Over-expression of Metastasis-associated in Colon Cancer-1 (MACC1) Associates with Better Prognosis of Gastric Cancer Patients
Shao-hua Ge, Xiao-jiang Wu, Xiao-hong Wang, Xiao-fang Xing, Lian-hai Zhang, Yu-bing ZhuHong Du, Bin Dong, Ying Hu, Jia-fu Ji*
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education),1Department of Gastrointestinal Surgery,2Department of Pathology,3Tissue Bank, Peking University School of Oncology,
Beijing Cancer Hospital & Institute, Beijing 100142, China
Over-expression of Metastasis-associated in Colon Cancer-1 (MACC1) Associates with Better Prognosis of Gastric Cancer Patients
Shao-hua Ge1, Xiao-jiang Wu1, Xiao-hong Wang1, Xiao-fang Xing1, Lian-hai Zhang1, Yu-bing Zhu1Hong Du1, Bin Dong2, Ying Hu3, Jia-fu Ji1*
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education),1Department of Gastrointestinal Surgery,2Department of Pathology,3Tissue Bank, Peking University School of Oncology,
Beijing Cancer Hospital & Institute, Beijing 100142, China
Objective:The aim of this study was to detect metastasis-associated in colon cancer-1 (MACC1) expression in Chinese gastric cancer and analyze the relationship between MACC1 expression and postoperative survival.
Methods:The expression of MACC1 and c-MET protein in a sample of 128 gastric cancer tissues was detected by immunohistochemistry. A retrospective cohort study on the prognosis was carried out and data were collected from medical records.
Results:The positive rate of MACC1 protein expression in gastric cancer was 47.66%, higher than that in adjacent noncancerous mucosa (P<0.001). MACC1 protein expression was not related to the clinicopathological variables involved. Kaplan-Meier analysis revealed that the survival of MACC1 positive group tended to be better than that of MACC1 negative group, particularly in patients with stage III carcinoma (P=0.032). Cox regression analysis revealed that MACC1 protein over-expression in gastric cancer tended to be a protective factor with hazard ratio of 0.621 (P=0.057). Immunohistochemical analysis showed that the positive rate of c-MET protein expression was much higher in cases with positive MACC1 expression in gastric cancer (P=0.002), but P53 expression was not associated with MACC1 expression.
Conclusion:MACC1 over-expression implies better survival and may be an independent prognostic factor for gastric cancer in Chinese patients.
MACC1; Gastric cancer; Prognosis
INTRODUCTION
Gastric cancer is one of the most common carcinomas and one of the leading causes of cancer death in China. Because of the heterogeneity in gastric cancer cells, the type of cells is of great importance in the prognosis of patients with the same stage of carcinoma and who are receiving similar treatment. And it is difficult to give proper personalized treatment to each patient and to identify the patients with cancer relapse and metastasis at the earliest possible time. There are many factors that may affect the prognosis[1], and serum biomarkers CEA[2-4], CA19-9[5-7], CA72-4[8-11], CA242[12]and a combination of the all[13,14]are widely usedprognostic factors. When anastomotic recurrence or distant metastasis is revealed by assistance of tumor markers or medical imaging, gastric cancer can not usually be cured by further surgery. Subsequently, it is of great importance to find new markers that will be helpful in gastric cancer monitoring and prognosis evaluation.
Metastasis-associated in colon cancer-1 (MACC1) gene was identified by differential display real-time polymerase chain reaction (RT-PCR) in primary colon cancer by Stein et al[15]. As for MACC1 translation, the predicted MACC1 consensus coding sequence consists of 2,559 nucleotides encoding a protein with 852 amino acids[16]. MACC1 protein contains several functional motifs, starting with ZU5 domain, Src-Homology (SH3) binding motif followed by a variant SH3 domain and two death domains from the N-terminal region[17]. MACC1 gene is located on 7p21.1, mapped on the same chromosomes as c-MET and hepatocyte growth factor (HGF) involved in the HGF-MET signal pathway. Functional study revealed that the HGF receptor c-MET was the transcriptional target of MACC1. In MACC1-transfected SW480 colon cancer cells, MACC1controls c-MET expression via a specific consensus sequence described as transcription factors specificity protein 1 (Sp1)[18]. However, the mechanism of how MACC1 binding to the Sp1 site is not yet clear. Putative factors found by PROMO software include Sp1, transcription factors ETF, E2F-1, p53 and Pax-5. As a direct interaction of Sp1 and p53 has been reported previously[19], tumor suppressor gene p53 may, therefore, play a role in the function of MACC1.
It was reported by Stein that increased MACC1 mRNA expression in primary colon cancer was related to metastasis-free survival in patients with stage I-III carcinoma[15]. Data from our microarray also suggested that MACC1 mRNA expression in gastric cancer cells is much higher than that of adjacent noncancerous mucosa[20]. On the basis of these researches, our study was the first attempt to investigate the relationship of MACC1 expression and gastric cancer prognosis. MACC1 protein expression was analyzed and the relationship between MACC1 expression and survivals was studied. To elucidate the molecular mechanism of MACC1 involved in gastric cancer, the expression of c-MET and p53 in gastric cancer cells were also analyzed.
MATERIALS AND METHODS
Patients and Tissue Specimens
Tissue specimens were obtained with informed written consent from 128 gastric cancer patients who were treated at the Peking University Beijing Cancer Hospital between January 2000 and December 2002. The investigation was approved by the Ethics Committee of Peking University. All patients (83 males, 45 females, mean age = 57 years, range 26-81 years) were diagnosed as having gastric cancer without preoperative chemotherapy or radiotherapy. A number of clinicopathological variables such as gender, age, tumor size and location, Borrmann classification, histological type, tumor-node-metastasis (TNM) stage, depth of tumor invasion, lymph node metastasis, distant metastasis and vascular invasion were included for survival analysis. p53 protein expression in clinical pathological reports was also included. Postoperative staging of gastric cancer was classified according to the 2002 tumor-node-metastasis (TNM) classification system recommended by the American Joint Committee on Cancer[21]. There were 14 patients with stage I, 20 patients with stage II, 56 patients with stage III and 38 patients with stage IV carcinoma. After gastrectomy, resected specimens of gastric cancer were routinely processed for macroscopic pathological assessment and fixed with 10% formalin in phosphate buffered saline (PBS) for immunohistochemistry. The patients were followed from a period of 1.23 months to 97.47 months (mean: 31.09 months). Follow-up was managed through correspondence, over the telephone or in the clinic every 3 to 6 months for 3 years and half a year thereafter. In the clinic, a complete history, physical examination, complete blood count, chemistry profile, imaging studies and endoscopy were routinely completed. One hundred and twenty-eight gastric cancer patients in our study were followed up regularly and follow-up information is complete. The primary endpoint of the follow-up was death of gastric cancer patients. Patients who did not die as a result of gastric cancer were excluded.
Immunohistochemistry
Formalin-fixed paraffin sections of 4μm thickness were mounted on poly-L-lysine-coated slides. The samples were then deparaffinized in xylene and rehydrated in graded alcohol. After hydration, endogenous peroxidase activity was blocked with 3% (v/v) hydrogen peroxide (H2O2) for 20 minutes at room temperature. Standard antigen retrieval was then performed with heat induced epitope retrieval (HIER) by heating the slides immersed in retrieval solution (pH 6.0) in a pressure boiler. After boiling, the slides remained in the pressure boiler for 3 minutes and then gradually cooled at room temperature. After washing with PBS three times, the sections were incubated with primary antibody anti-MACC1 (2.50μg/ml, 5197, ProSci, USA) or anti-c-MET (18-2257, Invitrogen) at 4°C overnight. After rinsing, the slides were incubated with peroxidaselabeled polymer conjugated to poly Peroxidase-anti-Mouse/Rabbit IgG (PV-9000, Zhongshan Biotechnology Company, Beijing, China) at 37°C. Diaminobenzidine (DAB) staining reaction was then performed and followed by Meyer hematoxylin counterstain. The slides were then dehydrated, cleared and mounted as normal. For negative controls, the primary antibody was replaced by non-immune rabbit serum to confirm the specificity. Internal positive control was used for quality assurance.
MACC1 staining was principally evaluated according to the scoring criteria. The information recorded was: subcellular location (nuclear and/or cytoplasmic), intensity of staining (negative, weak, moderate or strong) and percentage of positive immunoreactive cells. The positive group referred to the cases with >20% cells having positive immunoreactivity. The rest were defined as negative. The slide evaluation was performed by two board-certificated pathologists, and both pathologists gave almost identical reports with only minor differences. A consensus regarding controversial cases was reached after discussion.
Statistical Analysis
Regarding MACC1 expression and the clinicopathological variables, data were cross-tabulated and a Chi-square test was performed, except for the age parameter which was assessed by Student'sttest. Cumulative survival was estimated by the Kaplan-Meier method and comparisons between groups were done with a log-rank test. Postoperative survival was measured from the date of first surgery to the date of death caused by gastric cancer, or the last date of information collection if no end event was documented. A multivariate analysis of Cox proportional hazards regression model (backward, stepwise) was analyzed to assess the influence of each variable on survival.P<0.05 was considered statistically significant.
RESULTS
MACC1 Expression in Primary Gastric Cancer
MACC1 protein expression was found in the cytoplasm of both adjacent noncancerous mucosa and gastric cancer cells. However the positive rate of MACC1 expression in gastric cancer cells was much higher than that in adjacent noncancerous mucosa (47.66% versus 23.33%,P<0.001, Figure 1A-D).
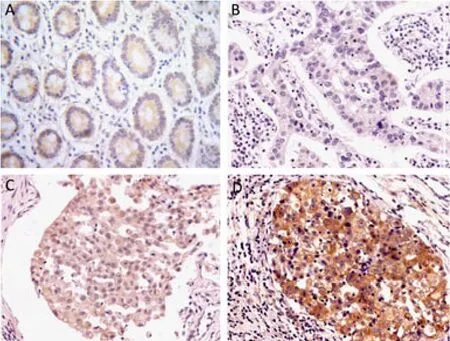
Figure 1.MACC1 protein expression determined by immunohistochemical staining in adjacent noncancerous mucosa and gastric cancers. (A) MACC1 protein expression in adjacent noncancerous mucosa (×200). (B) Negative expression in gastric cancer (×200). There was no MACC1 expression. (C) Weak positive expression in gastric cancer (×200). (D) Moderate positive expression in gastric cancer (×200). MACC1 protein was expressed in cytoplasm of the cells in (A), (C) and (D).
The Association of MACC1 Protein Expression with Clinicopathological Variables and Postoperative Survival in Gastric Cancer
We investigated the association of MACC1 protein expression with clinicopathological variables and postoperative survival. It suggested that MACC1 protein expression had no association with clinicopathological variables (Table 1), especially with the variables obviously related to the prognosis of gastric cancer patients.
Kaplan-Meier analysis showed that the postoperative survival of the MACC1 positive group tended to be better than that of the MACC1 negative group, but the difference was not statistically significant (P=0.249). For all the patients with stages I-IV carcinoma, the 5-year survival rates of MACC1 positive and negative groups were 40.10% and 34.30%, respectively, with respective median survival times of 42.10 months (95%CI: 16.90-67.26) and 23.57 months (95%CI: 14.73-32.41, Figure 2A).
The relationship of MACC1 protein expression and postoperative survival was also analyzed according to TNM stages and combined TNM stages. The difference in postoperative survival between the MACC1 positive group and negative group was not statistically significant in patients with stage I-II carcinoma (P=0.408, Figure 2B). Among patients with stage III carcinoma, the postoperative survival of the MACC1 positive group was much better than that of MACC1 negative group (P=0.032). The 5-year survival rates of the MACC1 positive group and negative group was 50.30% and 24.80%, with median survival time of MACC1 negative group 19.90 months (95%CI: 14.03-25.77, Figure 2C). The difference in postoperative survivals between MACC1 positive and negative groups was also not statistically significant in patients with stage IV carcinoma (P=0.670, Figure 2D). Different TNM stages were combined and the postoperative survivals were analyzed. Among patients with stages I, II and III carcinoma combined, the postoperative survival of the MACC1 positive group tended to be higher (P=0.105). The 5-year survival rates of MACC1 positive and negative groups were 58.00% and 46.50%, respectively, with mean survival times of 66.42 months (95%CI:54.50-78.34 mon) and 48.96 months (95%CI:38.52-59.41 mon), respectively (data not shown).

Figure 2.Postoperative survival curves in patients with Stage I-IV carcinoma. (A) Stage I-IV carcinoma; (B) Stage I-II carcinoma; (C) Stage III carcinoma; (D) Stage IV carcinoma. MACC1 protein positive expression refers to the cases showing weak and moderate staining.
Clinicopathological variables that may affect the prognosis of gastric cancer patients were analyzed by Cox regression analysis which showed that MACC1 expression, TNM stage and curative surgery were independent predictors of postoperative survival. The best mathematical multivariate Cox regression model consisted of three factors: MACC1 expression, TNM stage and curative surgery. Expression of MACC1 protein in gastric cancer cells tended to be a protective factor with hazard ratio of 0.621 (P=0.057, Table 2) in Chinese gastric cancer patients.
c-MET Expression in Gastric Cancer and the Association of MACC1 and c-MET Co-expression with Postoperative Survival
A previous study has reported that c-MET is the target of the MACC1 gene which controls c-METpromoter activity and expression[18]. To clarify whether MACC1 acts via regulating c-MET, the expression of c-MET protein was also detected and analyzed in 128 gastric cancer tissue samples (c-MET expression was available in 116 cases). The positive rate of c-MET protein expression in gastric cancer cells was not different from that in adjacent noncancerous mucosa (67.77% versus 63.77%,P=0.575). The positive rate of c-MET protein expression was elevated much higher in cases with positive MACC1 expression in gastric cancer cells (P=0.002). Concurrent expressions of MACC1 and c-MET proteins (both negative and positive expression) were found in 72 out of 116 (62.07%) gastric cancer tissue samples (Figure 3A).
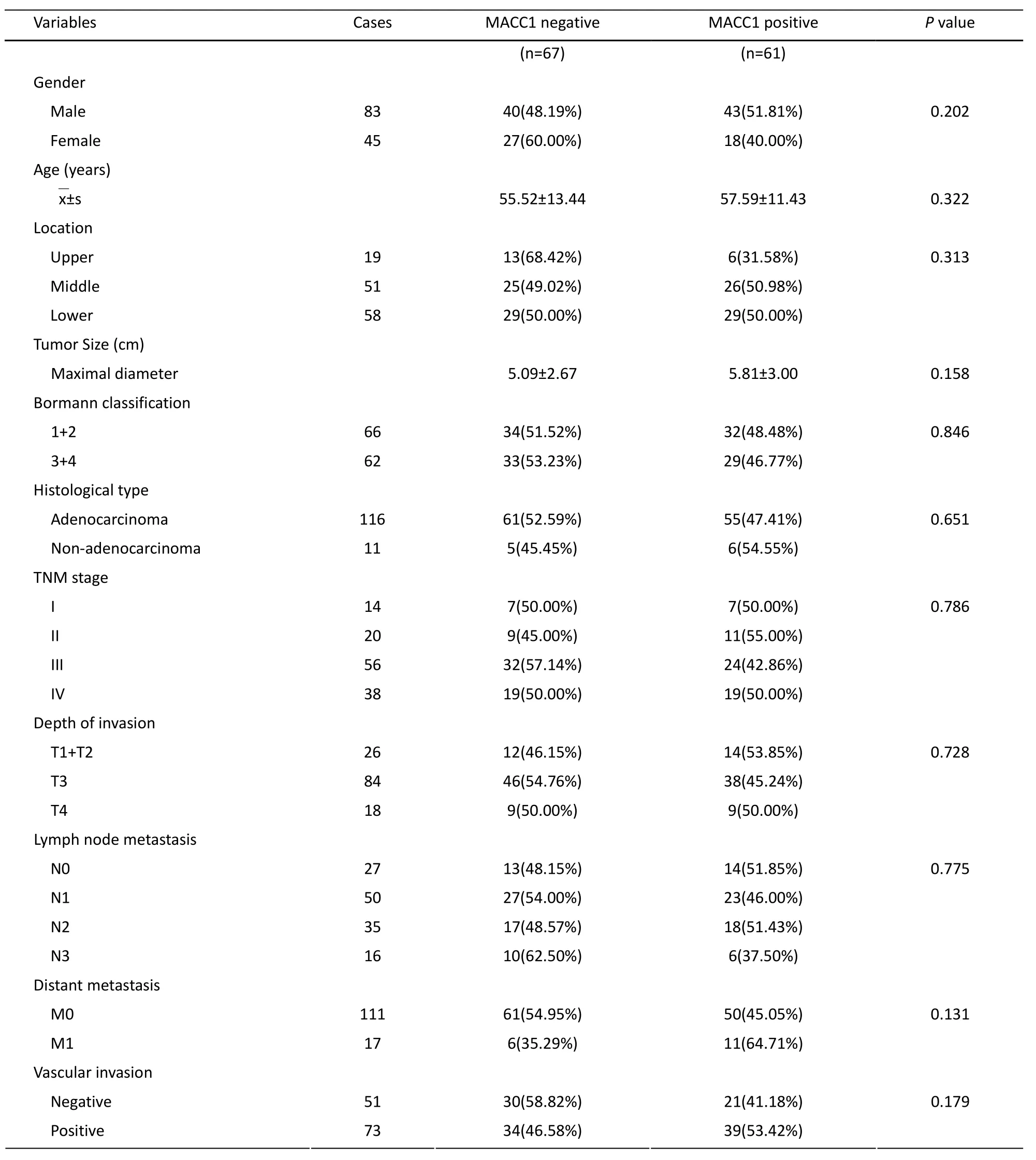
Table 1.Association of MACC1 protein expression with clinicopathological variables of gastric cancer patients

Figure 3.MACC1 and c-MET co-expression determined by immunohistochemical staining in gastric cancer and their relationship with the postoperative survival. A: MACC1 expression determined by immunohistochemical staining in gastric cancer. B: c-MET expression determined by immunohistochemical staining in the same position. C: Postoperative survival curves of patients with Stage I-IV carcinoma based on MACC1 and c-MET co-expression. D: Postoperative survival curves of patients with Stage III carcinoma based on MACC1 and c-MET co-expression.
Kaplan-Meier analysis showed that the difference between the survival of the c-MET positive group and the negative group was not statistically different in patients with stage I-IV carcinoma and stage III carcinoma (stage I-IV:P=0.822; stage III:P=0.272). The relationship between c-MET and MACC1 co-expression and postoperative survival was also analyzed in patients positive for both proteins (group A) and negative for both proteins (group B). The difference between the survival rates of two groups was not statistically significant in patients with stage I-IV carcinoma (P=0.413). The 5-year survival rates of group A and group B were 34.20% and 29.10%, respectively, and the median survival times of group A and group B were 42.10 months (95%CI: 18.28-65.92) and 23.57 months (95%CI: 12.94-34.21), respectively (Figure 3B). As for patients with stage III carcinoma, the survival of group A tended to be better than group B (P=0.093, Figure 3C). The 5-year survival rates of group A and group B were 50.60% and 26.80%, respectively, with the median survival time of group B of 16.77 months (95%CI: 13.47-20.07).
Expression of p53 in Gastric Cancer and the Relationship of p53 Expression and MACC1 Expression
The expression of p53 in 30 cases was analyzed in the 128 gastric cancer patients. The positive rate of p53 protein expression in gastric cancer cells was 36.67%. The positive rate of MACC1 expression was 45.45% in the positive p53 expression group and 57.89% in the negative p53 expression group. The difference of MACC1 expression between the p53 expression positive group and the negative group was not statistically significant (P=0.707) (Table 3).
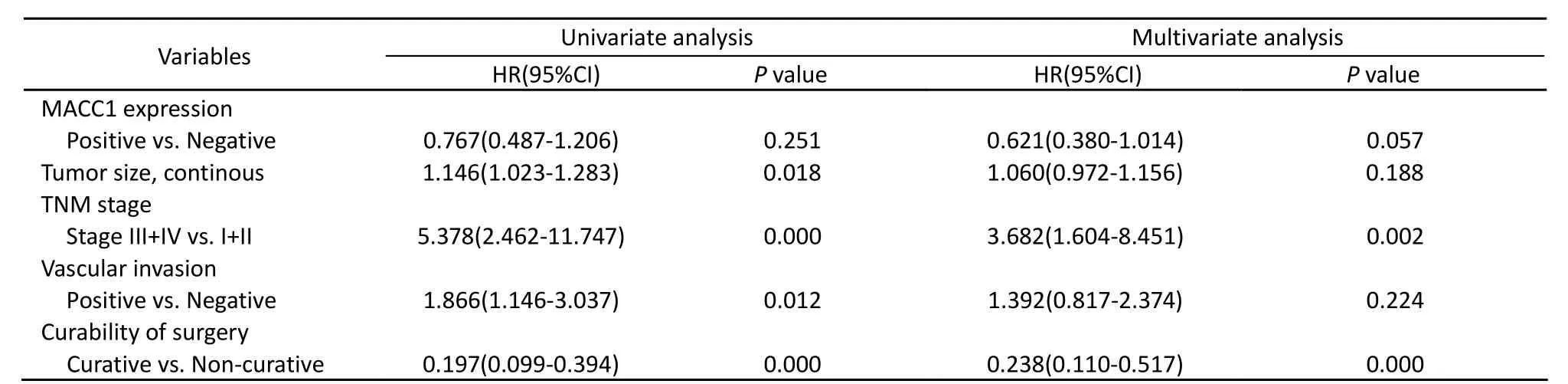
Table 2. Multivariate survival analysis of the prognostic factors by Cox regression analysis
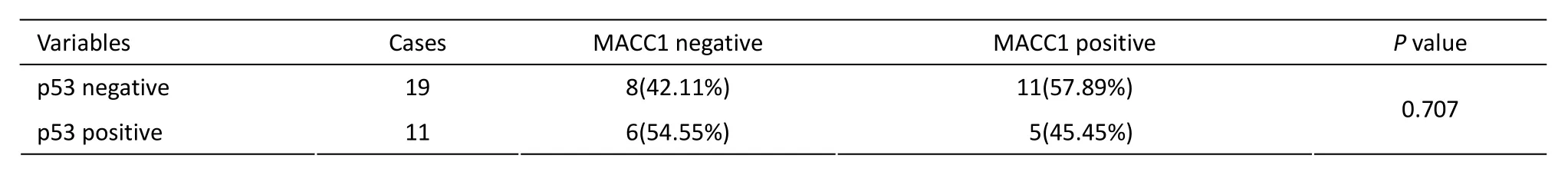
Table 3.The relationship of MACC1 expression and p53 in 30 patients with p53 expression in pathological reports
DISCUSSION
Our study showed that the positive rate of MACC1 protein expression in gastric cancer cells was much higher than in adjacent noncancerous mucosa, as was mRNA expression confirmed by microarray data[20]. The results suggest that MACC1 protein expression is consistent with mRNA expression and indicate that there are certain functions of MACC1 protein that are highly expressed in gastric cancer cells, which was confirmed by postoperative survival analysis in our study.
The clinical significance of MACC1 protein expressionwas studied. The results revealed that MACC1 protein over-expression had no association with clinicopathological variables related to the prognosis of gastric cancer patients, such as tumor size, TNM stage, histological type and vascular invasion[22-24].
Survival analysis using Kaplan-Meier method showed that MACC1 over-expression was related to improved postoperative survival of gastric cancer patients. Although the survival difference between the positive MACC1 expression group and the negative expression group was not statistically significant in 128 gastric cancer patients, there was a difference between the survival of MACC1 positiveand negative- expressing patients with stage III carcinoma. Because of the tissue difference between gastric cancers and colon cancers, the outcome of the survival analysis in our study was not the same as in Stein et al[15]. As for the survival of patients with stage I, stage II and stage IV carcinomas, there was no difference in the survival rates between the two groups. Possible explanations for this are as follows: Firstly, there were relatively few patients with early stage cancer: 14 patients with stage I carcinoma and 20 patients with stage II. As for patients with stage IV carcinoma, a number of them had already been suffering from distant metastasis and the effect of MACC1 over-expression on postoperative survival was not as important as the TNM stage as was shown in the survival analysis of 118 patients with M0 (M0: there is no distant metastasis,P=0.071). Cox analysis revealed that MACC1 expression, TNM stage and curative surgery were independent prognostic factors of gastric cancer. Tumor size, tumor location, vascular invasion and postoperative chemotherapy was not found to be independent prognosis factors, which is not consistent with the findings of Maehara et al[25]. Secondly, the differences could be as a result of the different number of cases studied and patient race. Overall, MACC1 over-expression in gastric carcinoma cells was a protective factor independent of TNM stage and vascular invasion.
c-MET is a high affinity receptor of HGF and plays a crucial role in embryonic development and tissue repair. It is expressed in normal tissues and overexpressed in a variety of cancer cells, including gastric cancer[26]. Many cytokines, such as HGF, Shc, Src[27], Grb2 and p85 regulatory subunit PI3K[28], fibroblast growth factor (FGF)[29]and Sprouty-2[30]could affect the function of c-MET in gastric cancer. c-MET over-expression in gastric cancer is related to histological type[31], advanced cancer stages and the survival of the patients. In gastric cancer cell lines, c-MET over-expression was seen to be related to the proliferation and invasion of gastric cancer[32]. Until now, there have been few studies indicating the relationship between c-MET and the survival of gastric cancer patients. However, it has been shown that c-MET over-expression was associated with liver metastasis[33]and the survival of gastric cancer patients[34-36]. According to Stein et al, c-MET was the target of MACC1 gene and c-MET expression was also controlled and regulated by MACC1. Therefore, c-MET protein expression in gastric cancer was detected and its relationship with postoperative survival of patients was also analyzed. The positive rate of c-MET expression in gastric cancer tissue samples was 67.77%. Concurrent expression of MACC1 and c-MET proteins was found in 62.07% of the patients, which indicated that there was a correlation between MACC1 and c-MET protein expression. Survival analysis revealed that c-MET expression was not associated with postoperative survival, nor did it change the relationship of MACC1 expression and postoperative survival.
p53 is a tumor-suppressor gene and maybe related to MACC1 function as reported by Stein et al[18]. In this study, p53 expression in clinical pathological reports was also collected and analyzed. It showed that there was no correlation between p53 expression and MACC1 over-expression. A possible explanation could be that the number of cases investigated was not enough to show a statistical difference. More cases were required to confirm the relationship between p53 and MACC1 in gastric cancer. A function study indicated that MACC1 protein expression was related to c-MET protein expression, but the latter did not affect the postoperative survival of gastric cancer patients compared with MACC1 only. It was shown that the prognosis of gastric cancer was associated with MACC1 expression but not with c-MET expression.
In conclusion, MACC1 protein was highly expressed in gastric cancer cells and was related to the postoperative survival of patients independent of the clinicopathological variables. Function analysis revealed that c-MET expression related to MACC1 expression but did not change the relationship of MACC1 expression and postoperative survival. Both MACC1 and c-MET are potential candidate biomarkers for gastric cancer diagnosis, but MACC1 is a biomarker significant in the prognosis of gastric cancer patients. There were also some limitations in our study. Firstly, the study focused on MACC1 protein expression because it was closely related to its function and there have been fewer studies on MACC1 mRNA. MACC1 protein in the serum of gastric cancer patients was not involved in our study yet. Secondly, we preliminarily studied the molecular mechanism of MACC1 protein in gastric cancer, however it is still unclear as there were few studies on the function of MACC1. The results of our study showed that it is a protective factor for MACC1 protein, which provides more information to help understand the new gene MACC1.
REFERENCES
1. Siewert JR, Bottcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998; 228: 449-61.
2. Iwanicki-Caron I, Di Fiore F, Roque I, et al. Usefulness of the serum carcinoembryonic antigen kinetic for chemotherapy monitoring in patients with unresectable metastasis of colorectal cancer. J Clin Oncol 2008; 26: 3681-6.
3. Ishigami S, Sakamoto A, Uenosono Y, et al. Carcinoembryonic antigen messenger RNA expression in blood can predict relapse in gastric cancer. J Surg Res 2008; 148: 205-9.
4. Yamamoto M, Baba H, Toh Y, et al. Peritoneal lavage CEA/CA125 is a prognostic factor for gastric cancer patients. J Cancer Res Clin Oncol 2007; 133: 471-6.
5. Kim HJ, Lee KW, Kim YJ, et al. Chemotherapy- induced transient CEA and CA19-9 surges in patients with metastatic or recurrent gastric cancer. Acta Oncol 2009; 48: 385-90.
6. Takahashi Y, Takeuchi T, Sakamoto J, et al. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients:a prospective clinical study. Gastric Cancer 2003; 6: 142-5.
7. Kochi M, Fujii M, Kanamori N, et al. Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer 2000; 3: 177-86.
8. Ubukata H, Katano M, Motohashi G, et al. Evaluation of CA72-4 as a tumor marker in patients with gastric cancer. Gan To Kagaku Ryoho 2003; 30: 1821-4.
9. Sheng SL, Wang Q, Huang G. Development of time-resolved immunofluorometric assays for CA 72-4 and application in sera of patients with gastric tumors. Clin Chim Acta 2007; 380: 106-11.
10. Goral V, Yesilbagdan H, Kaplan A, et al. Evaluation of CA 72-4 as a new tumor marker in patients with gastric cancer. Hepatogastroenterology 2007; 54: 1272-5.
11. Fernandes LL, Martins LC, Nagashima CA, et al. CA72-4 antigen levels in serum and peritoneal washing in gastric cancer. Correlation with morphological aspects of neoplasia. Arq Gastroenterol 2007; 44: 235-9.
12. Ozkan H, Kaya M, Cengiz A. Comparison of tumor marker CA 242 with CA 19-9 and carcinoembryonic antigen (CEA) in pancreatic cancer. Hepatogastroenterology 2003; 50: 1669-74.
13. Ucar E, Semerci E, Ustun H, et al. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther 2008; 25: 1075-84.
14. Gao YC, Yuan ZB, Yang YD, et al. Effect of freeze-thaw cycles on serum measurements of AFP, CEA, CA125 and CA19-9. Scand J Clin Lab Invest 2007; 67:741-7.
15. Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 2009; 15: 59-67.
16. Stein U, Dahlmann M, Walther W. MACC1-more than metastasis? Facts and predictions about a novel gene. J Mol Med 2009; 88: 11-8.
17. Kokoszynska K, Krynski J, Rychlewski L, et al. Unexpected domain composition of MACC1 links MET signaling and apoptosis. Acta Biochim Pol 2009; 56: 317-23.
18. Stein U, Smith J, Walther W, et al. MACC1 controls Met: what a difference a Sp1 site makes. Cell Cycle 2009; 8: 2467-9.
19. Wierstra I. Sp1: emerging roles--beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun 2008; 372:1-13.
20. Yuezheng Zhang, Lianhai Zhang, Yang Gao, et al. Discovery and validation prognostic markers in gastric cancer by genome-wide expression profiling. World J Gastroenterol 2010.
21. American Joint Committee on Cancer: Gastric Cancer. AJCC Cancer Staging. Manual, 2002, 6th ed. pp. 99-103. New York: Springer-Verlag.
22. Zhang M, Zhang H, Ma Y, et al. Prognosis and surgical treatment of gastric cancer invading adjacent organs. ANZ J Surg 2010; 80: 510-4.
23. Moghimi-Dehkordi B, Safaee A, Zali M R. Survival rates and prognosis of gastric cancer using an actuarial life-table method. Asian Pac J Cancer Prev 2008; 9: 317-21.
24. Santoro R, Carboni F, Lepiane P, et al. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg 2007; 94: 737-42.
25. Maehara Y, Kakeji Y, Oda S, et al. Time trends of surgical treatment and the prognosis for Japanese patients with gastric cancer. Br J Cancer 2000; 83: 986-91.
26. Prat M, Narsimhan RP, Crepaldi T, et al. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer 1991; 49: 323-8.
27. Okamoto W, Okamoto I, Yoshida T, et al. Identification of c-Src as a potential therapeutic target for gastric cancer and of MET activation as a cause of resistance to c-Src inhibition. Mol Cancer Ther 2010; 9: 1188-97.
28. Benvenuti S, Comoglio PM. The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol 2007; 213: 316-25.
29. Shimazaki K, Yoshida K, Hirose Y, et al. Cytokines regulate c-Met expression in cultured astrocytes. Brain Res 2003; 962: 105-10.
30. Holgren C, Dougherty U, Edwin F, et al. Sprouty-2 controls c-Met expression and metastatic potential of colon cancer cells: sprouty/c-Met upregulation in human colonic adenocarcinomas. Oncogene 2010; 29: 5241-53.
31. Kuniyasu H, Yasui W, Kitadai Y, et al. Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun 1992; 189: 227-32.
32. Kaji M, Yonemura Y, Harada S, et al. Participation of c-met in the progression of human gastric cancers: anti-c-met oligo- nucleotides inhibit proliferation or invasiveness of gastric cancer cells. Cancer Gene Ther 1996; 3: 393-404.
33. Amemiya H, Kono K, Itakura J, et al. c-Met expression in gastric cancer with liver metastasis. Oncology 2002; 63: 286-96.
34. Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 1999; 85: 1894-902.
35. Drebber U, Baldus SE, Nolden B, et al. The over-expression of c-met as a prognostic indicator for gastric carcinoma compared to p53 and p21 nuclear accumulation. Oncol Rep 2008; 19: 1477-83.
36. Uen YH, Lin SR, Wu CH, et al. Clinical significance of MUC1 and c-Met RT-PCR detection of circulating tumor cells in patients with gastric carcinoma. Clin Chim Acta 2006; 367: 55-61.
10.1007/s11670-011-0153-9
2010-10-15;Accepted2011-02-17
This work was supported by the grants of the Foundation from Beijing Municipal Committee of Science and Technology (No. D0905001040631), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars from Ministry of Education of China.
*Corresponding author
E-mail: jiafuj@gmail.com
© Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2011
 Chinese Journal of Cancer Research2011年2期
Chinese Journal of Cancer Research2011年2期
- Chinese Journal of Cancer Research的其它文章
- Effects of an Engineered Anti-HER2 Antibody chA21 on Invasion of Human Ovarian Carcinoma Cell In Vitro
- Reduction of Plasma MicroRNA-21 is Associated with Chemotherapeutic Response in Patients with Non-small Cell Lung Cancer
- The Use of CT Perfusion to Determine Microvessel Density in Lung Cancer: Comparison with FDG-PET and Pathology
- Immediate Versus Delayed Treatment with EGFR Tyrosine Kinase Inhibitors after First-line Therapy in Advanced Non-small-cell Lung Cancer
- DNA Repair Gene Polymorphisms in the Nucleotide Excision Repair Pathway and Lung Cancer Risk: A Meta-analysis
- Clinical Impact of t(14;18) in Diffuse Large B-cell Lymphoma
