阴外动脉灌注脂多糖对泌乳奶牛乳的成分和脂肪酸组成的影响
张养东 王加启 胡 涛 李珊珊 卜登攀 金 迪 孙 鹏 周凌云
(1.东北农业大学动物营养研究所,哈尔滨 150030;2.中国农业科学院北京畜牧兽医研究所,北京 100193)
阴外动脉灌注脂多糖对泌乳奶牛乳的成分和脂肪酸组成的影响
张养东1,2王加启2*胡 涛2李珊珊2卜登攀2金 迪2孙 鹏2周凌云2
(1.东北农业大学动物营养研究所,哈尔滨 150030;2.中国农业科学院北京畜牧兽医研究所,北京 100193)
本试验旨在研究阴外动脉灌注脂多糖(LPS)对泌乳奶牛乳成分、乳脂合成前体物和脂肪酸组成的影响。选用6头处于泌乳期第(185±30)天、体重(576±36)kg的经产的荷斯坦奶牛,随机分成2组。采用交叉试验设计,试验组阴外动脉灌注LPS(Escherichia coliO111∶B4,0.01μg/kg BW),对照组灌注生理盐水。试验分2期,每期试验7 d,2期间隔14 d。结果表明:泌乳奶牛干物质采食量受LPS影响差异不显著(P>0.05);LPS显著提高乳中乳蛋白率(P<0.05),对乳脂率无显著影响(P>0.05);LPS极显著降低血浆中乳脂合成前体物非酯化脂肪酸的含量(P<0.01),β-羟丁酸含量呈先降低后升高的趋势(P>0.05);LPS不同程度地降低了乳脂中饱和脂肪酸(P>0.05)和短链脂肪酸(P>0.05)的含量,提高了乳脂中不饱和脂肪酸(P>0.05)和中长链脂肪酸(P>0.05)的含量,并影响脂肪酸的去饱和作用。结果提示,LPS是诱发乳脂合成发生变化的主要激发因子之一。
阴外动脉;泌乳奶牛;脂多糖;乳成分;乳脂肪酸组成
脂多糖(lipopolysaccharide,LPS)是革兰氏阴性细菌崩解时释放的一种主要成分,是诱发动物机体产生炎症反应的主要诱因之一[1-3],在机体发生炎症反应的同时,机体脂类代谢也受相应的调控[4-5]。在啮齿类动物上的研究发现,LPS能调控乳腺中乳脂肪合成的关键酶,如脂肪酸合成酶和乙酰辅酶羧化酶等[6-7],下调脂蛋白脂肪酶的活性[8],从而抑制乳腺吸收脂肪酸用于乳脂肪的合成[9]。但目前LPS影响乳腺内乳脂肪合成的研究仅局限于啮齿类动物,在反刍动物上,更多是局限于将LPS作为乳腺内乳房炎的激发因子基础上的研究。Zebeli等[3]研究表明,随着泌乳奶牛饲粮中谷物比例的不断提高,瘤胃中的LPS随之上升,LPS不但介导了机体的炎症反应,且与瘤胃中乳脂率呈负相关,LPS可能是预防反刍动物乳脂降低综合症的一种新的举措[3];但LPS在降低乳脂的同时,对乳脂脂肪酸组成的影响却鲜有研究。同时,LPS在胃肠道中迁移位点研究不尽一致,有研究者认为LPS是从瘤胃迁移到外周血液中[10],也有研究者认为LPS是从肠道尤其是肠道末端迁移到外周血液中[11-13]。因此,本试验在避开反刍动物胃肠道的基础上,直接通过阴外动脉灌注LPS更加直观的研究LPS对乳脂脂肪酸组成的影响。
1 材料与方法
1.1 试验动物与试验饲粮
选用6头泌乳期第(185±30)天,体重(576±36)kg的经产的荷斯坦奶牛,以玉米、豆粕、麦麸、青贮、苜蓿干草及羊草为主要原料配制基础饲粮,满足中国农业行业标准(NY/t 34—2004)推荐的营养需求。饲粮组成及营养水平见表1。
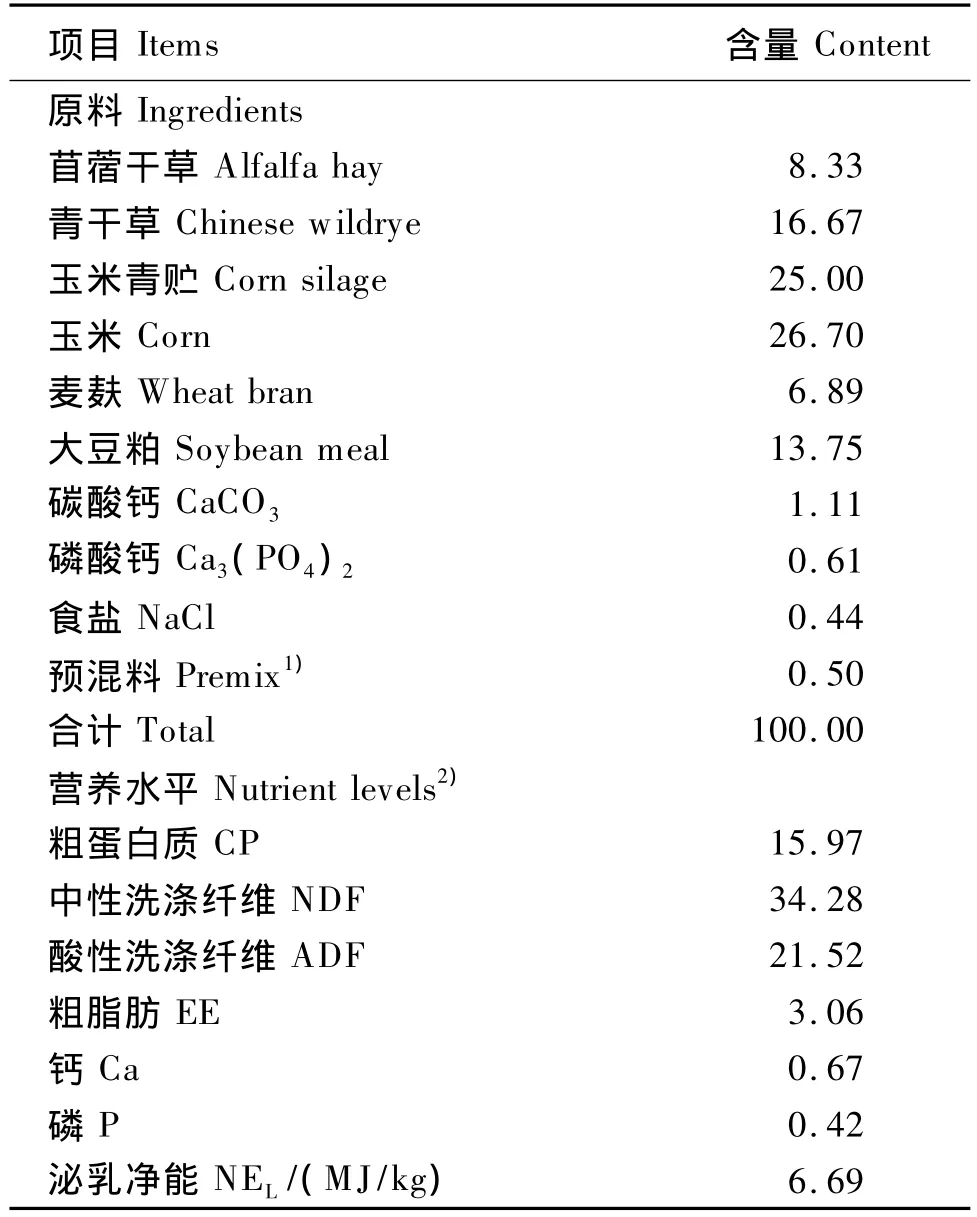
表1 饲粮组成及营养水平(干物质基础)Table 1 Composition and nutrient levels of the diet(DM basis) %
1.2 试验设计与饲养管理
1.2.1 灌注液准备
生理盐水灌注液:10 m L生理盐水(无致热源的灭菌生理盐水)。
LPS 灌注液:LPS(Escherichia coliO111∶B4,美国sigma公司)用生理盐水配制成0.5 mg/m L储备溶液,再用生理盐水按照0.01μg/kg BW 配制成终体积10 m L LPS溶液。
1.2.2 试验设计
试验采用交叉试验设计,6头奶牛随机分成2组,每组3头,试验按时间顺序为7 d预试期,7 d第1正试期,14 d间隔期,7 d第2正试期。预试期内奶牛接受1次阴外动脉生理盐水灌注,使动物机体适应灌注条件,减轻灌注对动物机体的应激。
第1正试期一组奶牛灌注LPS 0.01μg/kg BW(LPS组),另一组为对照组,奶牛灌注生理盐水10 m L;第2正试期,2处理交叉。于正试期第1天07:00灌注。
配制好的溶液经预热达到37℃时,经右侧阴外动脉一次性灌注,灌注时要尽量保持试验动物安静,必要时给试验动物套上“防踢棒”,灌注缓慢而均匀进行,在5 m in内灌注完毕,随后再用10 m L无致热源的灭菌生理盐水冲洗灌注管道,以保证灌注液全部进入试验动物动脉血管中。
1.2.3 饲养管理
试验动物采用栓系式饲养,日饲喂2次(07:00和19:00),自由饮水,挤奶采用利拉伐手推式挤奶机进行。试验期间每天记录采食量和产奶量,并观测试验动物的精神状态。
1.3 样品的采集与测定方法
生鲜乳样品的采集:于灌注后 0、6、12、24、36、48、60、72、84、96 h 分别采集生鲜乳样。乳样分为2份,一份添加防腐剂(bronopol tablet,加拿大D&F Control System公司),4℃保存,用于测定乳成分。另一部分-20℃保存,用于乳脂脂肪酸组成测定。
血浆样品的采集:于灌注后 0、6、12、24、72 h分别利用真空采血管(血浆管)采集尾动脉血样,4℃静置过夜后,3 000×g、4℃离心15 m in分离血浆。分离后的血浆于-20℃保存。
乳脂肪含量、乳蛋白质含量利用Foss-MilkoscanTMMinor乳成分分析仪(Minor Scan FOSS,丹麦)测定。利用正己烷与异丙醇的混合液提取牛奶上清液中的脂肪,然后对溶有脂肪的正己烷液体进行酸碱甲酯化,再测定脂肪酸组成,具体方法参见 Bu 等[14]文献。
血浆中非酯化脂肪酸(NEFA)和β-羟丁酸(BHA)的测定采用 Beckman Synchron CX5PRO分析系统,试剂盒购自英国RANDOX公司。
1.4 数据处理
所有数据以SAS 8.2软件M IXED模块进行统计学检验。统计模型中包含试验牛的随机因素以及试验期、试验处理、试验时间和试验处理与试验时间的交互效应等的固定因素。变量的统计结果均以最小二乘均数形式列表,显著水平为P<0.05,极显著水平为P<0.01。
2 结果
2.1 阴外动脉灌注 LPS对泌乳奶牛干物质采食量和乳成分的影响
由表2可知,泌乳奶牛干物质采食量受LPS影响差异不显著(P>0.05)。灌注LPS显著提高乳蛋白率(P<0.05),对乳脂率无显著影响(P>0.05)。

表2 阴外动脉灌注脂多糖对泌乳奶牛乳成分的影响Table 2 Effects of infusion LPS into external pudendal artery on m ilk com position in dairy cow s
2.2 阴外动脉灌注LPS对泌乳奶牛动脉血浆中乳脂合成前体物的影响
由表2和图1可知,与对照组相比,阴外动脉灌注LPS极显著降低血浆中乳脂合成前体物非酯化脂肪酸的含量(P<0.01);对血浆中乳脂合成前体物BHA有增加趋势(P>0.05),但随着灌注后时间的延续呈先降低后升高的趋势(P>0.05)。

表3 阴外动脉灌注脂多糖对动脉血浆中乳脂合成前体物的影响Table 3 Effects of infusion LPS into external pudendal artery on contents of synthesis precursors ofm ilk fat in artery plasma of dairy cows

图1 阴外动脉灌注脂多糖对泌乳奶牛动脉血浆中乳脂合成前体物β-羟丁酸的影响Fig.1 Effects of infusion LPS into external pudendal artery on the content of BHA in artery plasma of dairy cow s
2.3 阴外动脉灌注LPS对泌乳奶牛乳脂肪酸组成的影响
由表4可知,与对照组相比,阴外动脉灌注LPS降低了乳脂中饱和脂肪酸的含量(P>0.05),提高了乳脂中单不饱和脂肪酸、多不饱和脂肪酸和总不饱和脂肪酸的含量(P>0.05)。阴外动脉灌注LPS降低了乳脂短链脂肪酸的含量(P>0.05),增加了中链脂肪酸和长链脂肪酸的含量(P>0.05)。
2.4 阴外动脉灌注LPS对泌乳奶牛乳脂去饱和指数的分析
由表5可知,与对照组相比,阴外动脉灌注LPS 增加了 C16∶1/C16∶0、C18∶1/C18∶0 和c-9,t-11-CLA/t-11-C18∶1(P> 0.05),降低了 C14∶1/C14∶0(P>0.05)。
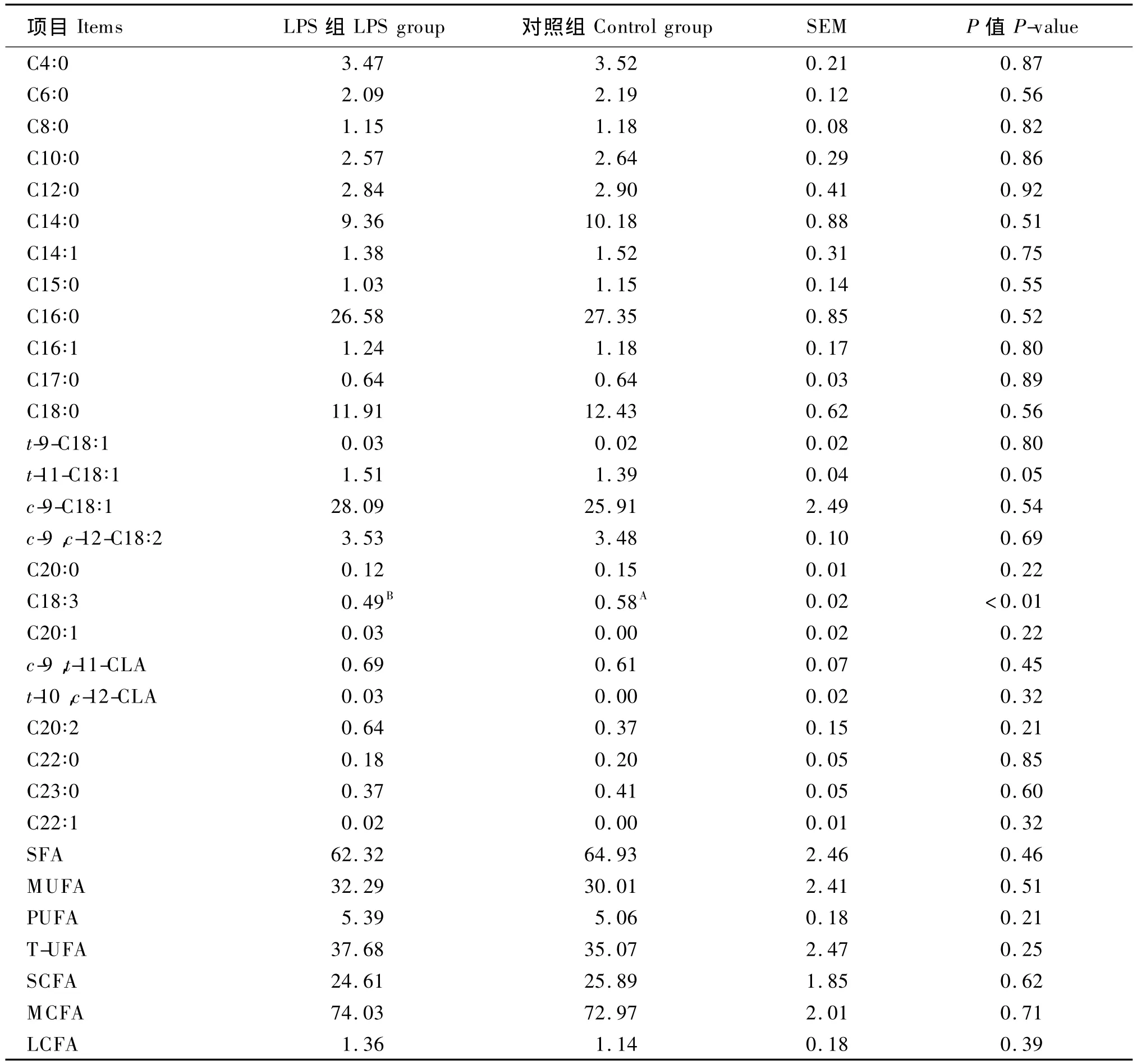
表4 阴外动脉灌注脂多糖对泌乳奶牛乳脂肪酸组成的影响Table 4 Effects of infusion LPS into external pudendal artery on m ilk fat constituents in dairy cows
3 讨论
3.1 阴外动脉灌注LPS对泌乳奶牛乳成分的影响
研究发现,谷物诱导的泌乳奶牛亚急性瘤胃酸中毒随着诱导时间的延续,外周血浆中LPS含量随之升高,12 h后达到每毫升0.81内毒素单位(endotoxin unit)[12];乳汁中乳脂率受到显著抑制(P<0.01),但有增加乳蛋白率的趋势(P=0.08)[15]。本试验结果与之相似,虽然本试验阴外动脉灌注的LPS剂量与Khafipour等[12]报道的血液中LPS含量相近,但本试验在抑制乳脂合成上未达到显著差异(P>0.05),极显著提高了乳汁中的乳蛋白率(P<0.01)。这可能与试验动物的泌乳阶段有关,Khafipour等[12]选用的试验动物为泌乳盛期奶牛[第(84±29)天],而本试验选用的试验动物为泌乳中后期的奶牛[第(185±30)天]。

表5 阴外动脉灌注脂多糖对泌乳奶牛去饱和指数分析Table 5 Effects of infusion LPS into external pudendal artery on the de-saturated indices in dairy cows
3.2 阴外动脉灌注LPS对泌乳奶牛乳脂合成前体物的影响
乳汁C4~C15、50%的C16脂肪酸是乳腺内源合成的脂肪酸,乳汁中50%的C16和C16以上的脂肪酸是乳腺直接从血浆中摄取转运[16]。内源合成的脂肪酸的前体物为瘤胃发酵产生的BHA和乙酸,乳腺摄取的脂肪酸来源于血浆中NEFA和富含三酰甘油的脂蛋白[17]。本试验结果表明,阴外动脉灌注LPS显著降低了泌乳奶牛动脉血浆中NEFA浓度(P<0.05),这与乳汁中乳脂率的降低相吻合;血浆中的BHA浓度(P>0.05)虽然有所增加,但随着灌注时间的延续,呈现先降低后升高的趋势,这与Waldron等[18]报道结果相同,Waldron等[18]研究发现,泌乳初期奶牛乳腺内注射LPS时,血浆中NEFA浓度和BHA浓度极显著降低(P<0.01),血浆中BHA浓度在注射后9 h达到最低[18];本试验血浆中BHA浓度在灌注后6 h达到最低,这可能与采样时间点不同有关。但Myers等[19]和 Husier等[20]分别在猪、犊牛上试验发现,LPS未能显著影响血液中NEFA的浓度(P>0.05)。这可能与试验动物的区别有关,本试验选择试验动物为泌乳奶牛,血浆中NEFA除用于生理活动外,还用于乳的生产,而Myers等[19]和Husier等[20]选择的试验动物猪、犊牛血浆中NEFA主要用于生理活动。
3.3 阴外动脉灌注LPS对泌乳奶牛乳脂肪酸组成的影响
研究发现,高精料饲粮条件下瘤胃液pH降低,革兰氏阴性菌溶解,释放大量的LPS[11-12,21-23],可能降低了脂类水解[24-25],加快食糜外流速度,缩短微生物对不饱和脂肪酸的作用时间,因此降低了生物氢化效率[26],增加了乳中不饱和脂肪酸的含量[24,27]。本试验结果也证实LPS影响乳脂脂肪酸的组成,增加了乳脂中不饱和脂肪酸的含量。虽然以前的研究重点在饲粮经瘤胃、肝脏、乳腺直到乳汁等一系列过程中发生的变化,LPS是否是主要的增加乳中不饱和脂肪酸的因子有待研究,本试验越过瘤胃、肝脏等复杂的器官,直接从供给乳腺供血的阴外动脉灌注LPS研究其对乳脂中乳脂肪酸的影响,结果证实了LPS是引起乳脂中不饱和脂肪酸提高的主要诱因之一。
3.4 阴外动脉灌注LPS对泌乳奶牛乳脂中去饱和指数的分析
不饱和脂肪酸的去饱和作用需要△去饱和酶系的参与[28],而乳腺中△9去饱和酶活性可用C14∶1/C14∶0、C16∶1/C16∶0、C18∶1/C18∶0 和c-9,t-11-CLA/t-11-C18∶1 等间接反映[29]。本试验结果表明,与对照组相比,阴外动脉灌注LPS组提高了 C16∶1/C16∶0、C18∶1/C18∶0 和c-9,t-11-CLA/t-11-C18∶1,这与乳脂脂肪酸中不饱和脂肪酸的含量提高相吻合;但C14∶1/C14∶0却有降低的趋势,这可能是C14∶1是短链脂肪酸,血浆中合成前体物供给不足所致,具体原因待进一步研究确证。
4 结论
本试验条件下,LPS影响乳汁中乳成分组成、乳脂合成前体物的生成以及乳脂肪酸的含量与比例,是诱发乳脂合成发生变化的主要激发因子之一。
[1]GUIDRY A J,OST M,MATHER IH,et al.Sequential response ofm ilk leukocytes,album in,immunoglobulins,monovalent ions,citrate,and lactose in cows given infusions ofEscherichia coliendotoxin into themammary gland[J].American Journal of Vet-erinary Research,1983,44(12):2262-2267.
[2]CARROLL JA,ARTHINGTON JD,Jr,CHASE C C,etal.Early weaning alters the acute-phase reaction to an endotoxin challenge in beef calves[J].Journal of Animal Science,2009,87(12):4167-4172.
[3]ZEBELIQ,AMETAJ B N.Relationships between rumen lipopolysaccharide and mediators of inflammatory response with m ilk fat production and efficiency in dairy cows[J].Journal of Dairy Science,2009,92(8):3800-3809.
[4]GRUNFELD C,FEINGOLD K R.Regulation of lipid metabolism bycytokines during host defense[J].Nutrition,1996,12(Suppl.1):S24 - S26.
[5]GRUNFELD C,MARSHALL M,SHIGENAGA J K,et al.Lipoproteins inhibit macrophage activation by lipoteichoic acid[J].Journal of Lipid Research,1999,40(2):245-252.
[6]LOPEZ-SORIANO F J,W ILLIAMSON D H.Acute effects of endotoxin(lipopolysaccharide)on tissue lipid metabolism in the lactating rat.The role of delivery of intestinal glucose[J].Molecular and Cellular Biochem istry,1994,141(2):113-120.
[7]PEKALA P H,KAWAKAM IM,ANGUS C W,et al.Selective inhibition of synthesis of enzymes for de novofatty acid biosynthesis by an endotoxin-induced mediator from exudate cells[J].Proceedings of the National Academy of Sciences of the United States of America,1983,80(9):2743-2747.
[8]KHOVIDHUNKITW,KIM M S,MEMON R A,et al.Thematic review series:the pathogenesis of atherosclerosis.Effects of infection and inflammation on lipid and lipoproteinmetabolism mechanisms and consequences to the host[J].Journal of Lipid Research,2004,45(7):1169-1196.
[9]MERKEL M,ECKEL RH,GOLDBERG IJ.Lipoprotein lipase:genetics,lipid uptake,and regulation[J].Journal of Lipid Research,2002,43(12):1997-2006.
[10]EMMANUEL D G V,MADSEN K L,CHURCHILL T A,et al.Acidosis and lipopolysaccharide fromEscherichia coliB∶055 cause hyperpermeability of rumen and colon tissues[J].Journal of Dairy Science,2007,90(12):5552-5557.
[11]GOZHO G N,KRAUSE D O,PLAIZIER JC.Rum inal lipopolysaccharide concentration and inflammatory response during grain-induced subacute rum inal acidosis in dairy cows[J].Journal of Dairy Science,2007,90(2):856-866.
[12]KHAFIPOUR E,KRAUSE D O,PLAIZIER JC.A grain-based subacute rum inal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation[J].Journal of Dairy Science,2009,92(3):1060-1070.
[13]GHOSHAL S,W ITTA J,ZHONG J,et al.Chylom icrons promote intestinal absorption of lipopolysaccharides[J].Journal of Lipid Research,2009,50(1):90-97.
[14]卜登攀,王加启,DHIMAN T R,等.植物油来源亚油酸和亚麻酸对乳脂CLA合成的影响[J].畜牧兽医学报,2007,7(6):63 -71.
[15]FAIRFIELD A M,PLAIZIER JC,DUFFIELD T F,et al.Effects of prepartum administration of a monensin controlled release capsule on rumen pH,feed intake,and milk production of transition dairy cows[J].Journal of Dairy Science,2007,90(2):937-945.
[16]哈斯额尔敦.十二指肠灌注游离十八碳脂肪酸对泌乳奶牛生产性能和乳脂肪酸组成的影响[D].博士学位论文.北京:中国农业科学院,2010.
[17]BAUMAN D E,MELLENBERGER RW,INGLE D L.Metabolic adaptations in fatty acid and lactose biosynthesis by sheep mammary tissue during cessation of lactation[J].Journal of Dairy Science,1974,57(6):719-923.
[18]WALDRON M R,KULICK A E,BELL A W,et al.Acute experimental mastitis is not causal toward the development of energy-related metabolic disorders in early postpartum dairy cows[J].Journal of Dairy Science,2006,89(2):596-610.
[19]MYERSM J,FARRELL D E,EVOCK-CLOVER C M,et al. Long-term recombinant porcine somatotropin(PST)treatment m itigates the responses to subchronic lipopolysaccharide in sw ine[J].Domestic Animal Endocrinology,2003,24(2):155-170.
[20]HUSIER B R,BLUM JW.Metabolic and endocrine changes in response to endotoxin adm inistration with or without oral arginine supplementation[J].Journal of Dairy Science,2002,85(8):1927-1935.
[21]NAGARAJA T G,LECHTENBERG K F.Acidosis in feedlot cattle[J].Veterinary Clinics of North America:Food Animal Practice,2007,23(2):333 -350.
[22]PLAIZIER JC,KRAUSED O,GOZHO G N,etal.Subacute rum inal acidosis in dairy cow s:the physiological causes,incidence and consequences[J].The Veterinary Journal,2008,176(1):21 -31.
[23]ANDERSEN P H,BERGELIN B,CHRISTENSEN K A.Effect of feeding regimen on concentration of free endotoxin in rum inal fluid of cattle[J].Journal of Animal Science,1994,72(2):487-491.
[24]LATHAM M J,STORRY J E,SHARPE M E.Effect of low-roughage diets on them icroflora and lipidmetabolism in the rumen[J].Applied and Environmental Microbiology,1972,24(6):871-877.
[25]DOREAU M,FERLAY A.Digestion and utilisation of fatty acids by rum inants[J].Animal Feed Science and Technology,1994,45(3/4):379-396.
[26]KALSCHEUR K F,TETER B B,PIPEROVA L S,et al.Effectof dietary forage concentration and buffer addition on duodenal flow oftrans-C18∶1 fatty acids and m ilk fat production in dairy cows[J].Journal of Dairy Science,1997,80(9):2104-2114.
[27]PALMQUIST D L,DENISE B A,BARBANO D M.Feed and animal factors influencing m ilk fat composition[J].Journal of Dairy Science,1993,76(6):1753-1771.
[28]DE B,JA G.Historical perspective and recent developments in identifying the cause of diet-induced m ilk fat depression[C]//Proceedings of the cornell nutrition conference for feed manufacturers.Ithaca,N.Y.:Cornell University,2000:191 -202.
[29]BAUMAN D E,GRIINARIJM.Regulation and nutritional manipulation of milk fat[M]//MOL J A,CLEGG R A.Biology of the Mammary Gland.New York:Academ ic/Plenum Publishers,2002:209-216.
*Corresponding author,professor,E-mail:wang-jia-qi@263.net
(编辑 王智航)
Infusion of Lipopolysaccharide into External Pudendal Artery of Lactating Dairy Cows:Effects on Milk Composition and Milk Fat Constituents
ZHANG Yangdong1,2WANG Jiaqi2*HU Tao2LIShanshan2BU Dengpan2JIN Di2SUN Peng2ZHOU Lingyun2
(1.Institute of Animal Science,Northeast Agricultural University,Harbin150030,China;2.Institute of Animal Science of the Chinese Academy of Agricultural Sciences,Beijing100193,China)
The study was designed to evaluate m ilk composition,content of synthesis precursors and m ilk fat constituents in response to the infusion of lipopolysaccharide(LPS)into external pudendal artery of lactating dairy cows.Six multiparous Holstein cows[(185±30)days of lactation,BW=(576±36)kg]were random ly divided into two group.A crossover trial design was used in the study,and cows in experimental group and control group were infused LPS(Escherichia coliO111∶B4,0.01 μg/kg BW)and physiological saline into external pudendal artery,respectively.The study consisted of 2 experimental periodswith 7 d each and a 14-day-interm ission between.The results showed as follows:dry matter intake(DM I)was not significantly affected by LPS challenge(P>0.01);m ilk protein percentage was significantly increased(P>0.05),but m ilk fat percentage was not significantly affected by LPS challenge(P>0.05);LPS challenge also significantly reduced the plasma non-esterified fatty acid(NEFA)content(P<0.01),which was the synthesis precursor ofm ilk fat,meanwhile,the hydroxybutyric acid(BHA)content tended to decrease at first and then increase(P>0.05);by the challenge of LPS,the contents of saturated fatty acids(P>0.05)and short-chain fatty acids(P> 0.05)were decreased,but the ones of unsaturated fatty acids and m iddle-chain fatty acids were increased(P>0.05),in addition,the desaturation of fatty acidswas also affected.It can be concluded that LPS is one of the major factors in affecting m ilk fat synthesis.[Chinese Journal of Animal Nutrition,2011,23(8):1317-1323]
external pudendal artery;lactating dairy cow;lipopolysaccharide;m ilk composition;m ilk fat constituents
S823
A
1006-267X(2011)08-1317-07
10.3969/j.issn.1006-267x.2011.08.010
2011-03-08
张养东(1982—),男,山东济宁人,博士研究生,从事反刍动物营养与牛奶质量研究。E-mail:zyd1982@yahoo.cn
*通讯作者:王加启,研究员,博士生导师,E-mail:wang-jia-qi@263.net

