Ni含量对Co1-xNixB催化水解放氢性能的影响
李 丽 王一菁 王亚平 任秋丽 焦丽芳 袁华堂
(南开大学新能源材料化学研究所,“先进能源材料化学”教育部重点实验室,天津 300071)
Hydrogen has attracted much attention due to the ever-growing demands in friendly environment,especially with the improvement of green-gas emission in the world.There are many methods of hydrogen production,such as reforming of natural gas, coal gasification,biomass pyrolysis and gasification and hydrolysis of complex hydrides.Recently,complex hydrides have been demonstrated to be promising hydrogen sources of portable application[1-3].Among all of the complex hydrides,sodium borohydride is a candidate for pure hydrogen supply to fuel cells at room temperature due to its high hydrogen storage capacity with a theoretical value of 10.8%(w,mass fraction).Sodium borohydride is stable,non-flammable,non-toxic in nature.Besides,it can release double amount of its stored high-purity hydrogen[4]. In addition,sodium borohydride solution reveals excellent stability under high pH value at room temperature,and hydrolysis reaction product of sodium borohydride is environ-mentally clean and can be recycled to generate the reactant[5].However,hydrogen generation rate is different with different catalysts.Hence,catalysts play a significant role in controlling hydrogen generation performance from hydrolysis of alkaline NaBH4solution.When catalysts are added into NaBH4alkaline solution,overall reactions can be depicted as shown in Eq.(1):
NaBH4+2H2O→NaBO2+4H2↑ (1)
A number of catalysts have been proved to be effective for accelerating hydrolysis reaction of NaBH4solution.Noble metal, including Ru,Pt,Pt-Pd,Pt-LiCoO2alloys,AgNi alloy[6-10],revealed excellent catalytic activity.However,these catalysts seem to be not practical for industrial application owing to their high cost and availability.On the other hand,some cheaper transition metal catalysts,such as Raney Ni and Co[11],carbon-supported Co-B[12],nickel and cobalt borides[13-14]and even metal salts,are generally used to accelerate the hydrolysis reaction of alkaline NaBH4.But catalytic performance still needs to be improved. Some catalysts have led to significant improvement in the catalytic activity of transition metal,like Co-Ni-P-B[15],Co-P-B[16], even comparable to that of noble metal catalyst[17-18].
In this paper,in order to improve hydrogen generation rate, we prepared Co1-xNixB alloys in ethanol solution.Effects of Ni content in the Co1-xNixB catalyst and temperature for the catalytic hydrolysis of an alkaline NaBH4solution were investigated.
1 Experimental
1.1 Preparation and characterization of Co1-xNixB alloys
Co1-xNixB amorphous alloys were systematically prepared by chemical reduction under vigorous stirring in the ice-water bath. Alkaline KBH4(99%)was used as reducing agent.Total amount of 0.005 mol metal salts(CoCl2·6H2O(98.0%-102.0%,Alfa Aesar)and NiCl2·6H2O(98%,Alfa Aesar))were dissolved in 50 mL ethanol to form a homogeneous solution.After 10 min stirring, 50 mL aqueous solution containing 0.02 mol KBH4and 0.002 mol NaOH(99%)were added into the mixture in the ice-water bath under vigorous stirring.The molar ratio of KBH4to metallic salts was 4∶1 in order to enable completion of the reduction reaction.The molar concentrations of CoCl2and NiCl2were adjusted to obtain different molar ratios of Ni/(Co+Ni)in the Co1-xNixB powder in order to enhance catalytic activity.Black precipitates were filtered and extensively washed with distilled water and ethanol(99%)three times,respectively.Finally,the products were dried at 323 K in vacuum for 24 h.For comparison,CoB and NiB powders were also synthesized by similar method.
The surface morphologies of catalyst alloys were studied by scanning electron microscopy(SEM,JEOL JSM-6700F field emission,Japan).Structure characterization of the CoB and Co0.85Ni0.15B catalysts was determined by X-ray diffraction(XRD, Rigaku D/Max-2500,Cu Kαradiation,Japan).Granular sizes were investigated by transmission electron microscopy(TEM,Tecnai 20,Netherlands).BET surface area was measured by Tristar 3000(USA).
1.2 Hydrogen generation measurement
The typical hydrogen generation measurement was as follow: 10mgas-preparedcatalystsdissolvedin10mLwaterwereplaced in a sealed flask with an outlet tube for collecting evolved H2gas.10 mL 0.2 mol·L-1NaBH4(98%,Alfa Aesar)solution containing 25 mmol NaOH was rapidly dropped into the sealed flask under mild stirring at controlled temperature.The volume of generated H2was measured by the water displacement method. After the hydrolysis reaction completed,the residual solution was filtered and the catalyst was reserved.
2 Results and discussion
SEM images of the as-synthesized CoB and Co0.85Ni0.15B catalysts are shown in Fig.1.The Co0.85Ni0.15B alloy was aggregated with fine powders.Compared with the morphology of CoB catalyst,Co0.85Ni0.15B alloy contained smaller,more regular,and welldispersed particles.XRD patterns show that CoB and Co0.85Ni0.15B catalysts are amorphous(Fig.2).
TEM images of CoB and Co0.85Ni0.15B catalysts are exhibited in Fig.3.Thereweremorewell-dispersedparticlesinCo0.85Ni0.15B catalyst than those in CoB catalyst.The particle sizes were 10-20 nm revealed in TEM images(Fig.3)which were equivalent to large active surface area.The BET surface areas were 120.74 for CoB and 124.98 m2·g-1for Co0.85Ni0.15B catalysts further confirmed it.
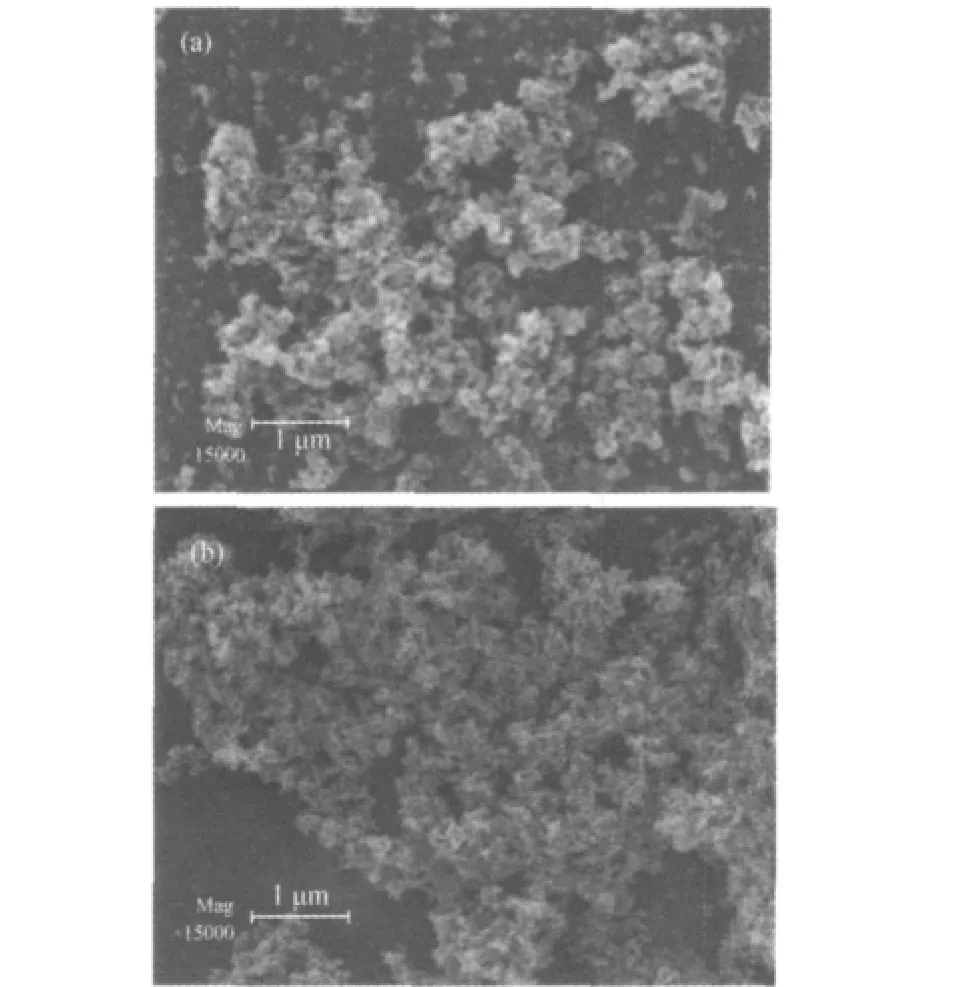
Fig.1 SEM images of the as-synthesized catalysts(a)CoB,(b)Co0.85Ni0.15B

Fig.2 XRD patterns of as-prepared CoB and Co0.85Ni0.15B alloy catalysts
The hydrogen generation volume as a function of reaction time by hydrolysis of alkaline NaBH4solution using different Ni/(Co+Ni)molar ratios in Co1-xNixB(x=0,0.05,0.10,0.15,0.20, 0.30,0.40,0.50,1)alloys at 298 K is shown in Fig.4.The relationship between the hydrogen generation rate and the Ni/(Co+ Ni)molar ratio(x)is shown in Fig.5.Increasing with Ni content in the Co1-xNixB catalyst,the hydrogen generation rate firstly increased,and it reached the highest at the molar ratio of x=0.15. But the hydrogen generation rate decreased when the molar ratio(x)was larger than 0.15.The maximum hydrogen generation rates of Co0.85Ni0.15B and CoB catalysts were 4228 and 3112 mL·min-1·g-1,respectively.The highest H2generation rate achieved by Co1-xNixB alloy could be understood by considering the fact that less active Ni sites were replaced by more active Co sites[16].
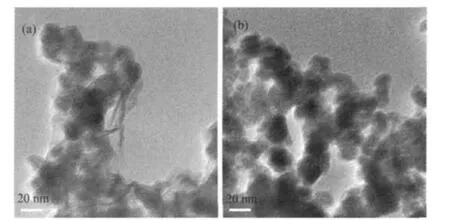
Fig.3 TEM images of the as-prepared catalysts(a)CoB,(b)Co0.85Ni0.15B

Fig.4 Hydrogen generation volume(V(H2))as a function of reaction time(t)by hydrolysis of alkaline NaBH4solution (0.2 mol·L-1)using CoxNi1-xB alloy catalysts at 298 K
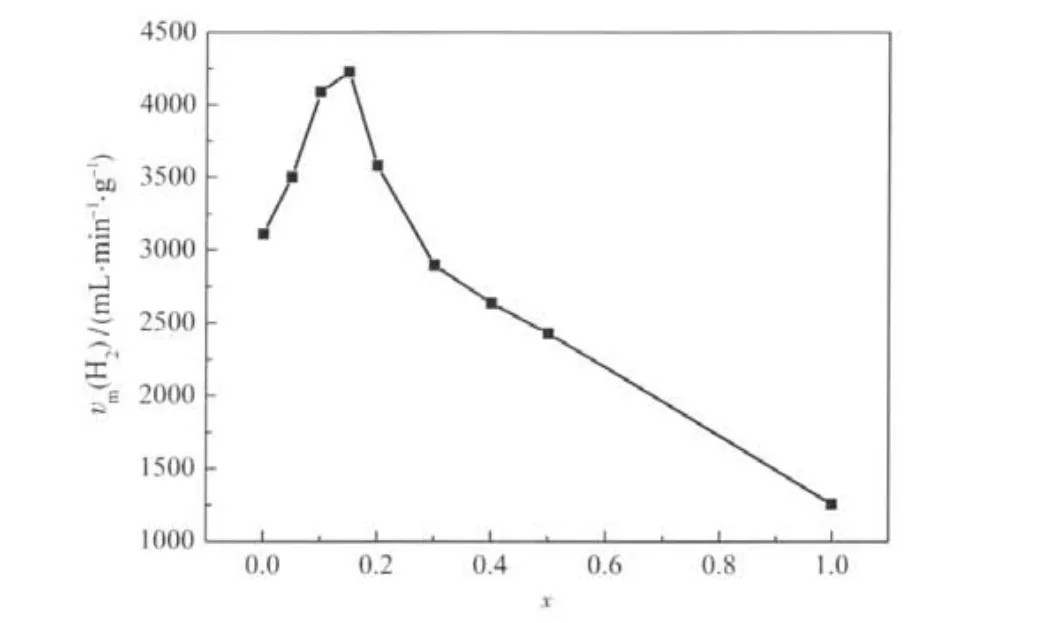
Fig.5 The maximum hydrogen generation rate(vm(H2))as a function of molar ratio(x)
In order to study the effect of solution temperatures,the hydrogen generation rates were tested in the range from 288 to 303 K using alkaline NaBH4(0.2 mol·L-1)solution and 10 mg catalyst of CoB and Co0.85Ni0.15B(Fig.6).Arrhenius plot of the hydrogen production rate gives activation energy(Ea)of 34.25 and 31.87 kJ·mol-1for CoB and Co0.85Ni0.15B,respectively(Fig.7).It indicates that this value for Co0.85Ni0.15B catalyst are lower than the previously reported value[3,19].The activation energy for CoB catalyst is also lower than those for the catalysts with structuredCoB[20-21]and carbon-supported CoB[12].Furthermore,the activation energies of CoB and Co0.85Ni0.15B catalysts are much lower than previous report[14]by using CoNiB powder catalyst for catalytic hydrolysis of alkaline sodium borohydride solution with identical molar ratio of Ni in Co1-xNixB catalyst.

Fig.6 H2generation volume as a function of reaction time at different temperatures(A)CoB,(B)Co0.85Ni0.15B;(a)303 K,(b)298 K,(c)293 K,(d)288 K

Fig.7 Arrhenius plots of the H2generation rates with CoB(A)and Co0.85Ni0.15B(B)alloy catalysts
3 Conclusions
By using chemical reduction method,Co1-xNixB catalysts with different Ni/(Co+Ni)molar ratios were synthesized in ethanol solution for catalyzing hydrogen generation from alkaline NaBH4solution.The reaction was carried out in ethanol solution that can decrease particles size and increase active surface area. Besides,ethanol solution can disperse catalyst well,which made more active sites well touch with the reactants.The assynthesized Co0.85Ni0.15B catalyst alloy revealed much higher catalytic performance than CoB catalyst.In summary,the low cost and the improved hydrogen generation rate of Co1-xNixB catalyst exhibits that it is very promising in the practical application for hydrogen generation.
1 Züttle,A.;Wenger,P.;Rentsch,S.;Sudan,P.;Mauron,P.; Emmenegger,C.J.Power Sources,2003,118:1
2 Anton,D.L.J.Alloy.Compd.,2003,356:400
3 Amendola,S.C.;Sharp-Goldman,S.L.;Janjua,M.S.;Spencer,N. C.;Kelly,M.T.;Petillo,P.J.;Binder,M.Int.J.Hydrogen Energy, 2000,25:969
4 Schlesinger,H.I.;Brown,H.C.;Finholt,A.E.;Gilbreath,J.R.; Hoekstra,H.R.;Hyde,E.K.J.Am.Chem.Soc.,1953,75:215
5 Li,Z.P.;Morigazaki,N.;Liu,B.H.;Suda,S.J.Alloy.Compd., 2003,349:232
6 Amendola,S.C.;Sharp-Goldman,S.L.;Janjua,M.S.;Kelly,M. T.;Petillo,P.J.;Binder,M.J.Power Sources,2000,85:186
7 Guella,G.;Zanchetta,C.;Patton,B.;Miotello,A.J.Phys.Chem. B,2006,110:17024
8 Peňa-Alonso,R.;Sicurelli,A.;Callone,E.;Carturan,G.;Raj,R. J.Power Sources,2007,165:315
9 Kojima,Y.;Suzuki,K.I.;Fukumoto,K.;Sasaki,M.;Yamamoto, T.;Kawai,Y;Hayashi,H.Int.J.Hydrogen Energy,2002,27:1029
10 Feng,R.X.;Cao,Y.L.;Ai,X.P.;Yang,H.X.Acta Phys.-Chim. Sin.,2007,23(6):932 [冯瑞香,曹余良,艾新平,杨汉西.物理化学学报,2007,23(6):932]
11 Liu,B.H.;Li,Z.P.;Suda,S.J.Alloy.Compd.,2006,415:288
12 Zhao,J.Z.;Ma,H.;Chen,J.Int.J.Hydrogen Energy,2007,32: 4711
13 Dai,H.B.;Liang,Y.;Wang,P.;Cheng,H.M.J.Power Sources, 2008,177:17
14 Fernandes,R.;Patel,N.;Miotello,A.;Filippi,M.J.Mol.Catal.AChem.,2009,298:1
15 Fernandes,R.;Patel,N.;Miotello,A.Int.J.Hydrogen Energy, 2009,34:2893
16 Patel,N.;Fernandes,R.;Miotello,A.J.Power Sources,2009, 188:411
17 Cho,K.W.;Kwon,H.S.Catal.Today,2007,120:298
18 Patel,N.;Guella,G.;Kale,A.;Miotello,A.;Patton,B.;Zanchetta, C.;Mirenghi,L.;Rotolo,P.Appl.Catal.A,2007,323:18
19 Kaufman,C.M.;Sen,B.J.Chem.Soc.Dalton Trans.,1985,2: 307
20 Lee,J.;Kong,K.Y.;Jung,C.R.;Cho,E.;Yoon,S.P.;Han,J.; Lee,T.G.;Nam,S.W.Catal.Today,2007,120:305
21 Krishnan,P.;Advani,S.G.;Prasad,A.K.Appl.Catal.B-Environ., 2009,86:137

