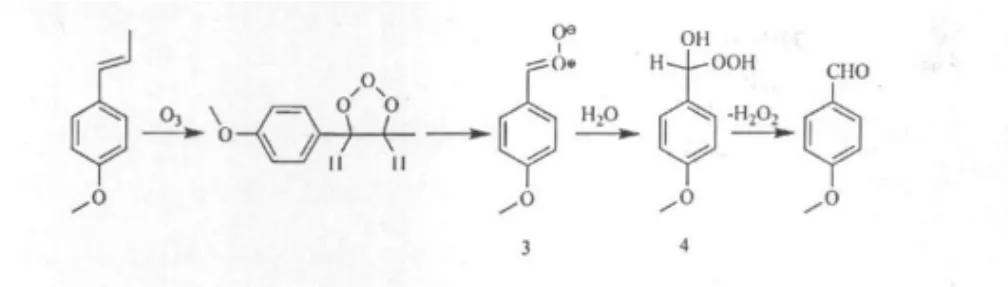
新体系下茴脑臭氧化反应制取茴香醛
于 静,沈敏敏,邓莲丽,甘 露,哈成勇
1中国科学院广州化学研究所纤维素化学重点实验室,广州510650;2中国科学院研究生院,北京100039
Introduction
Anisaldehyde is widely used as perfumes and essences,serving as an important role in chemical industry,food industry and cosmetics industry.[1-4]It is also an important intermediate of chemical products,drugs,pesticides and so forth.[5]It can be synthesized by oxidization of natural product essential oil anethole.In conventional methods,inorganic salts,such as chromium (VI),potassium permanganate and vanadium pentoxide-magnesium oxide,are usually used as oxidants. When these oxidants are used,anisic acid,which is the oxidation product of anisaldehyde,is usually produced as the main product.With these methods,the reactions are generally long and the yield of anisaldehyde is not high.[5-7]Furthermore,because of the side reactions and the use of acid and catalyst,these protocols produce significant quantities of chemical wastes.Ozonolysis reaction,[8-14]which has attracted many chemists’attention in recent years,can solve above problems.Gao et al reported a reaction to prepare anisaldehyde by the ozonolysis of anethole.[15]However,in order to obtain anisaldehyde with a yield of 73.6%,n-hexane/ethanol mixture was used as a solvent and sodium sulfite as a reductant,and the reaction temperature was 8.4℃.The reaction conditions were rigorous and the cost was high.Besides,a separate reduction step was needed.In this work,a solvent system composed of water and ethyl acetate was used,anisaldehyde was produced directly from the ozonolysis of anethole at low temperature avoiding the formation of ozonide.The ozonolysis reaction mechanism in this solvent system was investigated.Meanwhile,the influence of water/ethyl acetate mixture solvent on the ozonolysis of anethole was investigated using O3/O2as the oxidant.Moreover,other process parameters including solvent quantity,O3/O2flow rate,water content and reaction time were studied.The anisaldehyde produced in these reactions was monitored using gas chromatograph method.
Materials and Methods
Apparatus and testing conditions
GC spectra were recorded on a Shimadzu 2010 chromatograph with a FID(Flame Ionization Detector).The injection temperature was 250℃ and the column temperature was started from 50℃ to 260℃ at a rate of 10℃ per minute.The fuel gas of FID was hydrogen.The carrier gas of the chromatographic column was nitrogen.The air flow was 400 mL per minute,and the hydrogen 47 mL and the nitrogen 30 mL.1H NMR spectra were performed on a Bruker DRX-400 NMR spectrometer using TMS as the internal standard and CDCl3as the solvent.Infrared(IR)spectra were measured with an Analect RFX-65 spectrometer.MS experiment was carried out on a Shimadzu QP-2010A spectrometer with an ion source temperature at 200℃.
Preparation of anisaldehyde
All reagents were purchased from commercial sources and used as received without further purification.The anethole substrate 20 g was placed in a bubble column with water-jacketed condenser and dissolved in 15∶85 water/ethyl acetate(wt/wt).The solution was maintained at room temperature and a stream of O3/O2(0.06 m3·h-1,O3/O2ratio approximately 6%in mass fraction)was bubbled into the reaction solution through a bubbling pipe.A sample was taken out from the reactor at certain scheduled time using an injector and was analyzed by gas chromatography.When the reaction time was up,the ozonizer voltage was set to zero.Nitrogen was passed through the solution until the blue color was discharged.The crude reaction mixture was diluted with 50 mL ethyl acetate and 10 mL distilled water.The separated aqueous layer was extracted twice with 10 mL of ethyl acetate.The organic fractions were dehydrated with anhydrous sodium sulfite.The solvent of organic layers was evaparated under reduced pressure.Anisaldehyde product was obtained in a yield of 81.7%(16.4 g)with 99.5%purity.
Results and Discussion
Spectral data of anisaldehyde
Anisaldehyde:Yield:81.7%;purity 99.5%;colorless liquid;1H NMR(400MHz,CDCl3)δ:3.87(3H,s,CH3-O),6.98~7.82(4H,m,Ar-H),9.87(1H,s,CHO);IR(neat)νmax3076,2968,2842,2742,1685,1600,1512,1462,1261,1028,835 cm-1;MS(m/z,(rel int,%)):136([M]+,75),135(100),119 (2),107(19),92(21),77(38),65(12),64 (11),63(11),51(11),50(9),39(13),27(2).The data were consistent with those of anisaldehyde[17].
Comparison with traditional solvent systems
Five traditional solvent systems reported and four water/organic solvent systems were investigated for their influence to the yield of anisaldehyde.The mass fraction of water in water/organic solvent was 5%.When m (anethole)∶m(solvent)was 1∶3 and O3/O2flow rate was 0.04 m3/h,the yields of the anisaldehyde were varied in different solvent systems within 60 min reaction time.The results were shown in Table 1.
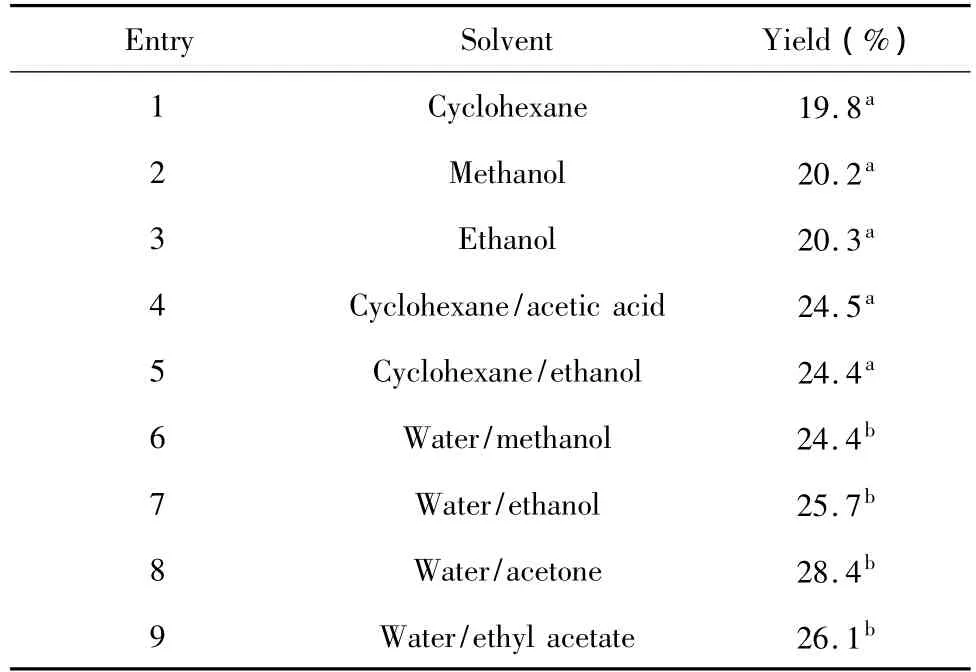
Table 1 Yield of anisaldehyde in different solvent systems
It was observed that the yield of anisaldehyde in water/ organic solvent system was obviously higher than those in traditional solvent systems.For the traditional solvent systems,the yields increased while the solvent polarity increasing.Because ozonide had good solubility in polar solvent,and then the dimer and polymer reactions were largely decreased.For water/organic solvent systems,ozone had good water solubility,and anethole and ozonide also had good solubility in organic solvent.So that anethole and ozone could react to produce ozonide in this solvent system.Besides,the addition of water to ozonide was believed to generate a gem-hydroperoxy alcohol,which could be decomposed to liberate anisaldehyde at room temperature.However,the yields of anisaldehyde in water/methanol and water/ethanol are slightly lower than those in water/acetone and water/ ethyl acetate.The reason was that methanol or ethanol could react with ozonide to generate hydroperoxyacetals.A separation step to obtain anisaldehyde was necessary.When water/acetone was used as solvent,the solution losing was large since acetone had a low boiling point.Therefore,part of the anethole was lost in the ozonolysis process.The results suggested that water/ ethyl acetate was the suitable solvent system for ozonolysis reaction of anethole.
Influence of water/ethyl acetate solvent quantity
The less solvent,the less ozone was dissolved in the solvent,which would lead to the decrease of anisaldehyde yield.However,a large solvent quantity would lead to a high cost and a long reaction time.In order to determine the optimum solvent quantity,the mass ratio of anethole and water/ethyl acetate solvent was studied (Table 2).
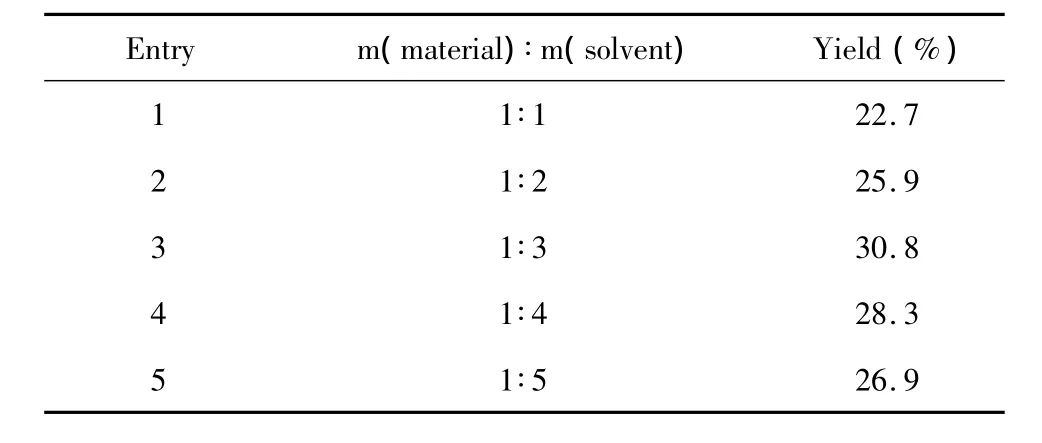
Table 2 Yield of anisaldehyde v.s.mass ratio of material and solvent
Table 2 shows the yields of anisaldehyde with different mass rations of anethole and water/ethyl acetate(water mass fraction was 5%)solvent from 1∶1 to 1∶5.The yield was low when the solvent too less,because more material was taken away by gas flow.The carboxyl oxide could be dimerized or polymerized easily.But the yield was low if too much solvent was used,because the efficiency of the ozone reacting with material was decreased.When the mass ration of anethole and water/ ethyl acetate solvent was 1∶3,a highest yield of anisaldehyde was achieved.Therefore,the mass ration of 1∶3 was selected in the following studies.
Influence of the O3/O2flow rate
The O3/O2flow rate was closely related to the quantity of ozone bubbling into the solution per hour.It was also related to the losing of solution taken away by O3/O2flow including the anethole and solvent.Therefore,it directly influenced the yield of anisaldehyde.The influence of O3/O2flow rate on the ozonolysis of anethole in the water/ethyl acetate solvent was investigated.The reaction time was 60 min.The results were summarized in Fig.1.

Fig.1 Influence of the O3/O2flow rate on the reaction
In Fig.1,as the O3/O2flow rate was increased from 0.04 to 0.07 m3/h,the anisaldehyde yield increased from 30.9%to 58.8%.However,when the O3/O2flow rate reached 0.08 m3/h,the anisaldehyde yield fell to 52.2%.The reason might be,with a large O3/O2flow bubbled into the reaction solution,the anethole in the solution was oxidized completely and some anisaldehyde products were peroxidized to anisic acid in 60 min.Furthermore,the losing of anethole and oxidized anethole show a sharp increase when the O3/O2flow rate was 0.07 m3/h.It could be explained as a large proportion of the solution including anethole was taken away by the large gas flow.The results demonstrated that the suitable rate of the O3/O2flow was 0.06 m3/h in this reaction.Under this condition,the anisaldehyde yield was comparatively high and the solution losing including anethole and water/ethyl acetate solvent was comparatively low.
Influence of water content in the water/ethyl acetate solvent
The influence of water content in the water/ethyl acetate solvent on the yield of anisaldehyde was showed in Table 3.The content of water in the water/ethyl acetate solvent affected the solubility of anethole.It was found that when the content of water in the mixture solvent exceeded 25%,the reaction solution became turbid.But,too less water would influence the yield of anisaldehyde since water played a role as a reagent in this reaction.In the present study,the anisaldehyde yield was investigated as the water content in mixture from 5%to 25%(Table 3).
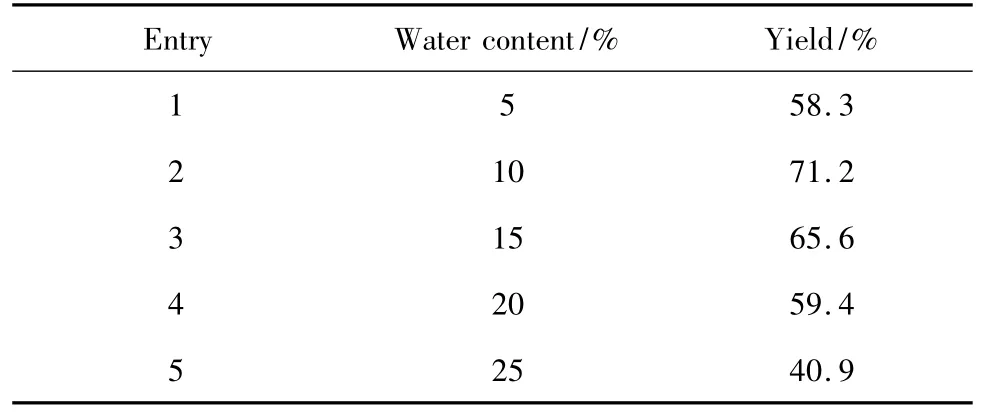
Table 3 Yield of anisaldehyde under different content of water in the mixture solvent
The results showed that the yield of anisaldehyde increases sharply as the content of water in the mixture solvent increased from 5%to 10%.When the content of water in the mixture solvent continuously increases from 10% to 25%,the yield gently decreased to 40.9%.By analyzing reaction mechanism and observing reaction phenomenon,we believed that water involved in the reaction with ozonides.Besides,when the water content increased,the solubility of anethole decreased.As a result,the reaction between anethole and ozone was affected.So,the optimum water content was 10%in the reaction solution.
Influence of reaction time
The reaction time would be another factor to affect the yield of anisaldehyde because it would be a decisive factor to the quantity of ozone in the reaction.Monitoring by the gas chromatograph,the yield of anisaldehyde was studied with different the reaction times from 20 min to 100 min.The results were shown in Fig.2.
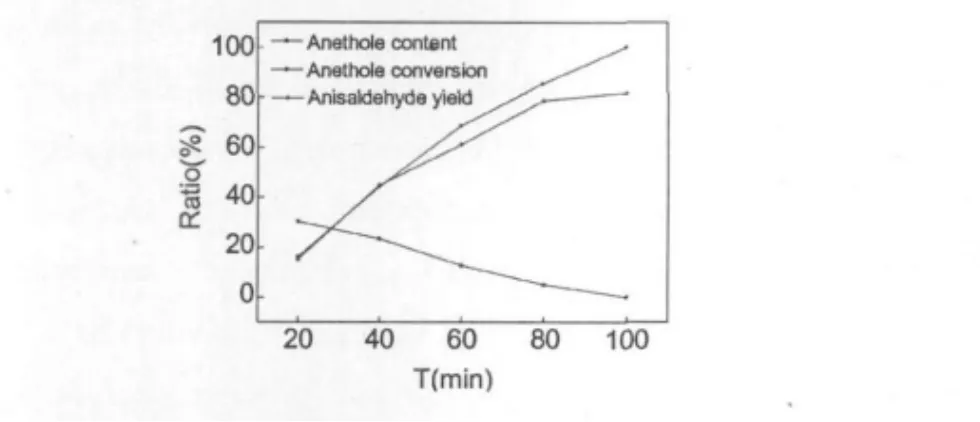
Fig.2 Influence of reaction time to the reaction
In Fig.2,it was found that the content of anethole in reaction solution decreased,while the reaction time increasing.When the reaction time reached at 100 min,the anethole in reaction solution was fully converted into anisaldehyde,and the yield of anisaldehyde reached the maximum 81.7%.
Reaction mechanism test and verify
According to the scheme 1,there was H2O2produced in the anethole ozonolysis reaction.H2O2could react with sulfur cyanogen ferrous to produce the thiocyanate iron complex,which had an obvious absorption at 475 nm.[18]
To testify above mechanism,ozonolysis was performed until the anethole was consumed.Product was isolated after extraction with no separate reduction.FTIR of this product showed no strong absorption at ca.1080-1110 cm-1,characteristic peak of the ozonides.On the contrary,there was an obviously carbonyl peak of aromatic aldehyde at 1685 cm-1.This just meant that no ozonide existed in the product,but anisaldehyde was produced directly.A more qualitative postreaction assay,performed on UV spectrum,tested the H2O2produced in ozonolysis of anethole in water/ethyl acetate mixture solvent.The water/ethyl acetate solvent and anethole/ water/ethyl acetate solution were ozonolysed respectively for 1 h.The nitrogen was bubbled to carry out the dissolved ozone.Some sulfur cyanogen ferrous was add-ed in above production respectively.Take some samples and detected on UV spectrum from 350 nm to 600 nm.If H2O2was existed in above system,an obvious absorption could be detected at 475 nm[18].The results were shown in Fig.3.
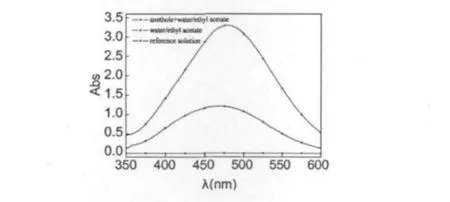
Fig.3 Analysis of H2O2generated in ozonolysis reaction
In Fig.3,the ultraviolet absorption curves are presented.The absorption value of reference solution was zero.The water/ethyl acetate mixture solvent oxidized by ozone for 60 min had a low absorption value.However,the anethole and water/ethyl acetate solution oxidized by ozone for 60 min had a high absorption value.It could be drawn that,in water/ethyl acetate solvent,ozone reacted with water to produce a small quantity of H2O2.In contrast,in anethole and water/ethyl acetate solution,ozone firstly reacted with anethole to generate carbonyl oxide 3,and then carbonyl oxide 3 reacts with water to produce gem-hydroperoxy alcohol 4,which decomposes with liberation of theanisaldehydeand H2O2.But the H2O2would be decomposed by ozone to H2O and O2,so that the anisaldehyde could not be oxidized to anisic acid.
Conclusions
A fast,convenient,and efficient one-pot synthesis method of anisaldehyde by ozonolysis of anethole without separation step was established.The optimal reaction conditions were as follows:water/ethyl acetate(10∶90,w/w)solution(mass ratio of anethole to solvent was 1∶3),O3/O2flow rate at 0.06 m3/h,the reaction carried out at room temperature for 100 min.The yield of anisaldehyde could reach 81.7%.
1 Kubo I,Kinst-Hori I.Tyrosinase inhibitors from anise oil.J Agric Food Chem,1998,46:1268-1271.
2 Nitoda T,Fan MD,Kubo I.Anisaldehyde,a melanogenesis potentiator.J Biosci,2007,62:143-149.
3 Cai J,Nie Q.Study on synthesis of anisaldehyde.Chin J Flavor Fragr Cosm,2005,2:21-22.
4 Shiseido Co.Ltd.Skin color improvement fragrance composition for use in cosmetics contains anisaldehyde as active ingredient.JP2004175951-A,2004-06-24.
5 Lin JC,Qiu L,Zhang LJ,et al.Studies on inhibitory effect of anisaldehyde and anisic acid on mushroom tyrosinase.Xiamen Daxue Xuebao(Ziran Kexue Ban),2007,46:254-257.
6 Tzedakis T,Savall A.Electrochemical regeneration of Ce (IV)for oxidation of p-methoxytoluene.J Appl Electrochem,1997,27:589-597.
7 Xiao Y,Huang HM,Yin DL,et al.Oxidation of anethole with hydrogen peroxide catalyzed by oxovanadium aromatic carboxylate complexes.Catal Commun,2008,10:29-32.
8 Elgendya EM,Khayyat SA.Oxidation reactions of some natural volatile aromatic compounds:anethole and eugenol.Rus J Org Chem,2008,44:823-829.
9 Bunnelle WH.Preparation,properties,and reactions of carbonyl oxides.Chem Rev,1991,91:335-362.
10 Kukovinets OS,Zvereva TI,Kabalnova NN,et al.Ozonolysis of verbenone in aprotic solvents.Mendeleev Commun,2009,19:106-107.
11 Criegee R.Mechanism of ozonolysis.Angew Chem Int Ed,1975,14:745-752.
12 Cremer D,Crehuet R,Anglada J.The ozonolysis of acetylene a quantum chemical investigation.J Am Chem Soc,2001,23: 6127-6141.
13 Kuczkowski RL.The structure and mechanism of formation of ozonides.Chem Soc Rev,1992,21:79-83.
14 Selcuki C,Aviyente V,Aplincourt P,et al.Effect of solvation on ozonolysis reaction intermediates and transition states.J Mol Model,2000,6:608-617.
15 Alam SH,Myeong YC,Keith TK,et al.Reaction of criegee intermediates with water vapor an additional source of OH radicals in alkene ozonolysis.J Phy Chem A,2003,107: 6176-6182.
16 Gao R,Li WH,Li D,et al.Preparation of anisaldehyde from star anise oil by ozonolysis reaction.Food Sci,2008,29:174-177.
17 SDBS Compounds and Spectral Search,http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi.
18 Liu JB,Zeng H,Liu XR.Determination of content of hydrogen peroxide with spectrophotometric method.J Jilin Teachers Coll,1997,18:69-70.
——李振声

