新型高耐热性环氧树脂的合成和表征
许元花, 罗 梦, 彭 桦, 王 曦, 苏胜培*
(1. 湖南省资源精细化与先进材料重点实验室,湖南师范大学,中国 长沙 410081;2. 湖南大学化学化工学院,中国 长沙 410081)
Epoxy resins have been extensively used in many applications, such as coatings, adhesives, composite materials and semiconductor encapsulation, because of their excellent electrical and chemical resistance, good mechanical properties, low shrinkage during curing, and great adhesion properties[1]. It is well acknowledged that diglycidyl ether of bisphenol A(DGEBA) is a kind of important versatile epoxy resin, which is accounted for 80% of the total thermosetting resins[2]. However, the conventional DGEBA was seriously confined in the applications such as integrated circuit packaging and advanced materials where high thermal and moisture resistance are required[3]. Therefore, the development of high thermal resistant and low moisture absorption epoxy resins for these applications has attracted a lot of interest from many researchers.
Many scientific efforts have been reported for improving the thermal resistance of epoxy resins by changing the structures of the backbone of resins, including increasing the crosslinking density of cured epoxy resin[4-7], introducing bulky and rigid structures such as biphenyl or naphthalene[8-9]and forming imide epoxy resin[10-11]. Panetal[12]have synthesized multifunctional epoxy resin from p-hydroxybenzaldehyde and bisphenol A, which showed higher glass transition temperature (Tg) and thermal stability than those of epoxy resin formed from benzaldehyde and bisphenol A. Duannetal[13]described that the thermal stability orTgof naphthalene-based epoxy resins with stronger rigid structure was higher than that of phenyl-based epoxy resins. Chinetal[14]have synthesized a novel curing agent bearing diimide-diacid structure; the cured epoxy resin has higherTgand thermal stability due to the introduction of imide structure compared with the products cured by phthalic anhydride. In general, to improve moisture resistance, hydrophobic groups were introduced to epoxy structure to decrease the hydrophilic sites such as polar groups. Renetal[15]have synthesized a novel novolac epoxy curing agent containing both naphthalene and dicyclopentadiene (DCPD) moiety; and the water absorption of obtained cured products could decrease to 0.66 % due to the introduction of hydrophobic aliphatic DCPD and naphthyl structure. Taoetal[16]proposed that a siloxane-containing cycloaliphatic epoxy compound, which has low polarity and hydrophobic nature, has low water absorption down to 0.48 %.
1,1-Bis(4-hydroxy-3-methylphenyl)cyclohexane (DMBPC) is a bisphenol-type compound. It has been applied in the synthesis of polycarbonate with improved water vapor transmission barrier properties[17], however, there is no report on its application in the epoxy resin. In this study, DMBPC will be used to replace bisphenol A in the synthesis of epoxy resin. It is expected that the rigid cyclic moieties and benzene rings in DMBPC would afford the novel epoxy resin with higher thermal properties and better water resistance due to the presence of the hydrophobic cyclohexyl moiety in the epoxy structure. The cure behaviors, thermal and water resistant properties of this novel epoxy resin will be explored and comparison experiments between this novel epoxy and the conventional DGEBA will be also performed.
1 Experimental
1.1 Materials
1, 1-Bis(4-hydroxy-3-methylphenyl)cyclohexane (DMBPC) was commercial grade and provided by Hunan Hongyue manufactory Co., Ltd. (Yueyang, China). Epichlorohydrin (ECH) and DGEBA with an epoxy value of 0.44 mol/100 g were purchased from Baling Petrochemical Co., Ltd. (Yueyang, China). The hardener, 4,4′-diaminodiphenylmethane (DDM), was purchased from Honghu BMI Resin Factory (Hubei, China). Sodium hydroxide was purchased from Huihong Chemical Reagent Co., Ltd (Changsha, China). Trimethyl benzyl ammonium chloride (TMBAC) was prepared from timethyl amine and benzyl chloride. All the agents were used without further purification.
1.2 Synthesis of 1, 1-bis(4-hydroxy-3-methylphenyl)cyclohexane epoxy resin[18-19]
88.8 g DMBPC (0.3 mol), 555 g ECH (6 mol) and 1.48 g TMBAC (7.5 mmol) was added to 1000 mL four-neck round-bottom flask, equipped with mechanical stirrer, thermometer, and a reflux condense 8. The mixture was stirred at 80 ℃ for 6 h, followed by adding 120 g sodium hydroxide aqueous solution (30 wt%) dropwise within 2 h at 70 ℃, and then the temperature of the system was maintained at 70 ℃ for an additional 3 h allowing the reaction to complete. After the completion of reaction, sodium chloride generated during the reaction was filtrated and the brine layer was removed. Finally, the product in the flask was washed with deionized water until no chloride ion could be detected by acidic AgNO3aqueous solution. After the excess epichlorohydrin was evaporated, a white solid product was obtained in 93.6% yield, epoxy value: 0.47 mol/100 g. The 1,1-bis(4-hydroxy-3-methylphenyl)cyclohexane epoxy resin (DMBPCEP) was prepared as shown in Scheme 1.
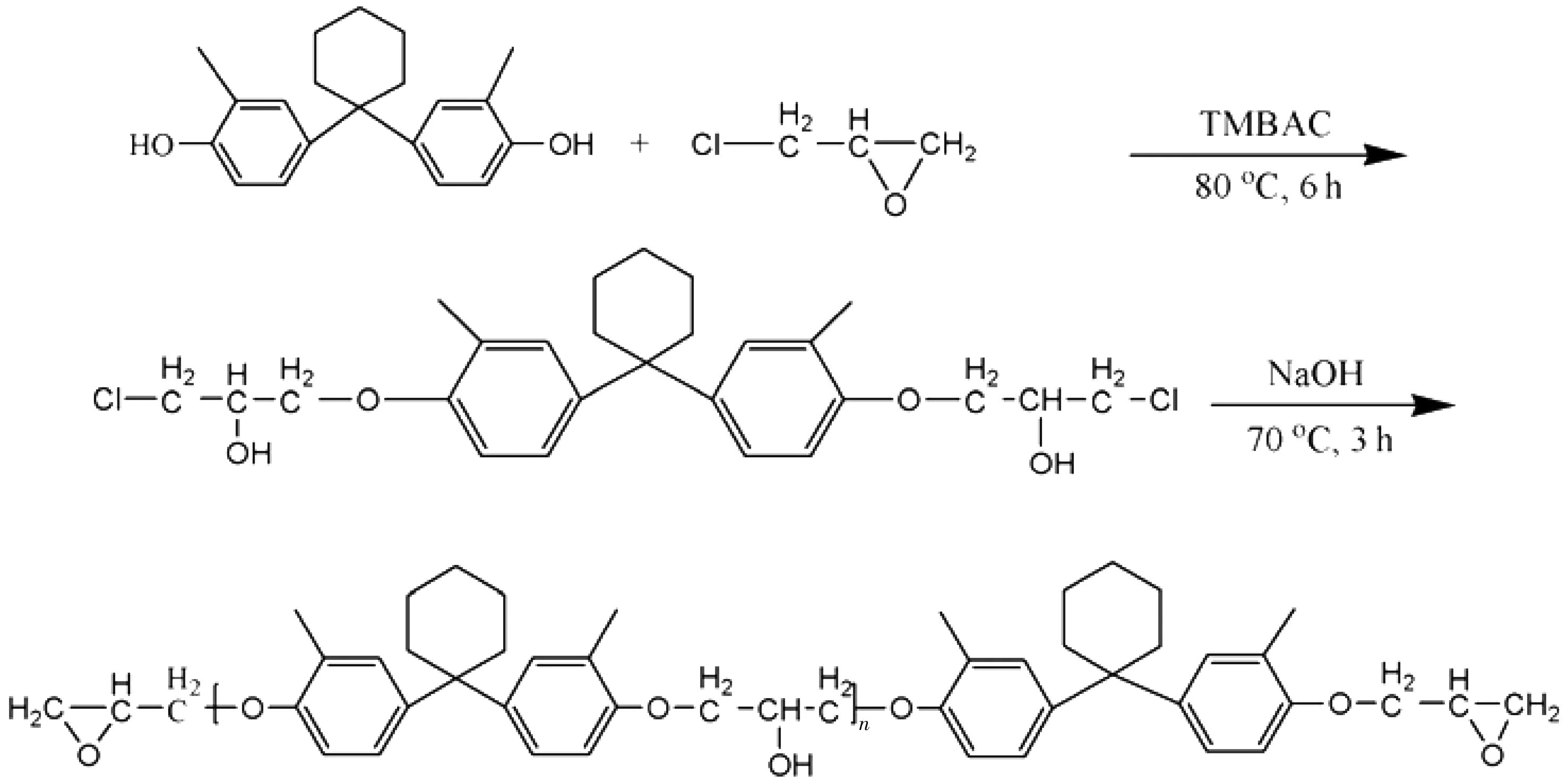
Scheme 1 Preparation of DMBPCEP
1.3 Instrumentation
FT-IR spectra were obtained using a WQF-200 instrument (Beijing, China) using conventional KBr pellets. A mixture of 1 mg of sample powder with 100 mg of dried KBr crystals was pressed into a pellet of 13 mm in diameter.1H NMR spectra were collected using a Varian INOVA-300FT-NMR spectrometer (Pola Alto, America) operating at 500 MHz using CD3COCD3as solvent. The epoxy value of obtained epoxy resin was determined by HCl/acetone titration method[20]. Curing behaviors of epoxy resin were performed on a Netzsch DSC200F3 at heating rate of 2.5, 5.0, 10, and 15 ℃/min.Tgof cured products were also characterized on the DSC fitted with a liquid nitrogen cooling system. Samples (8~12 mg) were ground to be powders, and then encapsulated in the aluminum pans. Two consecutive heating and cooling runs were performed using 10 ℃/min heating and cooling rates respectively. TGA experiments were conducted from 30 to 800 ℃ and 20 ℃/min scan rate on a Netzsch STA409PC instrument under nitrogen atmosphere with a 20 mL/min flow speed. TGA results are averages of a minimum of three determinations; temperatures are reproducible to ±1 ℃, while the error bars on the non-volatile material are ±1 %. Moisture absorption was determined as follows: the samples (50 mm × 15 mm × 6 mm) were dried under vacuum at 100 ℃ for 12 h, and then cooled to room temperature. The samples were weighed and placed in 100 ℃ water for certain hours and weighed again until the equilibrium water uptake was reached. The moisture absorption was calculated as follows: Moisture absorbance % = ((m-m0)/m0) × 100%, wheremis the weight of the sample after dipping in 100 ℃ water for certain hours,m0is the weight of the sample after placing in vacuum oven for 12 h.
2 Results and Discussions
2.1 Structure analysis of DMBPCEP
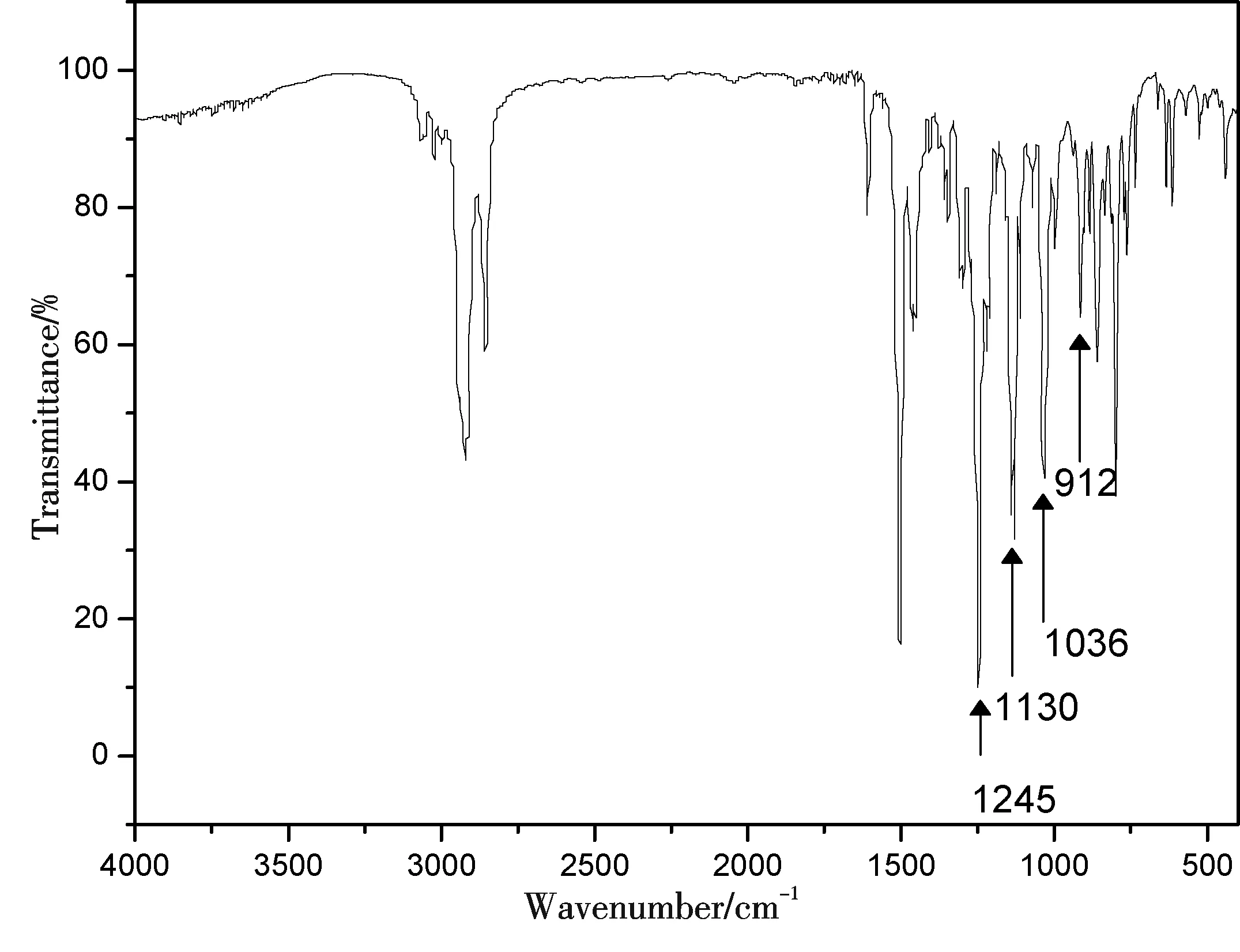
Figure 1 FT-IR spectrum of DMBPCEP
The FT-IR spectrum of DMBPCEP was shown in Fig. 1. As shown in Fig. 1, the bands at 912 cm-1and 1 130 cm-1were attributed to the stretching vibration of the oxirane ring and cyclohexyl respectively, which were the characteristic groups in the structure of DMBPCEP[21]. Besides, the absorption bands at 2 970~2 810 cm-1, which were attributed to the symmetric and asymmetric stretching vibration of methyl and methlene, the bands at 1 604 cm-1, 1 503 cm-1, and 1 455 cm-1, which were attributed to the stretching vibration of benzene rings, the bands at 1 245 cm-1and 1 036 cm-1, which were attributed to the symmetric and asymmetric stretching vibration of phenyloxyl, were all observed. It is surprisingly noted that no bands of hydroxyl were observed in the FT-IR spectrum. This can be explained from the average molecular weight and the value ofn. It is well known that the relationship betweennand epoxy value of DMBPCEP could be described as follows in theory: Epoxy value = 2×100/[407+352n]. The value ofncalculated was 0.05. This demonstrated that the amount of hydroxyl group was low, which could be ignorable and no bands near 3 500 cm-1in FT-IR spectrum were observed.1H NMR data of DMBPCEP was also used to characterize the chemical structure of the synthesized resin. The data was as follows (CD3COCD3;δ, ppm): 7.04~7.01 (m, 4H), 6.79 (d,J=8.5 Hz, 2H), 4.81~4.77 (m, 2H), 4.26~4.23 (m, 2H), 3.32~3.29 (m, 2H), 2.82 (d,J= 4.5 Hz, 2H), 2.71~2.69 (m, 2H), 2.16 (s, 4H), 2.07 (s, 6H), 1.43 (s, 6H). The FT-IR analysis and1H NMR results indicated that DMBPCEP was obtained.
2.2 Calculation of curing kinetics parameters of DMPCEP/DDM system

Figure 2 DSC curves of DMBPCEP/DDM at different heating rates
The cure of epoxy resins in which monomers or prepolymers with low molecular weight are incorporated into three-dimensional networks is a complex mechanism including many reactions. The kinetics of the curing reaction plays a crucial role in the formation of network structure, which dictates the physical and mechanical properties of the cured product[22]. In this work, DSC was chosen to monitor the curing behaviors of epoxy resin through the direct measurement of the heat flow which was considered to be proportional to the consumption extent of the reactive groups. For the DMBPCEP/DDM system, DSC curves at different heating rates were shown in the Fig. 2. The information obtained directly from the DSC curves such as the onset temperature (Ti), the peak temperature (TP), the terminal temperature (Tf), and reaction enthalpy (ΔH) were summarized in Table 1. It can be noted that DSC scans showed a wide exothermic peak at all heating rates. The curing temperature is shifted to the higher temperature with the increasing of heating rate due to thermal hysteresis[23].

Table 1 Characteristic temperature and total reaction enthalpy at different heating rates
To evaluate the kinetic parameters of DMPCEP/DDM system, Kissinger[24]and Crane methods[25]were applied to calculate activation energy and curing reaction order without the need of any assumption about conversion-dependent equation. Kissinger’s equation can be expressed as follows
(1)
Crane’s equation is depicted as Equation (2)
d (lnβ)/d (1/Tp)= -[ΔEa/nR+ 2Tp]
(2)
In the case of ΔEa/nR≫2Tp, Equation (2) could be presented as Equation (3)
d (lnβ)/d (1/Tp)= -ΔEa/nR
(3)


Figure 3 Plot of ln versus ( 1/Tp)

Figure 4 Plot of ln β versus (1/Tp)
2.3 Determination of the optimal curing process
An optimal curing process is determined by the curing kinetics and the curing mechanism. Generally, extrapolation method[28]based onT-βwas commonly employed to determine the manufacturing process. The line relationship betweenTandβwas descried as follows:T=A + Bβ. The fitting lines ofT(Ti,Tp,Tf) versusβwere shown in the Fig.5. If the value ofβwas equal to zero, the curing reaction would occur at isothermal temperature theoretically and the curing temperature of the pre-curing, curing, post-curing could be obtained for the curing process. They were 115.5, 142.1, and 174.4 ℃ respectively with corresponding linear correlations of 0.955, 0.965, and 0.975 which could be confirmed that the data were reasonable to the manufacturing process. Considering DSC experimental results, the curing reaction started in the range of 120~140 ℃ at different heating rates. Once the polymerization was initiated, the curing would be completed quickly at high temperatures. To prevent the occurrence of explosive polymerization, the curing process should be designed as slow heating rate or isothermal process for hours. Therefore, in this study, after DMBPCEP and melted DDM were mixed at 95 ℃ and degassed under vacuum, samples were cured following the sequence below: (a) 115 ℃ for 1 h; (b) 140 ℃ for 1 h; and (c) a post-cure temperature of 180 ℃ for 3 h.
2.3 Thermal and water resistance properties of cured DMBPCEP/DDM
2.3.1Tgand HDT
Tgwas generally used as a parameter of heat-resistance for polymer[29]. Fig. 6 showedTgtraces obtained from cured DMBPCEP/DDM and DGEBA/DDM. It can be seen thatTgof DMBPCEP/DDM was 171.9 ℃, significantly higher than 150.1 ℃ of the DGEBA/DDM. This improvement could be attributed to the rigidity increase and their ability to hinder the motion of the molecular chains and network junctions due to the introduction of cyclohexane structure in the cross-linking network. For the heat distort temperature (HDT), another important heat-resistant parameter, the difference between DMBPCEP and DGEBA were consistent with the change trend of the glass transition temperature. HDT of cured DMBPCEP and DGEBA was 160.7 ℃ and 147.0 ℃, respectively.
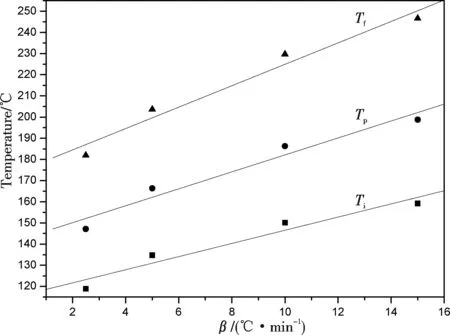
Figure 5 Relationship between β and Ti, Tp, Tf in the curing process of DMBPCEP/DDM

Figure 6 DSC traces obtained from the cured DMBPCEP/DDM and DGEBA/DDM
2.3.2 Thermal stability
The weight loss of the cured DMBPCEP resins at high temperature was shown in Fig. 7 and thus was compared with that of conventional DGEBA. TGA curves of both cured DMBPCEP and DGEBA products displayed one-step degradation. It can be seen that the 10 % weight loss temperature (T10) of cured DMBPCEP, a measure of the onset degradation, and the 50 % weight loss temperature (T50), the midpoint of the degradation process, was 377.6 ℃ and 410.6 ℃, respectively, which is comparable to DGEBA.
2.3.3 Water Resistance
It is well known that absorbed moisture has a detrimental effect, especially at elevated temperature, on the mechanical properties and electrical insulating performance of epoxy resins[30]. Therefore, moisture absorption is an important parameter to evaluate whether epoxy resin has high performance or not. Fig. 8 showed the moisture uptake of the cured products with the varying of time. It is noted that the moisture uptake of both cured DMBPCEP/DDM and DGEBA/DDM increased sharply in the initial stage of the absorption process, and then the increasing rate slowed down after 10 h, and finally lead to a plateau, corresponding to the water uptake at equilibrium. However, about 0.33 wt% of water uptake at equilibrium of the cured DMBPCEP/DDM was observed, which was 3 times lower than that of DGEBA/DDM. This improved moisture resistance of DMBPCEP/DDM was due to the hydrophobic nature of the cyclohexane rings compared with that of DGEBA/DDM. Meanwhile, cyclohexane rings in the network could be fused strain-free chair-form structure and essentially has no configuration strain. This could be effectively protected from the formation of cracking in the products, which hindered the diffusion of water[31].

Figure 7 TGA traces obtained from the cured DMBPCEP/DDM and DGEBA/DDM
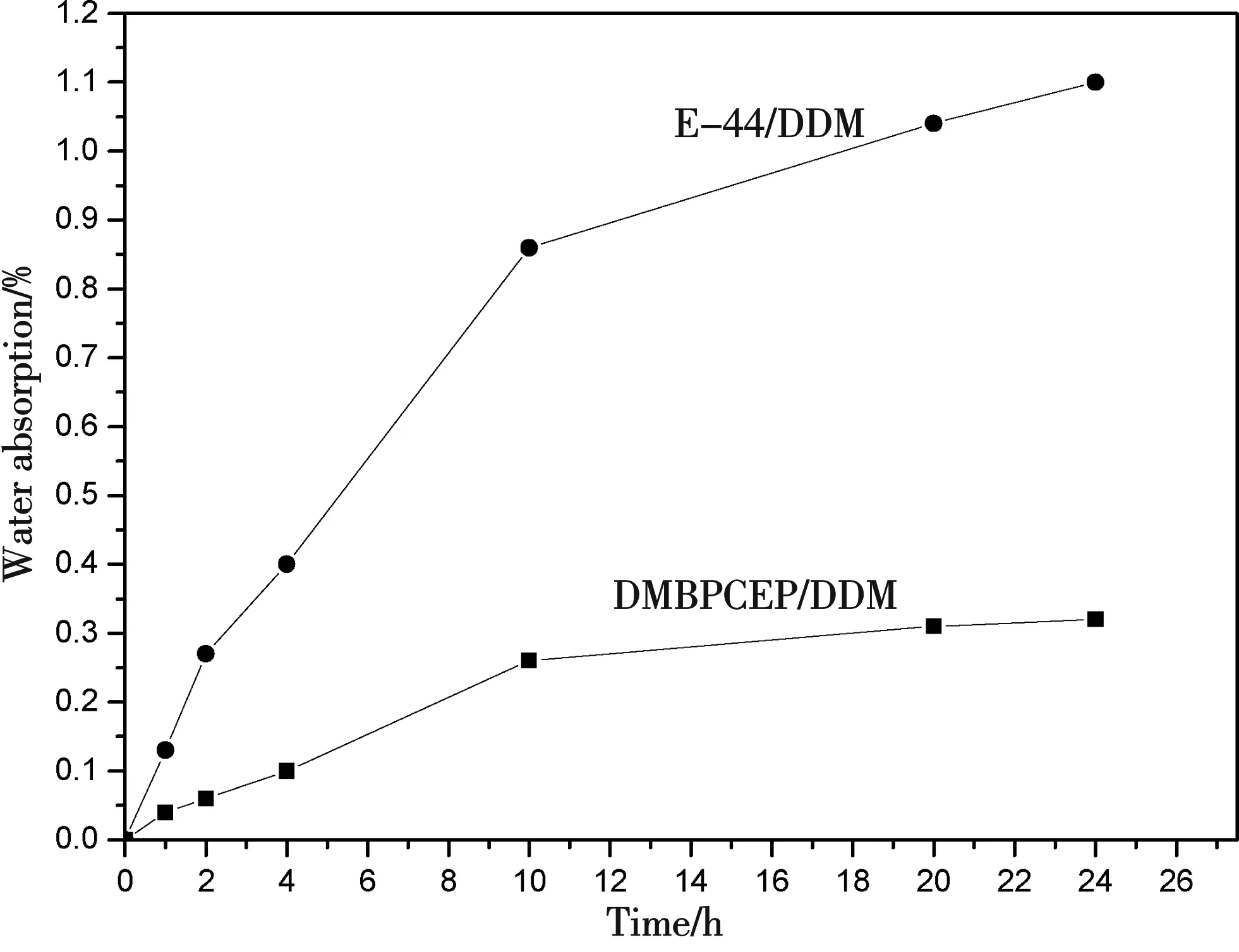
Figure 8 Water absorption curves of cured epoxy resin at 100 ℃
3 Conclusions
A novel bisphenol epoxy resin prepolymer containing cyclohexyl group was synthesized from DMBPC and ECH. The reactivity and curing behaviors of this resin with DDM as curing agent was comparable with that of conventional DGEBA.Tg, HDT, high-temperature stability, and water resistance for DMBPCEP/DDM resin system were all superior to conventional DGEBA/DDM system, which are attributed to the introduction of cyclohexyl units in the bisphenol structure. Evidences from experiments indicated that DMBPCEP could be a potential candidate of high heat-resistant and low water absorption resins.
[1] ZHANG X H, ZHANG Z H, XIA X N,etal. Synthesis and characterization of a novel cycloaliphatic epoxy resin starting from dicyclopentadiene [J]. Eur Polym J, 2007, 43(5): 2149-2154.
[2] WU L Y, SUN M L. Principle, technology and applications for epoxy resin [M].Beijing:China Machine Press, 2002.
[3] LEE J R, PARK S J, LEE S B. Method for preparing a high heat resistant epoxy resin composition comprising quinoxalinium salt containing benzyl group:US Patent, 6133383[P]. 2000-10-07.
[4] MUSTATA F, BICU I. Synthesis, characterization, and properties of multifunctional epoxy maleimide resins[J]. Macromol Mater Eng, 2006, 291(6): 732-741.
[5] MUSTATA F, BICU I. Multifunctional formaldehyde resins as curing agent for epoxy resins [J]. J Appl Polym Sci, 2010, 115(3): 1787-1796.
[6] WANG C S, LEE M C. Synthesis, Characterization, and properties of multifunctional naphthalene-containing epoxy resins cured with cyanate ester [J]. J Appl Polym Sci, 1999, 73(9): 1611-1622.
[7] MAO J, WANG J, DUAN H J. Effects of multi functional epoxy resin on thermal resistance of mixed epoxy resin system [J]. Thermosetting Resin, 2006, 21(1): 16-20.
[8] PAN G Y, DU Z J, ZHANG C,etal. Synthesis, characterization, and properties of novel novolac epoxy resin containing naphthalene moiety [J]. Polymer, 2007, 48(13): 3686-3693.
[9] REN H, SUN J Z, WU B J,etal. Synthesis and characterization of a novel epoxy resin containing naphthyl/dicyclopentadiene moieties and its cured polymer [J].Polymer, 2006, 47(25): 8309-8316.
[10] TANG H Y, SONG N H, GAO Z H,etal. Synthesis and properties of 1,3,4-oxadiazole-containing high-performance bismaleimide resins [J]. Polymer, 2007, 48(1): 129-138.
[11] DINAKARAN K, ALAGAR M. Studies on thermal and morphological properties of 1,1-bis(3-methyl-4-cyanatophenyl)cyclohexane-epoxy-bismaleimide matrices [J]. Polym Adv Technol, 2003, 14(8): 544-556.
[12] PAN G Y, LIU H P, DU Z J,etal. Synthesis, characterization and properties of multifunctional novolac epoxy resins [J]. Polym Mater Sci Eng, 2008, 24(10): 41-44.
[13] DUANN Y E, LIU T M, CHENG K C,etal. Thermal stability of some naphthalene-and phenyl-based epoxy resins [J]. Polym Degrad Stab, 2004, 84(2): 305-310.
[14] CHIN W K, HWU J J, SHAU M D. Curing behaviour and thermal properties of Epon 828 resin cured with diimide-diacid and phthalic anhydride [J]. Polymer, 1998, 39(20): 4923-4934.
[15] REN H, SUN J Z, WU B J,etal. Synthesis and curing properties of a novel novolac suring agent containing naphthyl and dicyclopentadiene moieties [J]. Chin J Chem Eng, 2007, 15(1):127-131.
[16] TAO Z Q, YANG S Y, CHEN J S,etal. Synthesis and characterization of imide ring and siloxane-containing cycloaliphatic epoxy resins [J]. Eur Polym J, 2007, 43(4): 1470-1479.
[17] MARK V, HEDGES C V. Polycarbonate compositions having improved barrier properties:US Patent,4304899[P].1981-12-08.
[18] LIN C H, YANG K Z, LEU T S,etal. Synthesis, characterization, and properties of novel epoxy resins and cyanate esters [J]. J Polym Sci Part A: Polym Chem, 2006, 44(11): 3487-3502.
[19] BHUVANA S, SAROJADEVI M. Synthesis and characterization of epoxy/amine terminated amide-imide-imide blends [J]. J Appl Polym Sci, 2008, 108(3): 2001-2009.
[20] YUAN H W, RAO Q H. Preparation of resorcinol diglycidyl ether and its application [J]. Thermosetting Resin, 2007, 22(5): 1-4.
[21] YAN H Q, CHEN S, QI G R. Synthesis, cure kinetics and thermal properties of the 2,7-dihydroxynaphthalene dicyanate [J]. Polymer, 2003, 44(26): 7861-7867.
[22] OPALICKI M, KENNY M, NICOLAIS L. Cure kinetics of neat and carbon-fiber-reinforced TGDDM/DDS epoxy systems [J]. J Appl Polym Sci, 1996, 61(6): 1025-1037.
[23] SU S P, JIANG D D, WILKIE C A. Study on the thermal stability of polystyryl surfactants and their modified clay nanocomposites [J]. Polym Degrad Stab, 2004, 84(2): 269-277.
[24] KISSINGER H. Reaction kinetics in differential thermal analysis [J]. Anal Chem, 1957, 29(11): 1702-1706.
[25] OH J H, JIANG J, LEE S H. Curing behavior of tetrafunctional epoxy resin/hyperbranched polymer system [J]. Polymer, 2001, 42(20): 8339-8347.
[26] ZHOU F, DENG J R, JIANG W H. Study on curing reactions of epoxy resin diluted by 1,2-cyclohexanediol diglycidyl ether [J]. Thermosetting Resin, 2007, 22(2): 23-26.
[27] LIU W B, QIU Q H, WANG J,etal. Curing kinetics and properties of epoxy resin-fluorenyl diamine systems [J]. Polymer, 2008, 49(20): 4399-4405.
[28] MA Z G, GAO J G. Curing kinetics of o-cresol formaldehyde epoxy resin and succinic anhydride system catalyzed by tertiary amine [J]. J Phys Chem B, 2006, 110(25): 12380-12383.
[29] SIMON S L, GILLHAM J K. Cure kinetics of a thermosetting liquid dicyanate ester monomer/high-Tg polycyanurate material [J]. J Appl Polym Sci, 1993, 47(3): 461-485.
[30] BOINARD P, BANKS W M, PETHRICK R A. Changes in the dielectric relaxations of water in epoxy resin as a function of the extent of water ingress in carbon fibre composites [J].Polymer, 2005, 46(7): 2218-2229.
[31] MAEHARA T, TAKENAKA J, TANAKA K,etal. Synthesis and polymerization of novel epoxy compounds having an adamantane ring and evaluation of their heat resistance and transparency [J]. J Appl Polym Sci, 2009, 112(1): 496-504.

