Effects of an Engineered Anti-HER2 Antibody chA21 on Invasion of Human Ovarian Carcinoma Cell In Vitro
Yi Gao, Qiang Wu, Zheng-sheng Wu, Gui-hong Zhang, An-li Zhang
Department of Pathology, Anhui Medical University, Hefei 230032, China
Effects of an Engineered Anti-HER2 Antibody chA21 on Invasion of Human Ovarian Carcinoma Cell In Vitro
Yi Gao, Qiang Wu*, Zheng-sheng Wu, Gui-hong Zhang, An-li Zhang
Department of Pathology, Anhui Medical University, Hefei 230032, China
Objective:HER-2 plays an important role in the development and progression of ovarian carcinoma. A number of monoclonal antibodies (MAbs) and engineered antibody fragments (such as scFvs) against the subdomain II or IV of HER-2 extracellular domain (ECD) have been developed. We investigated the effect of chA21, an engineered anti-HER-2 antibody that bind primarily to subdomain I, on ovarian carcinoma cell invasionin vitro, and explored its possible mechanisms.
Methods:Growth inhibition of SK-OV-3 cells was assessed using a Methyl thiazolyl tetrazolium (MTT) assay. The invasion ability of SK-OV-3 was determined by a Transwell invasion assay. The expression of matrix metalloproteinase-2 (MMP-2) and its tissue inhibitors (TIMP-2) was detected by immunocytochemical staining, and the expression of p38 and the phosphorylation of p38 were assayed by both immunocytochemistry and Western blot.
Results:After treatment with chA21, the invasion of human ovarian cancer SK-OV-3 cells was inhibited in doseand time-dependent manners. Simultaneously the expression of p38, phospho-p38, MMP-2 and the MMP-2/TIMP-2 ratio decreased, while TIMP-2 expression increased. Additionally, the decrease in phospho-p38 was much greater than that of p38.
Conclusion:chA21 may inhibit SK-OV-3 cell invasion via the signal transduction pathway involving MMP-2, TIMP-2, p38 and the activation of p38MAPK.
HER-2; Ovarian neoplasms; Invasion; p38 mitogen-activated protein kinase; Matrix metalloproteinase-2; Tissue inhibitor of matrix metalloproteinase-2
INTRODUCTION
The incidence and mortality of ovarian carcinoma ranks high among women malignancies. Invasion, metastasis, relapse as well as drug resistance are the main reasons for its treatment failure. Therefore, inhibition of invasion and metastasis is one of the strategies for successful treatment of ovarian cancer.
HER-2, also known as neu/c-erbB-2, is a member of the tyrosine kinase receptor family. It is a transmembrane protein consisting of an extracellular domain (ECD) with four subdomains(I/L1, II/S1, III/L2, IV/S2), a single transmembrane domain, and an intracellular tyrosine kinase domain[1]. HER-2 is overexpressed in many kinds 111111of cancer. It has been shown that HER-2 is overexpressed in approximately 15%-30% of ovarian carcinoma and plays an important role in tumor development and progression[2]. In addition, HER-2 overexpression often correlates with tumor metastasis and poor prognosis. The HER-2 ECD which has prognostic value was proposed as a real-time marker of HER2-positive breast cancer and an attractive target for cancer immunotherapy[3,4]. In recent years, a number of monoclonal antibodies (MAbs) and engineered antibody fragments (such as scFvs) targeting HER-2 ECD have been developed and evaluated for tumor diagnosis and therapy[5-7].
Trastuzumab (Herceptin), a recombinant monoclonal antibody against HER-2 protein, is the first approved targeted agent by Food and Drug Administration (FDA) for treatment of HER-2 overexpressing breast cancer[8,9]. It functions by binding to the juxtamembrane region in subdomain IV of HER-2 ECD. However, the majority of patients with metastatic breast cancer who initially respond to trastuzumab develop resistance within one year of treatment initiation. In the adjuvant setting, 15% of patients still relapse despite trastuzumab-basedtherapy[10-12]. A small number of early clinical trials suggested that trastuzumab would not be an effective treatment option for ovarian cancer patients[13]. Abuharbeid[14]found that SK-OV-3 cells which were treated with trastuzumab developed marked resistance to chemotherapeutics. Furthermore, trastuzmab also has found out cardiac toxicity[15]. Another therapeutic antibody pertuzumab (2C4), which binds epitopes within subdomain II of HER-2 ECD showed only part efficiency to some patients[16].
In recent years, a novel engineered monoclonal antibody against HER-2 named chA21 which directed binding activity to HER-2 extracellular subdomain I has been developed[17-18]. In previous study, we have found that chA21 inhibited cell proliferation and induced apoptosis of human ovarian carcinoma cells SK-OV-3[19]. In this study, we investigated the effect of anti-HER-2 engineered antibody chA21 on the invasion of SK-OV-3 in vitro, and the possible mechanisms of action.
MATERIALS AND METHODS
Cell Lines and Reagents
The human ovarian cancer cells SK-OV-3 were obtained from the Cell Bank of Shanghai Institutes for Biological Sciences (Shanghai, China). The cells were cultured in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) in an incubator (Kendro Laboratory, Canada) with 5% CO2and saturated humidity at 37°C. Engineering antibody chA21 was constructed, expressed and prepared as described in previous work[17]. Methyl thiazolyl tetrazolium (MTT) and Extracellular matrix (ECM) were purchased from Sigma (USA). MILLICELL-®PCF chambers were purchased from MILLIPORE (USA). The antibodies for Matrix metalloproteinase-2 (MMP-2) and its tissue inhibitors (TIMP-2) (clone 17B11 and clone 46E5), p38 (clone: H-147), phospho-p38 (clone: D-8) were purchased from Santa Cruz (California, USA).
Cell Proliferation Assay
SK-OV-3 (5×103per well) cells were seeded in 96-well plates (Corning-Coster Crop, USA) and cultured overnight. The medium was replaced with fresh DMEM or media containing chA21 at a concentration of 3, 6, 12 μg/ml for 24 h. Triplicate wells were set up in each sample. MTT (20 μl) was added to each well and incubated for an additional 4 h. Culture media was discarded followed by addition of 150 μl dimethyl sulfoxide (DMSO) and vibration for 10 minutes. The OD570 nm was measured by a multi-well scanning spectrophotometer (Multiskan MK3, Finland). The growth inhibitory rate was calculated as follows: (1-ODsample/ODcontrol)×100%. The experiments were repeated three times.
Cell Invasion Assay
Cell invasion was assayed using ECM-coated Transwell chambers (8 μm pore sizes). SK-OV-3 cells (5×104/ml) suspended in 100 μl serum-free DMEM were seeded to the upper part of each chamber, whereas the lower compartments were filled with 600 μl chemoattractant, obtained from the supernatant fluid of cultured NIH3T3 cells. chA21 (3, 6, and 12 μg/ml) and serum-free DMEM were added respectively to SK-OV-3 cells and chambers were incubated at 37°C for 24 h. Additionally, a separate chA21 6 μg/ml group was cultured for 19, 24, 29 h. After incubation, the cells remaining on the upper surface were completely removed using a cotton swab. The cells that migrated to the bottom chamber were stained with hematoxylin and eosin. The experiments were performed in triplicate and the migrated cells were counted microscopically (400×) in five different fields per chamber. The invasion inhibitory rate was calculated as follows: (control cell numbersexperimental cell numbers)/control cell numbers×100%.
Immunocytochemistry
SK-OV-3 cells (2.5×104per well) were seeded in 6-well plates with coverslips, and then cultured in medium with 12 μg/ml chA21 for 24 h. For the controls, the same volume of DMEM was added. Then the coverslips were taken out, washed, fixed, and stained according to the instruction manual of immunohistochemistry kits. In this study, p38, MMP-2 and TIMP-2 protein in fixed cells were detected by PV-9000 method, and phospho-p38 protein was detected with SABC method. p38, MMP-2 and TIMP-2 protein are localized in the cytoplasm, while phospho-p38 is found inside the nucleus. The immunostained sections were examined using an Eclipse E800 microscope (Nikon, Japan) coupled to a digital camera. Mean optical density (MOD) of microscopic images were quantitatively analyzed by an image analysis software program, Image-pro Plus 5.02 (Media Cybernetics Inc, USA).
Western Blot Analysis
Cells were treated with chA21 (0, 3, 6, or 12 μg/ml) in DMEM (10%NBS) for 24 h, and then washed using cold Phosphate buffered saline (PBS). Cells were lysed in Triton X-100 extraction buffer (20 mmol/L sodium phosphate (pH 7.4), 150 mmol/L NaCl, 1% Triton X-100, 5 mmol/L EDTA, 5 mmol/L phenylmethyl-sulfonyl fluoride, 10 mg/ml aprotinin, 10 mg/ml leupeptin, and 250 mg/ml sodium vanadate) for 10 minutes on ice. The lysates were collected, transferred to eppendorf tubes, and centrifuged for 20 minutes at 14,000r/min in 4°C. Protein concentration was assessed and equal amounts of protein (1.0 mg) were prepared. Then sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Sample Loading Buffer (5×) is diluted 1:4 with the protein samples and boiled for 5 minutes before flash cooling on ice. Equal amounts of protein were subjected to SDSPAGE at 100V, and transferred to 0.2 m polyvinylidene difluoride membranes (PVDF) sequencing grade for 5 h at 90mA. Blots were blocked in 5% milk, 0.1% Tween-TBS (blocker) for 2 h at room temperature. Primary antibody in optimal dilution (β-actin 1:5,000, p38 1:1,000, phospho-p38 1:1,000) was added in blocker and incubated overnight at 4°C. Secondary antibody inoptimal dilution (β-actin 1:10,000, p38 1:40,000, phospho-p38 1:25,000) was added in blocker, incubated for 2 h at room temperature. Blots were stained for 30 minutes at room temperature using Pierce Supersignal West Femto detection systems(USA).
Statistical Analysis
Data were expressed as mean ± standard deviation. Comparison between groups was made by one-way analysis of variance (ANOVA) and Pearson correlation.P<0.05 was considered statistically significant. All statistics were carried out using a SPSS 10.0 statistics software(SPSS Inc., IL, USA).
RESULTS
chA21 Inhibits the Growth of SK-OV-3 Cells
To evaluate the effect of chA21 on cell proliferation, human ovarian cancer cells SK-OV-3 over-expressing HER-2 were treated with different concentrations (3-12 μg/ml) of chA21 for 24 h. chA21 treatment resulted in a dose-dependent inhibition of SK-OV-3 cell proliferation. At concentrations of 3, 6, and 12 μg/ml, growth was inhibited 19.26%, 20.37% and 21.56% respectively. The chA21 treatment groups showed significant decrease in cell proliferation when compared with the control group.
chA21 Inhibits the Invasion of SK-OV-3 Cells
Cell invasion was assayed using ECM-coated Transwell chambers (8μm pores). SK-OV-3 cells were treated with chA21 (3, 6, or 12 μg/ml) for 24 h. The number of invaded cells was reduced with the increased concentrations of chA21 (Figure 1). At concentrations of 3, 6, and 12 μg/ml, invasion was inhibited 21.03%, 36.99% and 87.19% respectively (Figure 2). The inhibition of invasion occurred in a dose dependent manner.
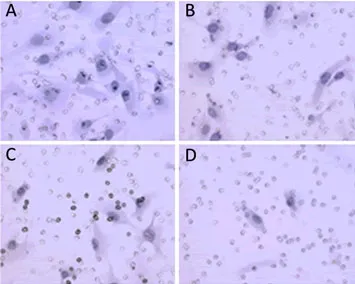
Figure 1.SK-OV-3 invasion after incubation with chA21 (3, 6 or 12μg/ml) for 24h(x400). The invaded cells were counted on the underneath of ECM-coated Millicell membrane. A. Control; B. chA21 (3μg/ml); C. chA21 (6μg/ml); D. chA21 (12μg/ml).
After treatment with chA21 (6μg/ml) for 19, 24, 29 h respectively, the number of migrated SK-OV-3 cells decreased compared with the control (Figure 3). Treatment times of 19, 24, 29 h inhibited invasion by 26.14%, 39.92% and 61.05% respectively (Figure 4, 5). Therefore, the inhibition of invasion occurred in a time dependent manner.
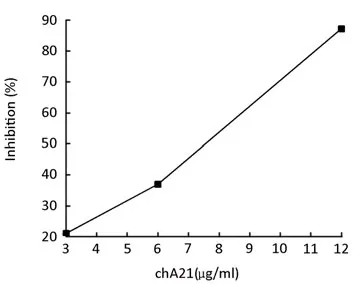
Figure 2.The inhibitory rate when SK-OV-3 cells were treated with chA21 (0, 3, 6, or 12 μg/ml) for 24h.
chA21 Inhibits the Expression of MMP-2, p38 and Phosphop38, while Upregulates TIMP-2 Expression in SK-OV-3 Cells
SK-OV-3 cells (2.5×104per well) were seeded in 6-well plates with coverslips, and then cultured in medium with 12 μg/ml chA21 for 24 h. The coverslips were harvested for immunocytochemistry. The expression of MMP-2, p38 and phospho-p38 were downregulated by chA21 treatment and TIMP-2 expression was upregulated (Figure 6, 7). As shown in Figure 8, MOD values of MMP-2, p38, phospho-p38 and MMP-2/TIMP-2 ratio in chA21 treatment group were significantly lower than those in control group, while MOD values of TIMP-2 were significantly higher than that in control group.

Figure 3.Invasion ability of SK-OV-3 cells incubated with chA21 (6μg/ml) for 19, 24 and 29h respectively (×400). A-C: Control group; A: 19h; B: 24h; C: 29h; D-F: chA21 group; D: 19h; E: 24h; F: 29h.
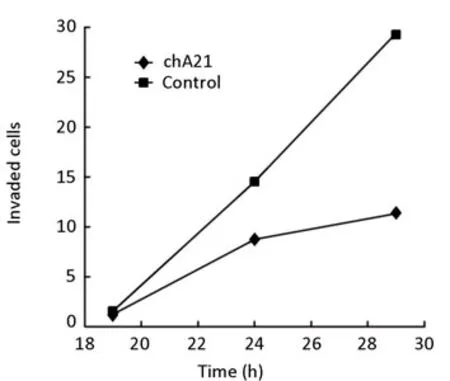
Figure 4.Effect on SK-OV-3 cells after treatment of chA21 (6μg/ml) for 19, 24, 29h.
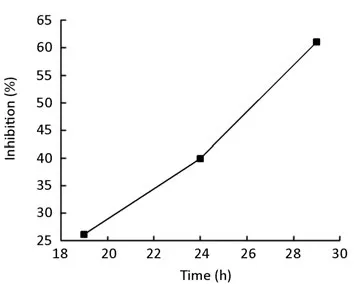
Figure 5.The inhibitory rate of SK-OV-3 cells exposed to chA21 (6μg/ml) for 19, 24, 29h.
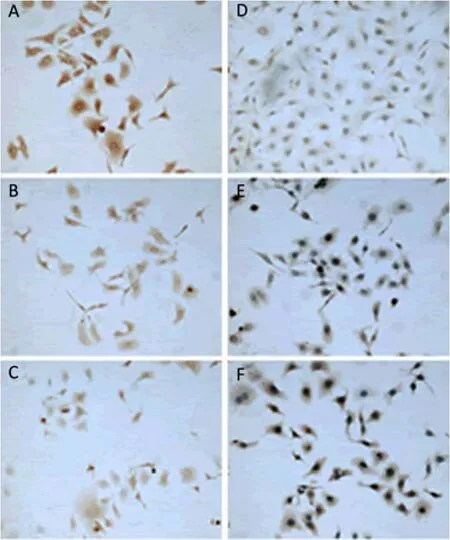
Figure 6.Effect of chA21 on MMP-2 and TIMP-2 expression in SKOV3 cells (two-step staining, ×100).

Figure 7.Effect of chA21 on p38 and phosphor-p38 expression in SK-OV-3 cells (p38: two-step method; phosphorp38: SABC method, x100)
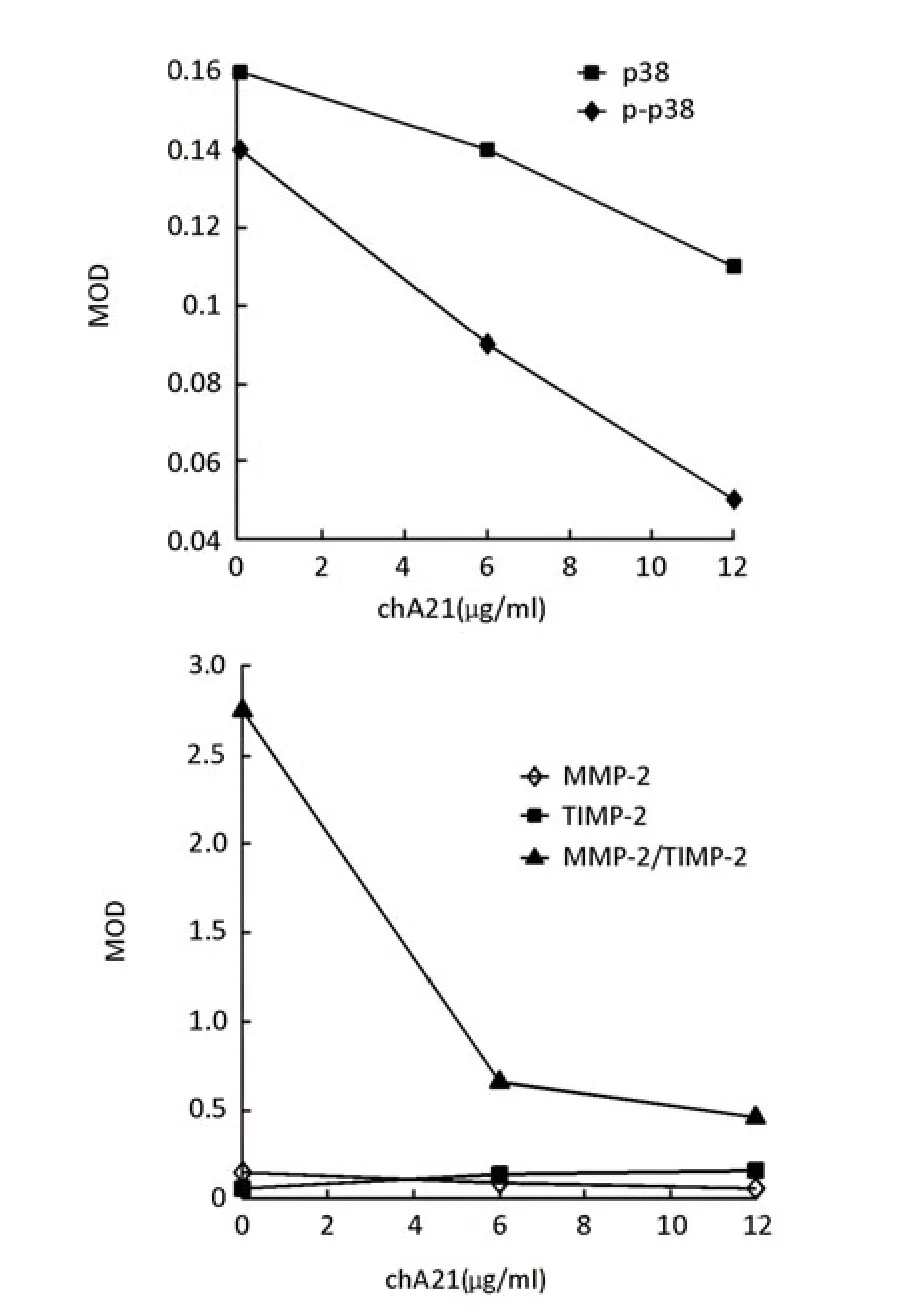
Figure 8.The change of MOD values of MMP-2, TIMP-2, p38, phosphor-p38 expression and MMP-2/TIMP-2 ratio after SK-OV-3 cultured in medium with 12 μg/ml chA21 for 24h in immunocytochemistry.
Western Blot Analysis of p38 and Phospho-p38
Cells were treated with chA21 (3, 6, or 12 μg/ml) and DMEM (10%NBS) for 24 h. p38 protein andphospho-p38 were then measured by Western blot. As shown in Figure 9, chA21 inhibited p38 and phospho-p38 in a dose-dependent manner. Relative MOD values were quantitatively analyzed by an image analysis software program, Image-pro Plus 5.02. This analysis showed a greater decrease in phospho-p38 with chA21 treatment than p38.
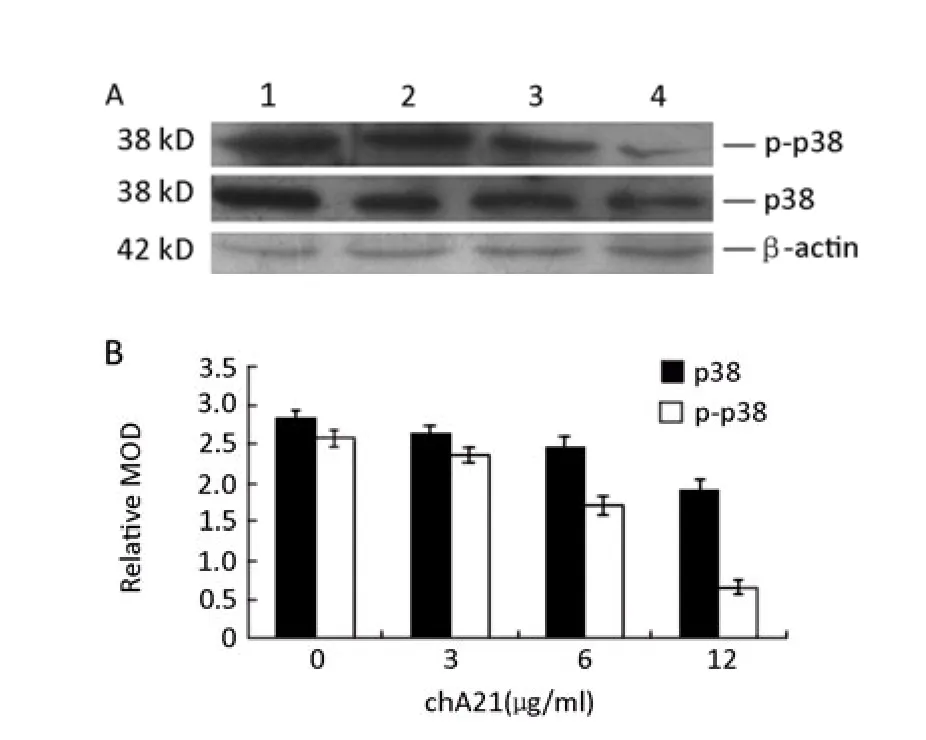
Figure 9.Western blot analysis of p38 and phospho-38 in the presence of chA21 (0, 3, 6, or 12μg/ml) for 24 h. 1: Control group; 2: chA21(3μg/ml) group; 3: chA21 (6μg/ml) group; 4: chA21(12μg/ml) group.
DISCUSSION
HER-2 has been identified as an important regulator of metastatic potential of breast cancer[20,21]. Zhang et al[22]found RNA interference (RNAi) targeting HER-2 gene inhibited the invasive and chemotactic ability of SK-OV-3 cells. The purpose of this study was to investigate the effect of anti-HER-2 engineered antibody chA21 on proliferation and invasion of SK-OV-3 cellsin vitro, and its possible mechanisms. It might be important for the treatment of ovarian cancer patients.
In the present study, the growth of SK-OV-3 cells declined with increasing concentrations of chA21 by MTT assay. In addition, the invasion ability was also inhibited with chA21 treatment by a Transwell assay. Comparing the results, we observed that with the same dose of chA21, the inhibitory effect on SK-OV-3 invasion was much greater than on cell growth. This suggests that the reduced number of cells underneath the ECM-coated membrane is mainly due to the inhibitory effect of chA21 on SK-OV-3 invasion. Furthermore, incubation with chA21 inhibited cell invasion in a time- and concentration-dependent manner. It showed that the invasion ability and the degraded ability to ECM of SK-OV-3 cells were inhibited evidently after subdomain I of HER-2 ECD was bound. In order to investigate the effect of subdomain I of HER-2 ECD in the invasion signal pathway of SK-OV-3, we examined the expression of p38MAPK, MMP-2, TIMP-2, which are downstream of HER-2.
The mitogen-activated protein kinase (MAPK) family has at least four members, namely p38, extracellular signal-related kinases 1 and 2 (ERK1 and ERK2), Jun amino-terminal kinases (JNKs), and ERK5. p38MAPK has been a key signal transduction pathway in inducing a wide variety of biological effects, such as cancer progression, invasion, apoptosis, cell cycle regulation and embryonic development[23,24]. The active form of p38, phospho-p38, is localized in the nucleus and affects many transcription factors such as transcription factor 2 (ATF-2), c-fos, c-Jun and c-myc in order to regulate cells[25,26]. Shen et al[27]found SB203580, a chemical inhibitor of p38, could significantly reduce the invasiveness and migration of human prostate cancer.
MMP-2 or gelatinase A is able to cleave some matrix components such as gelatin (collagen IV,V) and elastin[28]. MMP-2 and TIMP-2 play key roles in tumorigenesis, migration and invasion. Under normal conditions, there is a balance between MMP-2 and TIMP-2[29]. When the MMP-2/TIMP-2 ratio is disturbed, MMP-2 will begin to excessively degrade the ECM which promotes cell invasion[30-33].
Many studies have shown HER-2, p38MAPK and MMP-2/TIMP-2 system to be a circular signal transduction pathway. Overexpression of HER-2 protein can activate p38MAPK. The phospho-p38 protein can then upregulate MMP-2 expression which can promote HER-2 activation in turn[34-37]. Additionally, Yang et al[38]found the suppression of MMP-2 expression by MR-3, a methylated derivative of resveratrol, led to an inhibition of A549 cell invasion by inactivating the p38 MAPK signaling pathway.
Through immunocytochemistry and Western blot analysis, we found SK-OV-3 cells incubated with chA21 (3, 6 and 12μg/ml) for 24 h, had a slightly reduced p38 expression while phospho-p38 staining was evidently decreased. It showed that chA21 might mainly inhibit the activation of p38 protein.
Immunocytochemistry was performed to detect MMP-2 and TIMP-2 protein in SK-OV-3 in both control and chA21 treated groups. In the chA21 treatment group, the expression of MMP-2 and the MMP-2/TIMP-2 ratio were decreased, while TIMP-2 expression was increased. This result suggests that chA21 might change the MMP-2/TIMP-2 balance. This change may then serve to limit the invasion ability of SK-OV-3 cells.
In our study, the HER-2, p38MAPK and MMP-2/ TIMP-2 system seems to be very important to the invasion process of ovarian carcinomas. As suggested from the existing literature, HER-2 ECD is an important target for ovarian cancer therapy. Subdomain I of HER-2 ECD may mediate the expression of MMP-2 and TIMP-2 by activating p38MAPK protein. Subdomain I of HER-2 ECD may participate in the cell signal transduction pathway for SK-OV-3 cell invasion by regulating the expression of MMP-2, TIMP-2, p38 and the activation of p38MAPK.
Acknowledgements
We would like to thank Dr. Lian-sheng Cheng from University of Science and Technology of China for supplying chA21 antibody.
REFERENCE
1. Carpenter G. Receptors of epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem 1987; 56: 881-914.
2. Verri E, Guglielmini P, Puntoni M, et al. HER2/neu oncoprotein overexpression in epithelial ovarian cancer: evaluation of its prevalence and prognostic significance. Clinical study 2005; 68: 154-61.
3. Garoufali A, Kyriakou F, Kountourakis P. Extracellular domain of HER2: a useful marker for the initial workup and follow-up of HER2-positive breast cancer. J BUON 2008; 13: 409-13.
4. Bramwell VH, Doig GS, Tuck AB. Changes over time of extracellular domain of HER2 (ECD/HER2) serum levels have prognostic value in metastatic breast cancer. Breast Cancer Res Treat 2009; 114: 503-11.
5. Garrett JT, Rawale S, Allen SD, et al. Novel engineered trastuzumab conformational epitopes demonstrate in vitro andin vivoantitumor properties against HER-2/neu. J Immunol 2007; 178: 7120-31.
6. Liu CY, Yang W, Li JF. Effect of trastuzumab on tumor cell lines shedding high or low level of HER-2 ECD. Zhonghua Zhong Liu Za Zhi 2007; 29: 101-5.
7. Lurje G, Lenz HJ. EGFR Signaling and Drug Discovery. Oncology 2009; 77: 400-10.
8. Dean-Colomb W, Esteva FJ. Her2-positive breast cancer: herceptin and beyond. Eur J Cancer 2008; 44: 2806-12.
9. Shepard HM, Jin P, Slamon DJ, et al. Herceptin. Handb Exp Pharmacol 2008; 181: 183-219.
10. Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res 2006; 8: 215.
11. Piccart M. Circumventing de novo and acquired resistance to trastuzumab: new hope for the care of ErbB2-positive breast cancer. Clin Breast Cancer 2008; Suppl 3: S100-13.
12. Dieras V, Vincent-Salomon A, Degeorges A. Trastuzumab (Herceptin) and breast cancer: mechanisms of resistance. Bull Cancer(in French) 2007; 94: 259-66.
13. Wilken JA, Webster KT, Maihle NJ. Trastuzumab Sensitizes Ovarian Cancer Cells to EGFR-targeted Therapeutics. J Ovarian Res 2010; 3: 7.
14. Abuharbeid S, Apel J, Zugmaier G, et al. Inhibition of HER-2 by three independent targeting strategies increases paclitaxel resistance of SKOV-3 ovarian carcinoma cells. Naunyn Schmiedebergs Arch Pharmacol 2005; 371: 141-51.
15. Hattori K, Nishi Y, Nakamura S, et al. Evaluation of cardiac dysfunction after herceptin treatment in patients with metastatic breast cancer by echocardiography. Rinsho Byori 2007; 55: 120-5.
16. Takai N, Jain A, Kawamata N, et al. 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer 2005; 104: 2701-8.
17. Cheng LS, Liu AP, Yang JH, et al. Construction, expression and characterization of the engineered antibody against tumor surface antigen, p185(c-erbB-2). Cell Res 2003; 13: 35-48.
18. Hu S, Zhu Z, Li L, et al. Epitope mapping and structural analysis of an anti-ErbB2 antibody A21: Molecular basis for tumor inhibitory mechanism. Proteins 2008; 70: 938-49.
19. Zhang A, Xue H, Ling X, et al. Anti-HER-2 engineering antibody ChA21 inhibits growth and induces apoptosis of SK-OV-3 cells. J Exp Clin Cancer Res 2010; 29: 23.
20. Kim IY, Yong HY, Kang KW, et al. Overexpression of ErbB2 induces invasion of MCF10A human breast epithelial cells via MMP-9. Cancer Lett 2009; 275: 227-33.
21. Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res 2008; 14: 5158-65.
22. Zhang SL, Lu YM, Meng LR, et al. Inhibition of invasive and chemotactic abilities of SK-OV-3 cells by human epithelial growth receptor-2 small interfering RNA. Zhonghua Fu Chan Ke Za Zhi (in Chinese) 2007; 42: 48-53.
23. Bradham C, McClay DR. p38 MAPK in development and cancer. Cell Cycle 2006; 5: 824-8.
24. Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 2007; 1773: 1358-75.
25. Humar M, Loop T, Schmidt R, et al. The mitogen-activated protein kinase p38 regulates activator protein 1 by direct phosphorylation of c-Jun. Int J Biochem Cell Biol 2007; 39: 2278-88.
26. Fisher GJ, Talwar HS, Lin JY, et al. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skinin vivo. J Clin Invest 1998; 101: 1432-40.
27. Shen KH, Hung SH, Yin LT, et al. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem 2010; 333: 279-91.
28. Judith A. Matrix Metalloproteinases as Mediators of Primary and Secondary Cataracts. Expert Rev Ophthalmol. 2007; 2: 931–8.
29. Brun JL, Cortez A, Commo F. Serous and mucinous ovarian tumors express different profiles of MMP-2, -7, -9, MT1-MMP, and TIMP-1 and -2. Int J Oncol 2008; 33: 1239-46.
30. Chernov AV, Sounni NE, Remacle AG, et al. Epigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells. J Biol Chem 2009; 284: 12727-34.
31. Kim TJ, Rho SB Choi YL, et al. High expression of tissue inhibitor of metalloproteinase-2 in serous ovarian carcinomas and the role of this expression in ovarian tumorigenesis. Hum Pathol 2006; 37: 906-13.
32. Jinga DC, Blidaru A, Condrea I. MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: correlations with prognostic factors. J Cell Mol Med 2006; 10: 499-510.
33. Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit 2009; 15: RA32-40.
34. Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat Rev Mol Cell Biol 2001; 2: 127-37.
35. Kumar B, Koul S, Petersen J, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res 2010; 70: 832-41.
36. Ke Z, Lin H, Fan Z, et al. MMP-2 mediates ethanol-induced invasion of mammary epithelial cells over-expressing ErbB2. Int J Cancer 2006; 119: 8-16.
37. Lee EK, Lee YS, Lee H, et al. 14-3-3epsilon protein increases matrix metalloproteinase-2 gene expression via p38 MAPK signaling in NIH3T3 fibroblast cells. Exp Mol Med 2009; 41: 453-561.
38. Yang YT, Weng CJ, Ho CT. Resveratrol analog-3,5,4'-trimethoxytrans-stilbene inhibits invasion of human lung adenocarcinoma cells by suppressing the MAPK pathway and decreasing matrix metalloproteinase-2 expression. Mol Nutr Food Res 2009; 53: 407.
10.1007/s11670-011-0147-7
2010-10-12;Accepted2011-02-11
This work was supported by National “863” High-Tech R & D Program of China (No. 2004AA215260), Science Foundation for Young Teacher of Anhui Province (No.2008jq1062) and Research Foundation for Advanced Talents of the No.2 Hospital affiliated to the Anhui Medical University (No. 2009-08)
*Corresponding author
E-mail: aydjohn@yahoo.com
©Chinese Anti-Cancer Association and Springer Verlag Berlin Heidelberg 2011
 Chinese Journal of Cancer Research2011年2期
Chinese Journal of Cancer Research2011年2期
- Chinese Journal of Cancer Research的其它文章
- Reduction of Plasma MicroRNA-21 is Associated with Chemotherapeutic Response in Patients with Non-small Cell Lung Cancer
- The Use of CT Perfusion to Determine Microvessel Density in Lung Cancer: Comparison with FDG-PET and Pathology
- Immediate Versus Delayed Treatment with EGFR Tyrosine Kinase Inhibitors after First-line Therapy in Advanced Non-small-cell Lung Cancer
- DNA Repair Gene Polymorphisms in the Nucleotide Excision Repair Pathway and Lung Cancer Risk: A Meta-analysis
- Clinical Impact of t(14;18) in Diffuse Large B-cell Lymphoma
- Over-expression of Metastasis-associated in Colon Cancer-1 (MACC1) Associates with Better Prognosis of Gastric Cancer Patients
