvogel D, van Gelderen P, Immisch I, et al. The variability of serial fMRI data: correlation between a visual and a motor task. Neuroreport. 2000;11:3843-3847.
Cite this article as:Neural Regen Res. 2012;7(24):1889-1894.
Yong Hyun Kwon☆, Ph.D., Assistant professor, Department of Physical Therapy, Yeungnam College of Science & Technology, Daegu 705-703, Republic of Korea
Sung Ho Jang, M.D., Professor, Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, 317-1, Daemyung 5-dong, Namgu, Daegu 705-717, Republic of Korea
strokerehab@hanmail.net
2012-03-15
2012-06-30
(NY20120315004/H)
Kwon YH, Jang SH.
Onsite-effects of dual-hemisphere versus conventional single-hemisphere transcranial direct current stimulation: a functional MRI study. Neural Regen Res. 2012;7(24):1889-1894.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.24.007
(Edited by Kisiel-Sajewjcz K, Huang F, Wang JL/ Song LP)
- 中国神经再生研究(英文版)的其它文章
- Imaging changes in neural circuits in patients with depression using 1H-magnetic resonance spectroscopy and diffusion tensor imaging☆
- Protective effect of insulin and glucose at different concentrations on penicillin-induced astrocyte death on the primer astroglial cell line☆●
- Changes of auditory evoked magnetic fields in patients after acute cerebral infarction using magnetoencephalography**☆
- Outcomes of early physiotherapy in patients with cerebral aneurysms treated by surgical clipping or endovascular embolization☆●
- No association between a polymorphism of the adenylate cyclase type IX gene and major depressive disorder in the Chinese Han population*☆

