Niche partitioning between sympatric rhesus macaques and Yunnan snub-nosed monkeys at Baimaxueshan Nature Reserve, China
Cyril C GRUETER , LI Da-Yong, FENG Shun-Kai, REN Bao-Ping
(1. Anthropological Institute and Museum, University of Zurich, Zurich 8057, Switzerland; 2. Key Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, the Chinese Academy of Sciences, Beijing 100101, China; 3. College of Life Sciences, China West Normal University, Nanchong Sichuan 637002, China; 4. Baimaxueshan National Nature Reserve, Tacheng, WeixiYunnan 674400, China)
Niche partitioning between sympatric rhesus macaques and Yunnan snub-nosed monkeys at Baimaxueshan Nature Reserve, China
Cyril C GRUETER1,*, LI Da-Yong2,3, FENG Shun-Kai4, REN Bao-Ping2
(1.Anthropological Institute and Museum, University of Zurich, Zurich8057, Switzerland; 2.Key Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, the Chinese Academy of Sciences, Beijing100101,China; 3.College of Life Sciences, China West Normal University, Nanchong Sichuan637002,China; 4.Baimaxueshan National Nature Reserve, Tacheng, WeixiYunnan674400,China)
Here we provide a preliminary assessment of dietary and habitat requirements of two sympatric primate taxa, a “simple-stomached” and “complex-stomached” species (Rhinopithecus bietiColobinae vs.Macaca mulattaCercopithecinae), as a basis for illuminating how the two coexist. Ofca.22 plant food species consumed by the macaques, at least 16 were also eaten by the snub-nosed monkeys. Both species showed a preference for fruits. While the snub-nosed monkeys did not utilize any resources associated with human communities, rhesus macaques did occasionally raid agricultural crops. The mean elevation of the snub-nosed monkey group was 3,218 m, while the mean elevation of the macaque group was 2,995 m. Macaques were also spotted on meadows whereas snub-nosed monkeys evidently avoided these. For both species, mixed deciduous broadleaf/conifer forest was the most frequently used ecotype, but whereas evergreen broadleaf forest (Cyclobalanopsiscommunity) accounted for only 3% of the location records of the snub-nosed monkeys, it accounted for 36% of the location records of the macaques. Groups of the two species usually kept a considerable spatial distance from one another (mean 2.4 km). One close encounter and confrontation between groups of the two species resulted in the macaque group moving away. Our findings suggest that the coexistence of the two taxa is facilitated via differential macrohabitat use and spatial avoidance. Although divergent habitat-use strategies may reflect interspecific competition, they may also merely reflect different physiological or ecological requirements.
Macaca mulatta;Rhinopithecus bieti;Yunnan; Interspecific competition; Diet; Habitat use
Ecological niche theory posits that co-occurring species should evolve adaptations for avoiding orreducing interspecific resource competition (Hutchinson, 1959). A plethora studies have come to the conclusion that niche partitioning in sympatric species is mostly manifested in differential habitat use or differential diet selection (Ben-David et al, 1995; Churchfield & Sheftel, 1994; Gautier-Hion, 1978; MacKinnon & MacKinnon, 1980; Namgail et al, 2004; Porter, 2001; Rodman, 1991; Sachot et al, 2003; Tan, 1999; Wei et al, 2000).
Simple-stomached primates (cercopithecine monkeys) and complex-stomached primates (colobine monkeys) occur sympatrically in many regions of Subsaharan Africa and South/Southeast Asia. Differing food requirements and digestive strategies between them have been assumed to lead to little competition over food sources (Bennett & Davies, 1994; Oates, 1994). Nevertheless, partly overlapping dietary spectra create a potential for food competition between colobines and cercopithecines (e.g. Davies, 1991; MacKinnon & MacKinnon, 1980; Yeager, 1996).
In this article, we address the question of how sympatric rhesus macaques (Macaca mulatta vestita) and Yunnan snub-nosed monkeys (Rhinopithecus bieti) in the Baimaxueshan Nature Reserve manage to coexist. Both species are medium-sized to large and are semiterrestrial or semiarboreal foragers, i.e. both can get access to resources on the ground and in the tree layer.
Rhesus macaques are among the most widely distributed primate species, and their occurrence in diverse types of environments —from mangrove swamps to high-altitude pine-oak-spruce forests (e.g. Teas, 1978; Teas et al, 1980) — makes them prone to be called ecological generalists. The subspeciesvestitais distributed in southeast Tibet and northwest Yunnan (Brandon-Jones et al, 2004). Rhesus macaques exhibit a preference for grasses and clover in the deciduous forests of the Himalayan foothills (Goldstein & Richard, 1989), but are largely frugivorous in other habitats (Lindburg, 1971). In many parts of their range, rhesus macaques have become “Kulturfolger” and venture into urban environments (e.g. parkland adjacent to temple complexes) where they may live close to and commensally with humans and rely heavily on agricultural crops and food from people, garbage, grains and other hand-outs (Fooden, 2000; Goldstein & Richard, 1989; Richard et al, 1989; Southwick & Siddiqi, 1977).
Compared to rhesus macaques, the dietary and habitat requirements of the snub-nosed monkeys are narrower. This colobine has often been seen as a specialist, relying on tree lichens and dwelling in alpine fir forests (Kirkpatrick, 1996; Li et al, 1982). However, recent research has demonstrated greater ecological flexibility with regard to vegetation association and diet choice: they also occur in mixed deciduous broadleaf/conifer forests and forage on seasonally varying plant parts of angiosperm plants (Ding & Zhao, 2004; Grueter et al, 2009a,b; Li et al, 2008).
To elucidate how macaques and snub-nosed monkeys coexist and what the nature of their interspecific relations is, we compared macrohabitat use and diet of the two species based on a 2-year field study. Due to the fact that the study focused onRhinopithecus bieti, the data onRhinopithecusare more detailed and comprehensive while the data on Macaca are more cursory and preliminary.
1 Materials and Methods
1.1 Study region
We collected data across a 23-month period in 2005, 2006 and 2007 in the southern zone of the Baimaxueshan National Nature Reserve (27°34'N, 99°17'E), northwest Yunnan, PRC. The research area (ca 40 km2) ranges in altitude from 2,500 to 4,000 m and comprises a mixture of forest types interspersed with grassy alpine meadows. Topography consists of rugged mountains dissected by steep valleys. Annual rainfall averaged 1,004 mm over a 2-year period, with about 80% of this recorded from April to September. Snowfalls occurred in late winter. Monthly mean temperatures ranged from 6.6℃ in January 2007 to 21.5℃ in July 2006. Livestock grazing inside the forest and on meadows as well as extractive exploitation of herbal medicine, mushrooms and wood by local residents was widespread during the time of the study.
Six major vegetation communities, characterized by different species of dominant trees, were identified (Li et al, 2008): i) warm-temperate pine forest [main canopy species:Pinus yunnanensis(Pinaceae)] between 2,500 and 3,100 m; ii) mesophytic evergreen broad-leaved forest [main species:Cyclobalanopsisspp. (Fagaceae)] between 2,500 and 3,000 m; iii) montane sclerophyllous oak forest [main species:Quercus pannosa(Fagaceae)] between 3,200 and 3,500 m; iv) mixed deciduous broad-leaved/conifer forest [main species:Acanthopanax evodiaefolius(Araliaceae),Sorbusspp. (Rosaceae),Acerspp. (Aceraceae),Betula utilis(Betulaceae),Picea likiangensis,Tsuga dumosa(Pinaceae)] between 2,900 and 3,600 m; v) cool temperate fir forest between 3,500 and 4,000 m [main species:Abies georgei(Pinaceae)]. Bamboo [Fargesiaspp. (Gramineae)] and rhododendrons [Rhododendronspp. (Ericaceae)] are found in the undergrowth of all vegetation types.
Two primate species inhabit the study site,Macaca mulattaandRhinopithecus bieti. The focal snub-nosed monkey (SNM) group (Gehuaqing group) was composed ofca410 members. The group has become partially habituated after years of continued surveillance by reserve staff. Whereas only 1 group of SNM roamed the forest inside our study area, there were 3 groups of macaques, one in the central part of the study area and two in more peripheral areas.
1.2 Field work
The SNM were the primary focus of this research project and the study group was tracked whenever conditions were favorable. We established contact with the semi-habituated group (n=ca.410) on an average of 12 d per month. Every 30 min, we recorded the location and elevation of the group with a GPS receiver with an integrated barometrical altimeter as long as the group was within aural or visual reach. Forest type was recorded upon inspection of the main tree taxa. The feeding behaviour of the SNM was systematically sampled by means of group scans; additional data on the dietary spectrum was obtained viaad libitumobservations and by collecting partially consumed and discarded plant material in the group’s foraging path (for details see Grueter et al, 2009a,b).
The macaques were often aurally located, and indirect signs of their presence such as scat, foot/handprints etc. were encountered occasionally. The fact that the macaques were not habituated to people resulted in a limited direct contact time, totalingca. 3 h. The macaques’ alertness ruled out any detailed observations and allowed only very few direct feeding observations. Most data on food choice of macaques were based on indirect evidence, i.e. came from examination of food leftovers. Macaque faeces were cursorily inspected for seeds.
Macaque sightings usually happened accidentally when we were trailing the SNM. Days on which we located the SNM group and found fresh evidence of macaque whereabouts offered us an opportunity to estimate the distance between the two groups. Calculating the straight-line distance between the two groups on topographic maps would not take into consideration the hilly terrain would result in imprecise distance estimates. We therefore estimated the approximate walking distance rounded to the nearest 100 m.
As a means of sampling the vegetation, we established a total of 67 plots of 20 m × 20 m at 200-m altitude intervals; the placement of plots within the available habitat types was based on the relative contribution of different habitat types within the study area. We recorded the following variables for all trees with circumference >40 cm in the plots: tree height, crown diameter, bole height and girth. Crown diameter was paced off directly beneath the tree. For more details on the vegetation survey and calculation of structural properties of tress, see Li et al (2008). The data on use of altitudes were taken from (Li et al, 2008).
We performed non-parametric statistical tests and considered aPvalue <0.05 to be significant.
2 Results
We obtained 981 visual records of the SNM group’s location. For the macaques, we obtained 23 visual and 5 aural location records and found fresh scat on 18 occasions. Of these 46 records for the macaques, 3 records were from the Geluo group, 2 from the Lamasi group and the remainder from the Gehuaqing group.
for particular habitats are presented in Fig. 1. For both species, mixed deciduous broad-leaved/ conifer forest was the most frequently used ecotype, but the two species differed in their preference for subtropical evergreen broad-leaved forest. While this latter habitat made up only 3% of the SNM location records, it accounted for 36% of the macaques’ location records. Macaques were thus relatively much more associated with evergreen broad-leaved forest than SNM. Macaques were never encountered in fir forest and sclerophyllous oak forest and only once in pine forest. Macaques were once spotted on a meadow whereas SNM evidently avoided all open treeless areas unless they had to cross a gap in the canopy terrestrially.
The mean elevation used by the SNM group was 3,218 m (SD=188 m, range 2,625-3,793 m,n=981), the mean elevation of the macaques was 2,995 m (SD=215 m, range 2,600-3,600 m,n=46). The mean elevation used is significantly different between the two species (Mann-Whitney U,P<0.001,Z=-6.717,n1= 981,n2=46).
When comparing architectural features of trees inside the main habitat of SNM (mixed deciduous broad-leaved/conifer forest) and macaques (evergreen broad-leaved forest), there were significant differences in tree height (Mann Whitney U,P<0.001,Z=-4.024, (Tab. 2). A complete list of plant foods of the SNM is provided elsewhere (Grueter et al, 2009b). Both species appeared to incorporate animal matter into their diet. Amount and frequency of insect feeding, though, has not been evaluated. Whereas the SNM seemed to search for insects beneath the bark of trees, macaques were observed looking for unspecified animal matter in a small pond.n1=969,n2=249), crown volume (Z=-8.004,P< 0.001), and DBH (P=0.009,Z=-2.601) (Tab. 1). For all these variables, values were larger in evergreen forest.
The two primate species largely overlapped in their dietary spectrum. Ofca22 species eaten by the macaques, at least 14 were also consumed by the SNM
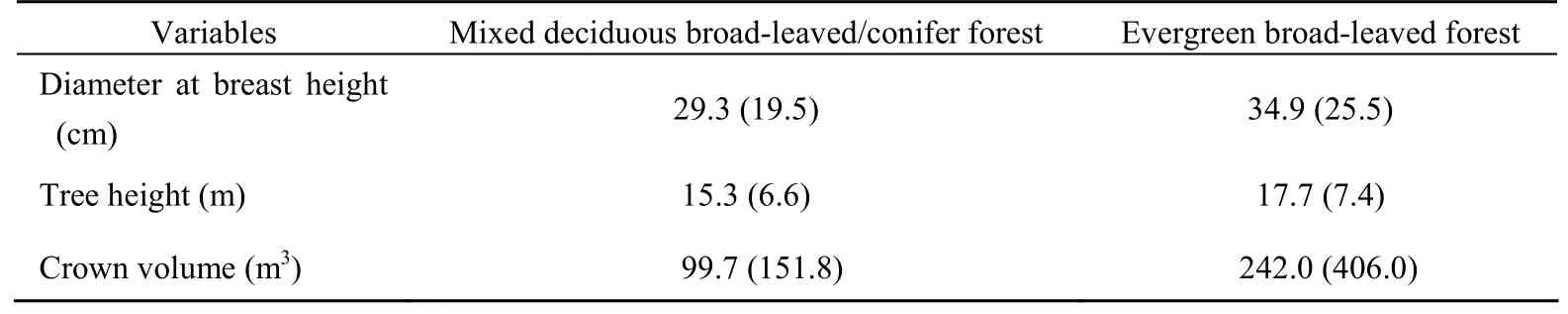
Tab. 1 Variables of measured trees in mixed deciduous broad-leaved/conifer forest vs. evergreen broad-leaved forest at Samage, Baimaxueshan Nature Reserve
The two species usually kept a considerable distance. On March 14th 2007, however, the 2 species were <400 m away; at that time, the SNM band had split into 2 subgroups of which the smaller subgroup was the one that was close to the macaque group. On 24 d, we obtained location records for both macaques and SNM: the mean distance was 2.35 km (SD=2.50 km, range 0.15-10.0 km). Only once were we able to witness a direct encounter between the macaques and the SNM. When the 2 species were at a distance ofca150 m, the macaques started uttering harsh calls, stayed highly alert and frequently sought exposed spots to monitor the SNM. The SNM group, however, remained rather indifferent. After several minutes of nervous vocalizing and being vigilant, the macaques retreated by moving downhill. The SNM group clearly outnumbered the macaque group.
3 Discussion
All the food items recorded for the rhesus macaques at Samage were ingested by the SNM as well (Grueter et al, 2009b), implying that diet choice does not distinguish specific niches. However, a thorough quantitative assessment of contribution of particular plant items to diet of rhesus monkeys is yet to be done to conclude whether interspecific food competition with SNM is of significance. This study was not aimed at obtaining an exhaustive list of food items consumed by the macaques, e.g. plant fiber residues were not extracted from feces.
SNM rely heavily on lichens throughout the year, and lichens form the majority of the diet in winter (Grueter et al, 2009a; Kirkpatrick, 1996; Xiang et al, 2007). Ecological niche theory posits that interspecific feeding competition is likely to be highest during periods of food 1996). This dependence on lichens most clearly distinguishes SNM from macaques and may permit coexistence during periods of overall food shortage, ie. the harsh winter period. However, it would be tempting to speculate that lichen consumption has evolved as a competition reducing strategy. Lichenivory has evolved as a response to the dearth of other fallback foods such as palatable mature leaves (Grueter et al, 2009a). Barbary macaques (Macaca sylvanus) do eat lichens in varying amounts (Ménard, 1985), and we would thus expect the scarcity and that dietary divergence will be most pronounced during this period (Schoener, 1974; Ungar, congeneric rhesus macaques to be anatomically and physiologically capable of digesting lichens as well. However, we have no indication that rhesus macaques at Samage feed on lichens, and how they cope with fruit scarcity in winter remains to be investigated. Other temperate macaques survive on buds, bark and mature leaves in winter [for a review see (Grueter et al, 2009a)].

Tab. 2 Plant food species and items consumed by rhesus macaques and SNM at Samage
Teas (1978) noted that the rhesus groups inhabiting mixed alpine forests in Nepal raided crops, and she surmised that the animals’ ability to survive at this high altitude was, at least in part, a result of the crop availability. Crop-raiding (corn, cabbage, potatoes, radishes) is also done by macaques at Samage and may not only help them to sustain through lean phases, but also makes them less dependent on resources utilized by the SNM. The SNM have never been witnessed to enter agricultural fields.
The taxonomic and physiognomic heterogeneity of the forest may promote coexistence (Hampton, 2004). Our preliminary findings show that coexistence is facilitated via differential habitat use in the forest mosaic present at Samage and spatial avoidance. Whereas SNM are primarily attached to mixed forest and rarely moved to evergreen forest, rhesus macaques were frequently spotted in the latter and did seem to have a preference evergreen oak forest at low altitudes. Moreover, macaques occasionally ventured into open areas such as river beds, edges of meadows and even centers of meadows. SNM, however, stayed away from clearings unless gaps in the canopy and lack of suitable arboreal routes forced them to enter clearings. (Goldstein & Richard, 1989) found that even in a forested habitat, rhesus macaques in Northwest Pakistan spent a disproportionately large amount of time in clearings and meadows. Whether there is competition for sleeping trees has not been assessed in this study. Previous work, however, has shown that some of the preferred trees for sleeping (firs, hemlocks, oaks) are among the commonest at the site (Li et al, 2007).
Resource partitioning is not necessarily an evolutionary mechanism to reduce interspecfic competition (Dueser & Shugart, 1978), but may also reflect divergent species-specific physiological, ecological and nutritional requirements and constraints (Maitz & Dickman, 2001; Walter, 1991), e.g. the fact that lichens are scarce in evergreen broad-leaved forest may partly account for the divergent macrohabitat utilization patterns. Furthermore, the supposedly thicker fur of the SNM may allow them to stay at higher elevations where they are exposed to colder temperatures.
In the observed direct encounter between the two species, the group size difference alone may have been responsible for the dominance of the SNM. The SNM group numbered about 410 while the size of the Gehuaqing macaque group was unclear: the highest number of individuals counted was 10, but since some individuals may likely have been missed in the dense vegetation, an estimate of 20 animals seems more reasonable. (MacKinnon & MacKinnon, 1980) provide circumstantial evidence that macaques are dominant over langurs in direct competitive conflicts, with body masses playing no role, but group size probably having an impact on the outcome of the contest. Yeager noted that in direct competition, long-tailed macaques (Macaca fascicularis) were often able to displace proboscis monkeys (Nasalis larvatus) (Yeager, 1989).
Within the ecological community at South Baimaxueshan, both macaques and snub-nosed monkeys have to share the available resources with a number of non-primate vertebrate competitors as well as humans, e.g. seeds such as acorns may also be used by squirrels and domestic sheep (cf. Emmons, 1980; Payne, 1980), bamboo shoots by red pandas (Ailurus fulgens), domestic cows and humans, fruits such asAcanthopanax(the single most important non-lichen food item the SNM (Grueter et al, 2009b) by some frugivorous birds such as blue-fronted redstart (Phoenicurus frontalis) and rufous-vented yuhina (Yuhina occipitalis) (Grueter, pers. obs.), and lichens by birds such as tits (Parusspp.) (used as material for nest building) and likely musk deer (Moschusspp.) (cf. Ustinov 1969).
Acknowledgements:We are grateful to LIU Si-kang, director of the Weixi section of Baimaxueshan Nature Reserve, for allowing us to carry out primatological research at the reserve. For assistance in the field, we thank FENG Xue-wen and FENG Xue-shang. FANG Zhen-dong is acknowledged for assistance in identifying botanical specimens. This paper was written while Grueter was working on his doctorate on socioecology ofRhinopithecus bietiwhich was sponsored by Janggen-Pǒhn-Stiftung, A. H. Schultz Stiftung, Zürcher Tierschutz, Zoological Society of San Diego, Primate Conservation, Inc., G. & A. Claraz-Schenkung, Goethe-Stiftung, Kommission für Reisestipendien der Schweizerischen Akademie der Naturwissenschaften SANW, Primate Action Fund of Conservation International, and one anonymous Swiss donor.
Ben-David M, Bowyer R, Faro J. 1995. Niche separation by mink and river otters: Coexistence in a marine environment[J].Oikos, 75: 41-48.
Bennett EL, Davies AG. 1994. The ecology of Asian colobines[M] // Davies AG, Oates JF. Colobine Monkeys: Their Ecology, Behaviour and Evolution. Cambridge: Cambridge University Press, 129-171.
Brandon-Jones D, Eudey AA, Geissmann T, Groves CP, Melnick DJ, Morales JC, Shekelle M, Stewart CB. 2004. Asian primate classification[J].Int J Primat, 25: 97-164.
Churchfield S, Sheftel B. 1994. Food niche overlap and ecological separation in a multispecies community of shrews in the Siberian taiga[J].J Zool, 234: 105-124.
Davies AG. 1991. Seed-eating by red leaf monkeys (Presbytis rubicunda) in dipterocarp forest of northern Borneo[J].Int J Primat, 12: 119-144.
Ding W, Zhao QK. 2004.Rhinopithecus bietiat Tacheng, Yunnan: Diet and daytime activities[J].Int J Primat, 25: 583-598.
Dueser RD, Shugart HH. 1978. Microhabitats in a forest-floor small mammal fauna[J].Ecology, 60: 108-118.
Emmons LH. 1980. Ecology and resource partitioning among nine species of African rainforest squirrels[J].Ecol Monogr, 50: 31-54.
Fooden J. 2000. Systematic review of the rhesus macaqueMacaca mulatta(Zimmermann, 1780) [J].Fieldian Zool, 96: 1-179.
Gautier-Hion A. 1978. Food niches and coexistence in sympatric primates in Gabon[M]// Chivers D, Herbert J. Recent Advances in Primatology. New York: Academic Press, 269-286.
Goldstein SJ, Richard AF. 1989. Ecology of rhesus macaques (Macaca mulatta) in northwest Pakistan[J].Int J Primat, 10:531-567.
Grueter CC, Li D, Ren B, Wei F, Xiang Z, van Schaik CP. 2009a. Fallback foods of temperate-living primates: A case study on snub-nosed monkeys[J].Am J Phys Anthrop, 140: 700-715.
Grueter CC, Li D, Ren B, Wei F, van Schaik CP. 2009b. Dietary profile ofRhinopithecus bietiand its socioecological implications[J].Int J Primat, 30: 601-624.
Hutchinson G. 1959. Homage to Santa Rosalia: why are there so many species? [J]Am Nat, 93:145-159.
Kirkpatrick RC. 1996. Ecology and Behavior of the Yunnan Snub-Nosed Langur (Rhinopithecus bieti, Colobinae) [D]. Ph.D. thesis, University of California, Davis.
Li D, Grueter CC, Ren B, Zhou Q, Li M, Peng Z, Wei F. 2007. Characteristics of night-time sleeping places selected by golden monkeys (Rhinopithecus bieti) in the Samage Forest, Baima Snow Mountain Nature Reserve, China[J].Integr Zool, 1: 141-152.
Li D, Grueter CC, Ren B, Long Y, Li M, Peng Z, Wei F. 2008. Ranging ofRhinopithecus bietiin the Samage Forest, China. II. Use of land cover types and altitudes[J].Int J Primat, 29: 1147-1173.
Li ZX, Ma SL, Hua CH, Wang YX, 1982. The distribution and habit of the Yunnan golden monkey,Rhinopithecus bieti[J].J Hum Evol, 11: 633-638.
Lindburg DG. 1971. The rhesus monkey in north India: An ecological and behavioral study[M]// Rosenblum LA. Primate Behavior: Developments in Field and Laboratory Research: Volume 2. New York: Academic Press, 1-106.
MacKinnon J, MacKinnon KC. 1980. Niche differentiation in a primate community[M]// Chivers DJ. Malayan Forest Primates. New York: Plenum Press, 167-190.
Maitz WE, Dickman CR. 2001. Competition and habitat use in two species of native AustralianRattus[J].Oecologia, 128: 526-538.
Ménard N. 1985. Le régime alimentaire deMacaca sylvanusdan diffé rent habitats d'Algérie: I-régime en chênaie décidue[J].Rev Ecol:Terre Vie, 40: 451-466.
Namgail T, Fox JL, Veer Bhatnagar Y. 2004. Habitat segregation between sympatric Tibetan argaliOvis ammonhodgsoniand blue sheepPseudois nayaurin the Indian Trans-Himalaya[J].J Zool, 262: 57-63.
Oates JF. 1994. The natural history of African colobines[M]//Davies AG, Oates JF. Colobine Monkeys: Their Ecology, Behaviour and Evolution. Cambridge: Cambridge University Press, 75-128.
Payne JB. 1980. Competitors[M]//Chivers DJ. Malayan Forest Primates. New York: Plenum Press, 261-277.
Porter LM. 2001. Dietary differences among sympatric Callitrichinae in Northern Bolivia:Callimico goeldii,Saguinus fuscicollisandS. labiatus[J].Int J Primat, 22: 961-992.
Richard AF, Goldstein SJ, Dewar RE. 1989. Weed macaques: the evolutionary implications of macaques feeding ecology[J].Int J Primat, 10: 569-594.
Rodman PS. 1991. Structural differentiation in microhabitats of sympatricMacaca fascicularisandM. nemestrinain East Kalimantan, Indonesia[J].Int J Primat, 12: 357-375.
Sachot S, Perrin N, Neet C. 2003. Winter habitat selection by two sympatric forest grouses in western Switzerland: implications for conservation[J].Biol Conserv, 112: 373-382.
Schoener TW. 1974. Resource partitioning in ecological communities[J].Science, 185: 27-39.
Southwick CH, Siddiqi MF. 1977. Population dynamics of rhesus monkeys in northern India[M]//Bourne GH. Primate Conservation. New York: Academic Press, 339-362.
Tan CL. 1999. Group composition, home range size, and diet of three sympatric bamboo lemur species (genusHapalemur) in Ranomafana National Park, Madagascar[J].Int J Primat, 20:547-566.
Teas J. 1978. Behavioral Ecology of Rhesus Monkeys (Macaca mulatta) in Kathmandu, Nepal[D]. Ph.D. thesis, Johns Hopkins University, Baltimore.
Teas J, Richie T, Taylor H, Southwick C. 1980. Population patterns and behavioral ecology of rhesus monkeys (Macaca mulatta) in Nepal[M]//Lindburg D. The Macaques: Studies in Ecology, Behavior and Evolution. New York: Van Nostrand Reinhold, 247-262.
Ungar PS. 1996. Feeding height and niche separation in sympatric Sumatran monkeys and apes[J].Folia Primat, 67: 163-168.
Ustinov S. 1969. On the feeding ofMoschus moschiferusL. and its adaptations to conditions for food searches[J].Zool Zh, 48:1558-1563.
Walter G. 1991. What is resource partitioning? [J].J Theoret Biol, 150: 137-143.
Wei FW, Feng ZJ, Wang ZW, Hu J. 2000. Habitat use and separation between the giant panda and the red panda[J].J Mamm, 80: 448-455.
Xiang Z-F, Huo S, Xiao W, Quan R-C, Grueter CC. 2007. Diet and feeding behavior ofRhinopithecus bietiat Xiaochangdu, Tibet: Adaptations to a marginal environment[J].Am J Primat, 69: 1141-1158.
Yeager CP. 1989. Feeding ecology of the proboscis monkey (Nasalis larvatus) [J].Int J Primat, 10: 497-530.
Yeager CP. 1996. Feeding ecology of the long-tailed macaque (Macaca fascicularis) in Kalimantan Tengah, Indonesia[J].Int J Primat, 17: 51-62.
云南白马雪山自然保护区猕猴和滇金丝猴的生态位分离
Cyril C GRUETER1,*, 黎大勇2,3, 蜂顺开4, 任宝平2
(1.Anthropological Institute and Museum, University of Zurich, Zurich8057, Switzerland; 2. 中国科学院动物研究所 动物生态和保护生物学重点实验室,北京 100101; 3. 西华师范大学 生命科学学院,四川 南充 710069; 4. 白马雪山国家级自然保护区, 云南 维西 674400)
原则上,食叶的滇金丝猴(Rhinopithecus bieti)和杂食的猕猴(Macaca mulatta)是可以同地共栖的,但这两种灵长类究竟是如何同地共存却一直是一个鲜见涉足的问题。该文初步通过分析它们的食性和生境需求来阐明两者共存的可能性。在猕猴取食约22种植物中,有16种也是滇金丝猴的取食对象。两种灵长类都显示出喜食果实。人们尚未发现滇金丝猴涉足人类作物等相关资源,但发现猕猴经常侵食庄稼。这与其生活海拔不同有关:滇金丝猴一般生活在平均海拔为3 218 m的山林中,而猕猴活动在平均海拔为2 995 m的林地。猕猴也会涉足牧场,而滇金丝猴回避这种场地。对于这两个种,混合的落叶阔叶/针叶林是最频繁使用的森林类型;滇金丝猴很少进入常绿阔叶林(青冈属群落,利用率仅3%),而猕猴相对进入这类林型的机会远比滇金丝猴高(36%)。两个物种的群体间常相互远离(2.4 km)。当其相遇时,常是猕猴主动回避。上述结果提示滇金丝猴和猕猴是通过大生境分化利用和空间避让来共存的。尽管不同的生境利用策略一定程度上会反映种间竞争的存在,但这种不同更可能反映着它们不同的生理/生态需求。
猕猴;滇金丝猴;云南;种间竞争;食性;生境利用
Q959.848;Q958.12
A
0254-5853-(2010)05-0516-07
;2010-03-29;接受日期:2010-08-19
10.3724/SP.J.1141.2010.05516
date: 2010-03-29; Accepted date: 2010-08-19
* Corresponding author (通信作者), current address: Max Planck Institute for Evolutionary Anthropology, Department of Primatology, Deutscher Platz 6, 04103 Leipzig, Germany; Email: cyril_grueter@eva.mpg.de
- Zoological Research的其它文章
- Rates and patterns of microsatellite mutations in common carp (Cyprinus carpio L.)
- Ethogram of Yangtze finless porpoise calves (Neophocaena phocaenoides asiaeorientalis)
- Embryonic development of the concave-eared torrent frog with its significance on taxonomy
- Seasonal variation and synchronization of sexual behaviors in free-ranging male Tibetan macaques (Macaca thibetana) at Huangshan, China
- Identification of zRAP55,a gene .preponderantly expressed in StagesⅠandⅡ oocytes of zebrafish
- 小杜鹃对强脚树莺的巢寄生及其卵色模拟

