川木通的化学成分及鉴别研究
刘晶晶,陈 幸,魏志奇,李 彬
1四川省中医药科学院中药研究所,成都 610041;2成都中医药大学药学院,成都 611730
川木通的化学成分及鉴别研究
刘晶晶1,2,陈 幸1*,魏志奇1,李 彬1
1四川省中医药科学院中药研究所,成都 610041;2成都中医药大学药学院,成都 611730
研究川木通的化学成分,根据结果对中国药典川木通的薄层色谱鉴别内容提出商榷意见。采用硅胶柱、SephadexLH-20分离及重结晶等方法,从川木通 Clem atis ar m andiiFranch.的乙醇提取物中,分离纯化得到四种化合物。通过波谱数据、理化性质和对照参考文献,分别鉴定为 9,12-octadecadienoic acid(Z,Z)-,2,3-dihydroxypropyl ester(1)、麦角甾醇 (2)、豆甾醇 (3)和β-谷甾醇 (4),其中 1和 2为首次分离得到。用 TLC和HPLC图谱比对发现采集的 11批川木通药材中均含有豆甾醇,而未发现齐墩果酸。中国药典以齐墩果酸为对照品薄层色谱法定性鉴别川木通的内容有待商榷。
川木通;麦角甾醇;豆甾醇;β-谷甾醇;鉴别
《中国药典》2005版收载川木通为毛茛科植物小木通 Clem atis ar m andiiFranch.或绣球藤 Clem atis m ontanaBuch.-Ham.的干燥藤茎,具有清热利尿、通经下乳之功效。主治水肿、小便不利与经闭乳少等症,是常用中药材[1]。中国药典规定以齐墩果酸为对照品薄层鉴别川木通。在我们以往的研究中,按药典方法试验,薄层色谱显示小木通和绣球藤均不含齐墩果酸[2]。本次经过成分分离、鉴定,从小木通的茎中分离得到 4个化合物,其结构分别鉴定为9,12-octadecadienoic acid(Z,Z)-2,3-dihydroxypropyl ester(1)、麦角甾醇 (2)、豆甾醇 (3)和β-谷甾醇(4),其中 1和 2为首次从小木通中分离得到。通过 HPLC和 TLC图谱比对,9批川木通和 2批绣球藤均含有豆甾醇而未发现齐墩果酸。因此我们认为中国药典以齐墩果酸作为对照品薄层鉴别川木通有待商榷。
1 仪器与材料
Auto Spec Ult ima-Tof质谱仪;Bruker Daltonics Bio TOF Q spectrometer高分辨质谱仪;Nist 98标准质谱图库;Varian INOVA-400型核磁共振仪;I R-8300型红外光谱仪;UV-2401PC型 (岛津)分光光度计。小木通 Clem atis ar m andiiFranch.采自四川省洪雅县、峨眉山鱼洞、峨眉四峨山,绣球藤 Clem atis m ontanaBuch.-Ham.采自四川省康定县,由四川省中药研究所生药室方清茂副研究员鉴定。
2 提取与分离
小木通木质藤茎 50 kg粉碎过 10目筛,用 95%乙醇回流提取两次,每次 2 h,滤过,减压浓缩,浓缩液用石油醚反复萃取,回收溶剂得浸膏 112.5 g。该浸膏经硅胶柱色谱层析分离,石油醚:乙酸乙酯 (25∶1-1∶1)梯度洗脱。流出组分经 TLC检测,合并得 5个洗脱部分。A部分 (7.8 g)经 RP-18色谱柱层析分离,甲醇洗脱,经过甲醇反复重结晶得到三个无色针状结晶 2、3和 4。B部分 (2.579 g)经 RP-18色谱柱层析分离,甲醇∶水 (75∶25)为流动相洗脱,再经过 Sephadex柱色谱层析纯化,甲醇洗脱,得化合物1。
3 结构鉴定
化合物 1 C21H38O4,无色油状物。UV(E tOH) λmax(logε):210.6 nm。IR(KBr)νmaxcm-1:3258, 2854,1715,1650。EI-MSm/z:354[M]+,336,262, 81,67(100)。1H NMR(CDCl3,400 MHz)δ:5.39 (2H,J=11.2,6.8 Hz),5.33(2H,J=10.8,6.4 Hz),4.16(2H,m),2.34(2H,t,J-7.6),0.89(3H,t, CH3);13C NMR(CDCl3,100 MHz)δ:174.3,130.2, 130.0,128.1,127.9,70.2,65.1,63.4,34.1,31.5, 29.6,29.3,29.1,29.1,27.2,25.6,24.9,22.5, 14.0。以上数据通过Nist 98标准质谱图库检索,并结合有关文献人工图谱解析,鉴定化合物 1为 9,12-Octadecadienoic acid(Z.Z)-,2,3-dihydroxypropyl ester。
化合物 2 C28H44O,白色粉末状结晶。EI-MS m/z:396[M]+(45),378[M-H2O]+(4),363(27), 337(12),271(26),253(16),157(18),143(22), 105(37),95(52),81(67),69(91),55(100)。13C NMR(CDCl3,100 MHz)δ:141.3(C-8),139.8(C-5),135.6(C-22),132.0(C-23),119.6(C-6),116.3 (C-7),70.5(C-3),55.8(C-17),54.6(C-14),46.3 (C-9),42.8(C-13),42.3(C-24),40.8(C-4),40.4 (C-20),39.1(C-21),38.4(C-1),37.0(C-10),33.1 (C-25),32.0(C-2),28.2(C-16),23.1(C-15),21.1 (C-11),21.1(C-12),19.9(C-28),19.6(C-27), 17.6(C-26),16.2(C-19),12.0(C-18)。以上数据与文献值一致[3],鉴定化合物 2为麦角甾醇 (ergosterol)。
化合物 3 C29H48O,无色针晶。mp.142~144℃(CH3OH)。EI-MSm/z:412[M]+(100),397 (9),394(28),379(10),327(3),300(40),273 (19),271(45),255(73),231(10),213(23)。13C NMR(CDCl3,100 MHz)δ:140.8(C-5),138.3(C-22),129.3(C-23),121.7(C-6),71.8(C-3),56.9 (C-17),56.0(C-14),51.2(C-24),50.2(C-9),42.3 (C-4),42.2(C-13),40.4(C-20),39.7(C-12),37.3 (C-1),36.5(C-10),31.9(C-25),31.9(C-7),31.9 (C-8),31.6(C-2),28.9(C-16),25.4(C-28),24.4 (C-15),21.2(C-27),21.1(C-11),21.0(C-21), 19.4(C-19),19.0(C-26),12.2(C-29),12.0(C-18)。以上数据与文献值一致[4],鉴定化合物 3为豆甾醇 (stigmasterol)。
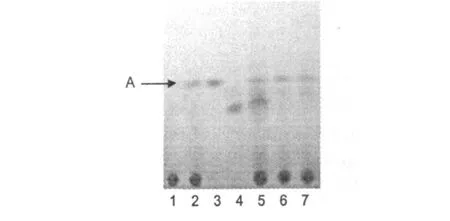
图 1 小木通、甾醇混合对照品、齐墩果酸对照品、白木通、绣球藤薄层色谱图Fig.1 TLC chromatogram of ergosterol,stigmasterol,β-sitosterol,the Caules ofC.ar m andiiandC.M ontana
化合物 4 C29H50O,无色针晶。mp.138~140℃(CH3OH)。EI-MSm/z:414[M]+(71),396 (51),381(21),329(20),273(17),255(23),231 (13),213(26),159(20),81(69),69(57),55 (100)。13C NMR(CDCl3,100 MHz)δ:140.8(C-5), 121.7(C-6),71.8(C-3),56.8(C-14),56.1(C-17), 50.2(C-9),45.9(C-4),42.3(C-13),39.8(C-12), 37.3(C-24),36.5(C-1),36.1(C-10),34.0(C-22), 31.9(C-8),31.9(C-20),31.6(C-7),29.2(C-2), 29.2(C-16),28.2(C-11),26.2(C-15),24.3(C-23),23.1(C-27),21.1(C-28),19.8(C-19),19.4 (C-21),19.1(C-25),18.8(C-29),12.0(26),11.9 (18)。以上数据与文献值一致[5,6],鉴定化合物 4为β-谷甾醇。
4 TLC鉴别
根据中国药典薄层色谱法(附录V IB),分别吸取样品和对照品溶液各 5μL,点于同一硅胶 G薄层板上,以环己烷-丙酮(4∶1)为展开剂,展开,取出,晾干,喷以 10%的硫酸乙醇溶液,在 105℃加热至斑点清晰。结果显示小木通和绣球藤均不含齐墩果酸,但在薄层色谱上显示出一紫红色共同斑点 (图 1中A点),经与对照品斑点比对,该斑点为麦角甾醇、豆甾醇、β-谷甾醇的混合物。
5 讨论
中国药典规定以齐墩果酸为对照品薄层鉴别川木通,试验结果表明不能检出齐墩果酸。该薄层条件下检出的主要斑点是麦角甾醇、豆甾醇、β-谷甾醇的混合物,因此我们认为药典以齐墩果酸为对照品薄层鉴别川木通,其内容有待商榷。
1 Committee of Chinese Phar macopoeia.Chinese Pharmacopoeia(中华人民共和国药典),2005,1:25.
2 LiB(李彬),Chen X(陈幸),LiWS(黎万寿).Discussion on ChuanMutong’s TLC essay contents recorded in Chinese pharmacopoeia.J Chin M ed M at(中药材),2005,28:1147-1148.
3 Wang F(王飞),Liu JK(刘吉开).The chemical constituents of basidiomyceteCalodon Suaveolens.Nat Prod Res Dev (天然产物研究与开发),2004,16:204-206.
4 Yan LH(闫利华),Xu LZ(徐丽珍),Zou Z M(邹忠梅),et al.Chemical constituents from stems ofClem atis ar m andii (Ⅰ).Chin Tradit Herb D rugs(中草药),2007,38:340-342.
5 Guo XM(郭学民),Hong YF(洪永福),Zhang L(张玲),et al.Studies on the chemical constituents of common floweringquince(Chaenom eles lagenaria).Chin Tradit Herb D rugs (中草药),1997,28:584-585.
6 Tian J(田军),Wu FE(吴凤锷),Qiu MH(丘明华),et al. Chemical constituents ofPterocephalus Hookeri.Nat Prod Res Dev(天然产物研究与开发),2000,12:31-38.
Chem ical Constituents and Identification of the Caules ofClem atis arm andiiFranch.andClem atis m ontanaBuch.-Ham.
L IU Jing-jing1,2,CHEN Xing1*,WEI Zhi-qi1,L IBin11Sichuan Academ y of ChineseM edicine Sciences,Sichuan 610041,China;2School of Phar m aceutical Sciences,Chengdu University of Traditional ChineseM edicine,Chengdu 611730,China
Four compounds,9,12-octadecadienoic acid(Z,Z)-,2,3-dihydroxypropyl ester(1),ergosterol(2),stigmasterol(3),andβ-sitosterol(4),were isolated from the ethanol extracts ofClem atis ar m andiiFranch.,and identified on the basis of the spectroscopic and physicochemical datawith those reported previously.Compounds 1 and 2 were isolated from the stems ofC.ar mandiifor the first time.However,oleanolic acid,an indexed component in Chinese Pharmacopoeia for the identification ofClem atisspp.was not detected in our conditions.To determinewhetheroleanolic acid presented inC.am andiiandC.M ontana,we collected 11 batch samples include nine forC.amandiiand two forC.M ontana.There was no trace of oleanolic acid in all 11 samples based on HPLC and TLC analysis,indicating the need for reinvestigation the chemical constituents and the indexed components ofC.spp..
Clem atis ar m andiiFranch.;Clem atis montanaBuch.-Ham.;ergosterol;stigmasterol;β-sitosterol;identification
1001-6880(2010)06-0998-03
2009-01-20 接受日期:2009-07-01
四川省中医药管理局项目
*通讯作者 Tel:86-28-85256143;E-mail:cjy55@163.com
Q946.91;R284.1
A

