从Mn3O4前驱体到MnO2纳米结构的形貌和结构变化
王 凡 王岩敏 文衍宣 粟海峰 李 斌
(广西大学化学化工学院,南宁 530004)
从Mn3O4前驱体到MnO2纳米结构的形貌和结构变化
王 凡*王岩敏 文衍宣 粟海峰 李 斌
(广西大学化学化工学院,南宁 530004)
锰氧化物是一类重要的且具有广泛应用背景的材料,控制合成不同形貌和组成的锰氧化物纳米结构将有助于拓宽其应用领域.本文报道了以Mn3O4为前驱体,通过水热法控制合成MnO2纳米结构的方法.用X射线衍射(XRD)、扫描电镜(SEM)、透射电镜(TEM)等手段对产物进行表征.在硫酸体系中,当反应温度为80和180℃时,所得产物分别为γ-MnO2海胆结构和β-MnO2单晶纳米棒.此外,MnOOH纳米线可以在稀酸溶液中合成.考察了反应温度、溶液酸度、反应时间对产物结构的影响,并提出了基于γ-MnO2为中间产物的反应机理.实验结果表明,水热体系促进了产物的各向异性生长并最终形成不同形貌和结构的锰氧化物.
纳米结构;水热生长;锰氧化物;MnO2纳米棒;MnOOH纳米线
Micro-and nano-structured inorganic materials with novel morphologies have drawn considerable attention because of their properties and potential applications in nanoscale devices. Recently,research on micro-and nano-structures is expanding rapidly into the assemblies of nano building blocks into ordered complex hierarchical superstructures,which are expected to have novel shape-dependent physicochemical properties.Due to their anisotropic structure,the oriented growth of nano building block,such as nanorods and nanowires,is difficult,and various methods,including capping agent and physical templates,have been employed to accomplish the controlled growth.However, these methods are complex and sometimes require further purification to remove the controlling agent or templates,which may introduce impurities and bring damages to the morphology of product.It has been and remains to be a challenge to find facile chemical methods for the synthesis and morphology control of hierarchical nanostructures under mild conditions.
Various polymorphic forms of manganese dioxides(MnO2), such as α-,β-,and γ-type,are of considerable interests in technological applications owing to their outstanding structural flexi bility,such as catalysis[1-2],magnetic materials[3],and super-capacitors[4].The performances of manganese dioxides in most of these applications strongly depended on the parameters,such as powder morphology,crystalline structure,and bulk density,and can be enhanced in hierarchical nanostructures with well-controlled composition,dimensions,and morphology.In addition, manganeseoxyhydroxide(MnOOH)wasused asan effective precursor to synthesize intercalation compound and lithium manganese oxide,which are seen as potentially low-cost,environmentally benign positive materials for rechargeable lithium-ion batteries.Thus,the synthesis approaches of manganese dioxide and oxyhydroxide in various morphologies and structures for a very wide variety of applications are of great significance.
Hydrothermal method has been facilely used to synthesize MnOOH and MnO2nanomaterials because of the simplicity and easily controlling.In this way,chemicals or precursors are important issues for the final composition and phase of the products.Based on the redox reactions of MnO-4and/or Mn2+,MnO2nanowires,nanotubes,and spherically hierarchical nanostruc tures had been hydrothermally prepared[5-10].Recently,the synthesis of MnO2nanostructures using commercial manganese oxide powder,likes MnO2and Mn2O3,as raw materials by facile hydrothermal method in the absence of templates or controlled agent has attracted attentions because it not only avoids complicated pre-treatment processes but also shows clearly the relationships between phases of the target products and the precursors[11-15].Generally,α-MnO2[11],β-MnO2[11],γ-MnO2[12-14],and MnOOH[15]nanorods/nanowires were successfully achieved. However,there is little evidence of morphological control in these syntheses with the shapes of final products being tuned to the precursors,and few studies are concentrated on synthetic route of MnO2hierarchical nanostructures from mineral precursors.Herein we present a simple surfactant free hydrothermal method to the synthesis of manganese oxide nanostructures using Mn3O4powder as raw materials.The structures and morphologies of the manganese oxide nanomaterials can be controlled by adjusting the acidity and temperature in the hydrothermal system.
1 Experimental
The raw Mn3O4materials were obtained from Qiulong chemical company,Shanghai.All chemicals were of analytical grade and used without further purification.In a typical synthesis,0.06 g of Mn3O4powder was added to 18 mL deionized water.Then, an appropriate amount of sulfuric acid was added dropwise to the mixture to adjust the pH value.The mixture was transferred to a 23 mL Teflon-lined autoclave and heated in an oven at 80-180℃.After the hydrothermal treatment,the resulting black product was collected and washed with deionized water and ethanol several times until pH is close to neutral.Finally,the products were dried in air at 80℃for characterization.
The samples were characterized by X-ray powder diffraction (XRD)with a Japan Rigaku D/max-2500 X-ray diffractometer equipped with graphite monochromatized high-intensity Cu Kαradiation(λ=0.154178 nm).The transmission electron microscopy (TEM)images were performed with a JEM-200CX instrument (JEOL Ltd.,Japan)using an accelerating voltage of 160 kV.The scanning electron microscopy(SEM)images were taken on a S-3400N SEM(Hitachi,Japan).The high-resolution transmission electron microscopy(HRTEM)imageswere carried out on a JEM-2010 HRTEM(JEOL Ltd.,Japan)at an acceleration voltage of 300 kV.
2 Results and discussion
2.1 XRD patterns of the precursor and the products
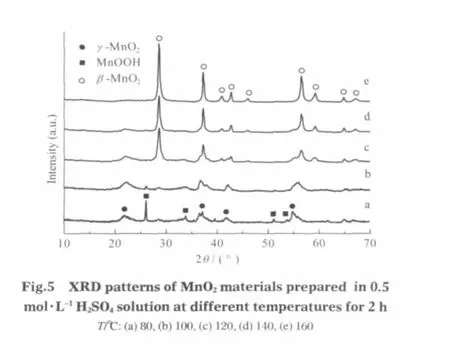
Fig.1 shows the XRD patterns of the commercial Mn3O4powder and the hydrothermal products.The XRD pattern of the Mn3O4powder(Fig.1(a))indicates that the raw material is well crystallized and is composed of hausmannite Mn3O4(JCPDS 80-0382)and MnOOH(JCPDS 74-1632).It can be found that the patterns of products are significantly different from that of the raw material.By hydrothermal treatment of Mn3O4powder in 0.5 mol·L-1H2SO4solution at 80℃ for 1 h,a mixture of γ-MnO2and MnOOH was obtained as shown in Fig.1(b).The calculated lattice constants of γ-MnO2(a=0.634 nm,b=1.017 nm,and c=0.410 nm)are in good agreement with JCPDS 14-0644(γ-MnO2).The intensity of MnOOH phase is gradually declined upon prolonging the reaction time.After 72 h,only the γ-MnO2phase is present(Fig.1(c)).

When the reaction temperature increases further to 180℃,the γ-MnO2intermediate is observed for the 1 h product(Fig.1(d)). As the reaction time is increased to 6 h,only the tetragonal β-MnO2(JCPDS 24-0735,a=0.4408 nm and c=0.2874 nm)is present(Fig.1(e)).No characteristic peaks for the impurities are observed.The sharp and intense peaks in the XRD pattern indicate good crystallinity of pure β-MnO2.Consequently,the Mn3O4powder was presumably transformed to the pure MnO2products upon prolonging hydrothermal treatment time.
2.2 Morphologies of the precursor and the products
The typical Mn3O4precursor and the obtained products were observed by SEM.Fig.2(a)displays a representative overview of the Mn3O4precursor.This raw material has irregular-shaped particles with inhomogeneous size distribution ranging from one to several ten micrometers.After hydrothermal treatment of these irregular particles in H2SO4solution,the shapes of the obtained products are greatly changed to some special nanostructures (Fig.2(b-d)).At 80℃in 0.5 mol·L-1H2SO4solution,the largescale urchin-like γ-MnO2nanostructures with diameters of 1-2 μm were obtained after 72 h(Fig.2(b)).The high magnification SEM image in the inset of Fig.2(b)shows the morphology of an individual urchin-like nanostructure.Large numbers of nanorods were found radiated from the center of the spherical particles and vertically aligned with high density.The uniform nanorods have lengths up to about 200 nm and width around 40 nm.The firm γ-MnO2nanostructures cannot be destroyed after a long time of ultrasonic treatment.In addition,the γ-MnO2nanorods are getting longer by the H+concentration.Shown in Fig.2(c) is the urchin-like nanostructures synthesized in 3 mol·L-1H2SO4solution at 80℃ for 72 h.The lengths of the building nanorods are about 500 nm.With a raised temperature of 180℃, large numbers of β-MnO2nanorods were observed.The asobtained nanorods have uniform diameter around 100 nm with lengths up to 1000 nm(Fig.2(d)).

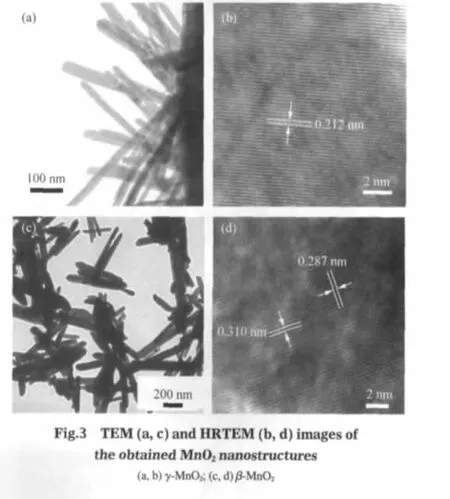
The morphology and structure of the as-obtained manganese oxide nanostructures were further characterized by TEM and HRTEM as shown in Fig.3.Fig.3(a)displays the magnified TEM image of the γ-MnO2urchin-like nanostructures,in which the nanorods were grown on the surface with different radial angles. It also can be found that the building nanorods have uniform diameters of 20-40 nm and lengths of 100-300 nm,confirming the SEM observation in Fig.2(b).The HRTEM image of a single building nanorod is shown in Fig.3(b).The lattice fringes have spacing of 0.212 nm,which matches well with separations betweentheneighboringlatticesofthe(300)planesofγ-MnO2.Fig.3 (c)shows a low-magnification TEM image of β-MnO2nanorods obtained at 180℃.The observed dimensions of individual nanorods are in good agreement with the estimated values from the SEM results.Fig.3(d)shows a typical HRTEM image of a single β-MnO2nanorod.The clear lattice image indicates the good crystallinity and single crystalline nature of the β-MnO2nanorods.The interplanar spacings of fringes are 0.310 and 0.287 nm,corresponding to the(110)and(001)planes of tetragonal β-MnO2,respectively.
2.3 Growth mechanism
To obtain one-dimensional(1D)nanostructures from mineral precursors,it is important to understand the formation mechanism involved in the hydrothermal process.Our results indicate that both reaction temperature and acidity of solution influence the transformation of Mn3O4critically.Therefore,the comprehension of such aspects may lead to the preparation of novel 1D nanostructures.
Numerous studies on the preparation of MnO2nanorods have been reported[2-3,5,7].However,the formation mechanism under hydrothermal conditions remains unclear since the results are somehow conflicting.Most works suggested that the formation of nanorods is assisted by the controlled agent[10,16],in which the rod-shaped micelles guide the product morphology.In the present work,we found that γ-MnO2acted as the important intermediate at the very beginning of the hydrothermal process,and the generation of MnO2nanostructures was tuned by the reaction temperature.
In acidic solution(pH<1),Mn3O4tends toward the following dismutation reaction:

The dismutation reaction is fast and most MnO6units,the bulding block for the construction of γ-MnO2and β-MnO2,will form and then adsorb on the surface of Mn3O4particles.These MnO6units will assembly together,provide the active sites and act as a director for assisting the formation of the nanorods. Through a condensation reaction,these MnO6aggregations may orient and grow in size by the continuous supply of MnO6units and form nanorods eventually on the edge of the particles.The γ-MnO2urchin-like nanostructures are stable because of the low synthesis temperature,and the length of nanorods increases with increasing the H+concentration(Fig.2(b,c)).
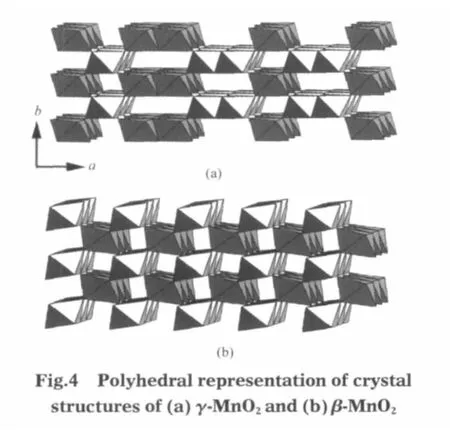
According to the XRD results,the transformation of Mn3O4at 180℃ comprises two distinct processes:the formation of γ-MnO2and then change to β-MnO2nanorods.As shown in Fig.1 (b-d),the(120)peaks of γ-MnO2are broad,which reveals that the tunnel structure of these γ-MnO2aggregations is poor at the beginning of the hydrothermal process.Fig.4 shows the structures of γ-MnO2and β-MnO2.β-MnO2is constructed of single chains of edge-sharing MnO6octahedra,forming a framework structure with 1×1 tunnel arrays[17].On the other hand,γ-MnO2is considered as a disordered intergrowth of the β-MnO2and ramsdellite structures,consisting of a random arrangement of single and double chains of MnO6octahedron[16].This disorder creates a great number of discordance on the structure and as a result,the metastable γ-MnO2will transform into β-MnO2with 1×1 tunnel structure by hydrothermal treatment at high temperature[17].Thus, the phase transformation of γ-MnO2to β-MnO2should include a rapid collapse of the 1×2 tunnel structure and then a short-distance self-assembly of MnO6octahedra.The symmetrical 1×1 tunnel structure acts as directing factor and orients the preferential growth direction of nanorods.With the developing of the dismutation reaction,the Mn3O4precursor powders become incompact,and ultimately change to γ-MnO2in acidic solution.At high temperature(180℃),the fresh γ-MnO2particles may redissolve into the acidic solution,and transform into β-MnO2nanorods owing to the topological convenience.
In these works,the hydrothermal product at 80℃is γ-MnO2, while the product at 180℃is β-MnO2.In fact,early synthesis studies of preparation of tunnel structure managanese oxides always suggest that if without hard cations,like K+,Mg2+,and Rb+acting as template,the tendency to form β-MnO2via homogeneous methods in acidic solution is prevailing[18].Fig.5 shows the XRD patterns of hydrothermal products obtained at different temperatures for 2 h.We can clearly see that the transformation of γ-MnO2to β-MnO2is getting fast with the increase of temperature.The γ-MnO2phase can still be found at 100℃(Fig.5b). As the reaction temperature increases further to 120℃,the β-MnO2starts to form (Fig.5c).At 160℃,only the β-MnO2phase ispresent(Fig.5e).Therefore,the role oftemperature isto tune the product types as well as accelerate the hydrothermal reaction[7-8]. In comparison with the hydrothermal synthesis,only irregularshaped particles were obtained at room temperature though the reaction time is prolonged to 6 d.
2.4 Formation of MnOOH nanostructures
In addition,we found that MnOOH phase products could likewise be selectively obtained in hydrothermal system in different H2SO4solutions.Fig.6(a)is the XRD patterns of the hydrothermal products in 5×10-4mol·L-1H2SO4solution(pH 3)for different time.It is clearly shown that the peaks of MnOOH become more sharp and intense with increasing reaction time,and consequently MnOOH becomes the main phase after 24 h at 180℃. The as-prepared product generally comprises mixed morphology of Mn3O4particles and MnOOH nanowires at 80℃after 24 h (Fig.6(b)).MnOOH nanowires have a diameter range of 100-300 nm and length of tens of micrometers.Interestingly,a large number of MnOOH multipods are found.A five-armed multipod is shown in Fig.6(c).We can clearly identify that the junction of the MnOOH multipod is a whole crystal.Further increase in hydrothermal temperature to 180℃formed mixtures of microrods and nanorods as shown in Fig.6(d).The microrods have diameters of 500 nm and lengths of 2-3 μm,while the nanorods have uniform diameters of 50 nm and lengths of 400 nm.The numerous multipods can still be found in these two microstructures.
It should be pointed out that the formation of MnOOH nanostructures is actually the outward embodiment of the nature of the initial crystal structure.The structures of MnOOH and β-MnO2are much alike in the arrangement manner of MnO6octahedra[19].In MnOOH,the MnO6octahedron is elongated with different Mn—O bonds due to an interaction of the Jahn-Teller effect(Mn4+is replaced by Mn3+).Thus,MnOOH is a distorted derivative of β-MnO2and can be viewed as equivalent by replacing O with OH in the crystal lattice.MnOOH nanowires have been obtained via redox reactions in neutral and dilute acid solution[19-22].Moreover,the effect of dilute acidity may lead to the slow dissolution of manganese oxide in these works.Therefore, the product with wide diameter distribution could be obtained by the slow yet stable crystal growth process.

The multipods can be looked upon as an ordered three-dimensional(3D)architectures,and may produce more active sites or exhibit more interesting electrical,optical,and magnetic properties than the two-dimensional(2D)or 1D architectures[23-24].The preparations of MnOOH multipods were often needed capping agent,like polyvinyl pyrrolidone(PVP)[23]and polyethylene glycol(PEG)[24].The role of the capping agents during the reaction is leading to competitive growth between the crystal planes due to the different adsorption of capping agents on the various crystal surfaces.In the present work,since no surfactant and capping agent were used,the surface defects might serve as active sites for the branch nanorods growth,which grow out from the main branch nanorod,bringing on hierarchical nanostructures[25].Hence, the formation of MnOOH multipods also reveals that the anisotropic crystal growth plays important roles when using mineral precursors to synthesize nanostructures in hydrothermal process.
3 Conclusions
In conclusion,a facile hydrothermal method for synthesizing MnO2nanostructures from Mn3O4solid precursors,which involved no catalysts or templates,has been developed.In the presence of H2SO4,different MnO2nanostructures can be selectively produced by adjusting the reaction temperature.The spherically urchin-like γ-MnO2nanostructures and β-MnO2nanorods are formed at 80 and 180℃,respectively.The possible growth mechanism of the MnO2nanostructures was proposed based on experimental results,and the γ-MnO2intermediate might play an important role in the formation of nanorods.In addition,MnOOH nanowires and multipods are formed at dilute acidic solution.Because of the flexible procedure of this method,the scale-up synthesis of MnO2with different phases and morphologies from single raw materials would be possible.
1 Son,Y.C.;Makwana,V.D.;Howell,A.R.;Suib,S.L.Angew. Chem.Int.Edit.,2001,40:4280
2 Zhang,W.X.;Yang,Z.H.;Wang,X.;Zhang,Y.C.;Wen,X.G.; Yang,S.H.Catal.Commun.,2006,7:408
3 Wang,G.L.;Tang,B.;Zhuo,L.H.;Ge,J.C.;Xue,M.Eur.J. Inorg.Chem.,2006:2313
4 Subramanian,V.;Zhu,H.W.;Vajtai,R.;Ajayan,P.M.;Wei,B.Q. J.Phys.Chem.B,2005,109:20207
5 Wang,X.;Li,Y.D.Chem.Eur.J.,2003,9:300
6 Wu,C.Z.;Xie,Y.;Wang,D.;Yang,J.;Li,T.W.J.Phys.Chem.B, 2003,107:13583
7 Li,W.N.;Yuan,J.K.;Gomez-Mower,S.;Sithambaram,S.;Suib, S.L.J.Phys.Chem.B,2006,110:3066
8 Song,X.C.;Zheng,Y.F.;Lin,S.;Wang,Y.Acta Phys.-Chim. Sin.,2007,23:258 [宋旭春,郑遗凡,林 深,王 芸.物理化学学报,2007,23:258]
9 Ni,J.P.;Lu,W.C.;Zhang,L.M.;Yue,B.H.;Shang,X.F.;Lv,Y. J.Phys.Chem.C,2009,113:54
10 Song,X.C.;Zhao,Y.;Zheng,Y.F.Cryst.Growth Des.,2007,7: 159
11 Wei,M.D.;Konishi,Y.;Zhou,H.S.;Sugihara,H.;Arakawa,H. Nanotechnology,2005,16:245
12 Yuan,Z.Y.;Zhang,Z.L.;Du,G.H.;Ren,T.Z.;Su,B.L.Chem. Phys.Lett.,2003,378:349
13 Zhang,G.Q.;Bao,S.J.;Zhang,X.G.;Li,H.L.J.Solid State Electrochem.,2005,9:655
14 Li,G.C.;Jiang,L.;Pang,H.T.;Peng,H.R.Mater.Lett.,2007, 61:3319
15 Yuan,Z.Y.;Ren,T.Z.;Du,G.H.;Su,B.L.Appl.Phys.A,2005, 80:743
16 Xiong,Y.J.;Xie,Y.;Li,Z.Q.;Wu,C.Z.Chem.Eur.J.,2003,9: 1645
17 Gao,T.;Fjellvag,H.;Norby,P.Nanotechnology,2009,20: 055610
18 Suib,S.L.J.Mater.Chem.,2008,18:1623
19 Zhang,Y.G.;Liu,Y.;Guo,F.;Hu,Y.H.;Liu,X.Z.;Qian,Y.T. Solid State Commun.,2005,134:523
20 Xi,G.C.;Peng,Y.Y.;Zhu,Y.C.;Xu,L.Q.;Zhang,W.Q.;Yu, W.C.;Qian,Y.T.Mater.Res.Bull.,2004,39:1641
21 Fang,Z.;Tang,K.B.;Gao,L.S.;Wang,D.;Zeng,S.Y.;Liu,Q.C. Mater.Res.Bull.,2007,42:1761
22 Zhang,Y.C.;Qiao,T.;Hu,X.Y.J.Solid State Chem.,2004,177: 4093
23 Zheng,D.S.;Yin,Z.L.;Zhang,W.M.;Tan,X.J.;Sun,S.X. Cryst.Growth Des.,2006,6:1733
24 Mi,Y.H.;Zhang,X.B.;Yang,Z.Q.;Li,Y.;Zhou,S.M.;Zhang, H.;Zhu,W.M.;He,D.L.;Wang,J.L.;Tendeloo,G.V.Mater. Lett.,2007,61:1781
25 Jia,Y.J.;Xu,J.;Zhou,L.H.;Liu,H.L.;Hu,Y.Mater.Lett.,2008, 62:1336
August 12,2009;Revised:November 23,2009;Published on Web:December 22,2009.
Structural and Morphological Transformation of MnO2Nanostructures from Mn3O4Precursor
WANG Fan*WANG Yan-Min WEN Yan-Xuan SU Hai-Feng LI Bin
(School of Chemistry and Chemical Engineering,Guangxi University,Nanning 530004,P.R.China)
Manganese oxides show huge structural flexibility and appear in various crystallographic polymorphs. Hence,morphological and phase control of desired manganese oxide nanostructures could enable their properties to be tuned with a greater versatility,and endow them with potential applications.Herein,we report a simple hydrothermal route for the synthesis of various MnO2nanostructures using Mn3O4powder as raw material.The obtained products were characterized by X-ray diffraction(XRD),scanning electron microscopy(SEM),and transmission electron microscopy(TEM).Results show that in H2SO4solution,urchin-like γ-MnO2nanostructures and single-crystal β-MnO2nanorods are obtained at 80 and 180℃,respectively.In addition,MnOOH nanowires were obtained in a dilute acid solution.The influence of synthetic parameters including temperature,acidity,and reaction time are discussed.The γ-MnO2intermediate might play an important role in the formation of nanorods.The evolution of phases and morphologies in the reaction process suggested the anisotropic crystal growth for the formation of nanostructures under acidic conditions.
Nanostructure;Hydrothermal growth;Manganese oxide;MnO2nanorod;MnOOH nanowire
O648;O614
*Corresponding author.Email:fanwang@gxu.edu.cn;Tel:+86-771-3233718.
The project was supported by the Natural Science Foundation of Guangxi Province,China(0832010).
广西自然科学基金(桂科青0832010)资助项目

