NF-E2:a Novel Regulator of Alpha-hemoglobin Stabilizing Protein Gene Expression△
Guo-wei Zhao ,Rui-feng Yang ,Xiang Lü ,Mitchell J.Weiss ,De-pei Liu *,and Chih-chuan Liang#
1National Laboratory of Medical Molecular Biology,Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100005,China
2Children’s Hospital of Philadelphia,Philadelphia 19104,PA,USA
β-thalassemia is a genetic anemia that is characterized by a relative excess of α-globin due to the defects of β-globin production.The cytotoxic free α-globin aggregates in cells and damages the cell membrane.Reactive oxygen species generated from the unstable α-globin further damages other cellular components.1α-hemoglobin stabilizing protein (AHSP),an abundant erythroid-specific protein,functions as a molecular chaperone to bind α-globin monomers,but not β-globin or hemoglobin A (Hb A).2,3The binding specificity of AHSP enables it to stabilize nascent α-globin subunits prior to Hb A assembly,facilitating their incorporation into Hb A.Studies with AHSP-null mice and the mice crossed with either β-thalassemia mice or α-thalassemia ones provide evidence of these functions of AHSP,indicating that AHSP may be an important modifier in both types of thalassemia.4,5
Given the essential role of AHSP in both normal and aberrant erythropoiesis,investigating its regulation is important for the understanding of these processes.Among the erythroid regulators,NF-E2 is a crucial one that consists of an 18kD subunit (NF-E2p18) of the widely expressed small Maf family and a hematopoietic-specific 45kD subunit (NF-E2p45) of the cap’n’ collar (CNC) family.6Although knock-out of either NF-E2p18 or NF-E2p45 in mice shows none or very slight abnormalities in erythroid phenotype,7,8the NF-E2p45-null erythroleukemia cell line CB3 fails to express both globins.9The NF-E2 heterodimer recognizes a consensus binding sequence (T/C) GCTGA(G/C) TCA (T/C),6and binds prominently to hypersensitive site (HS) 2 of β-globin locus control region (LCR) and HS 40 of α-globin major regulatory element (MRE).10,11In addition to the function as a direct transactivator,NF-E2 is also involved in extensive histone modifications.It interacts directly with methyltransferase complex MLL2,which methylates histone 3 at lysine 4 (H3K4) and has been implicated in mediating H3K4 trimethylation in the coding sequence of adult β-globin gene.12
Previous studies have shown that erythroid transcription factors GATA-1 and EKLF function as direct regulators of AHSP.13-15We therefore presumed that NF-E2 might also be involved in AHSP gene modulation,and explored the possible role of NF-E2 in AHSP regulation in this study.
MATERIALS AND METHODS
Cell culture and antibodies
DS19 cells and HeLa cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS) at 37°C in an incubator containing 5% CO2.16G1E-ER4 cells were grown as previously described.G1E-ER4 is a subline of G1E cells stably expressing an estrogen-activated form of GATA-1.GATA-1-ER fusion protein will relocate into nucleus upon estradiol treatment and reactivate GATA-1 function.17
Anti-NF-E2p18 (sc-477),anti-NF-E2p45 (sc-291),and anti-GATA-1 (sc-265) antibodies obtained from Santa Cruz Biotechnology (Santa Cruz,CA,USA) were applied in chromatin immunoprecipitation (ChIP) and Western blot.Anti-AcH3 (06-599),anti-AcH4 (06-866),and anti-H3K4me3(07-473) antibodies for ChIP were purchased from Millipore(Billerica,MA,USA).Anti-β-Actin antibody (internal control in Western blot),normal rabbit IgG (negative control in ChIP),and rat IgG (negative control in ChIP) were products of Sigma-Aldrich (St.Louis,MO,USA).Anti-AHSP and anti-α-globin antibodies for Western blot were gifts from Mitchell J.Weiss at Children’s Hospital of Philadelphia.
Retrovirus-mediated silencing of NF-E2p45 in DS19 cells
The small interfering RNA (siRNA) sequence for NF-E2p45 silencing (5’-GGAGAGATGGAACTGACTT-3’) was selected according to the algorithm provided by Invitrogen(Carlsbad,CA,USA).The oligonucleotide sequences for knockdown of NF-E2p45 (5’-gatccGGAGAGATGGAACTGACTTTTCAAGAGAAAGTCAGTTCCATCTCTCCTTTTTTg-3’;5’-aattcAAAAAAGGAGAGATGGAACTGACTTTCTCTTGAAAAGTTCAGTTCCATCTCTCCg-3’) were synthesized,annealed,and inserted into RNAi-ready pSIREN-Retro Q vector (Clontech,Mountain View,CA,USA).The recombinant retrovirus was generated by 293T cells after transient co-transfection with the recombinant retroviral plasmid and the helper plasmids pCGP and pVSV-G (Clontech) using calcium phosphate precipitation.18Spin-infection with the recombinant retrovirus of DS19 cells was carried out.After 48 hours,the infected cells were sub-cultured at an appropriate density(1∶10) and selected with puromycin.Stable puromycin-resistant cell pools were established within 10 days,and labeled as DS19shGFP (green fluorescent protein,served as irrelevant control) and DS19shp45 respectively.The DS19 cells were treated with dimethyl sulfoxide (DMSO)to increase baseline expression of AHSP and α-globin.
Reverse transcription-polymerase chain reaction(RT-PCR)
Total cellular RNA was extracted with TRIzol (Invitrogen)and cDNA was prepared using SuperScript II (Invitrogen)following the manufacturer’s instructions.PCR was performed with SYBR green dye on an ABI 7900HT Fast Real-Time PCR System (PE Applied Biosystems,Carlsbad,CA,USA).Samples were normalized to GAPDH.Primer sequences are available upon request.
ChIP assay
ChIP assays were performed as previously described19with minor modifications to examine NF-E2 occupancy across AHSP gene (locations 01-11) in DS19 cells before and after induction with 2% DMSO for 4 days,and to detect histone modifications at AHSP gene in DS19shGFP and DS19shp45 cells.
Previous studies show that GATA-1 co-occupies with NF-E2 in many erythroid genes,including β-globin gene and some erythrocyte membrane protein genes.20,21To examine whether the regulation of AHSP follows a similar pattern,ChIP was performed in G1E-ER4 cells in the presence or absence of estradiol induction (25 nmol/L for 24 hours).
All results were quantified and analyzed by real-time PCR.Signals were normalized to a standard curve generated with serial dilutions of input chromatin (1∶10,1∶102,1∶103,1∶104,and 1∶105).ChIP primer sequences are available upon request.
Luciferase reporter assay
The human AHSP promoter fragments as described in the study of Gallagher et al13were cloned into the pGL3-basic luciferase reporter (Promega,Madison,WI,USA).The recombinant constructs,named 1.1k (-912 bp-+254 bp),0.7k (-478 bp-+254 bp),and 0.4k (-184 bp-+254 bp)respectively,were transfected into HeLa cells at 50%confluence in 24-well plates.Briefly,cells were co-transfected with 0.25 μg pGL3-basic,2 ng Renilla luciferase pRL-CMV plasmid (Promega),and 1 μg indicated transcription factor construct or pcDNA4.GATA-1 and EKLF served as positive controls,and pcDNA4 was used as a negative control.Cells were harvested after 36-48 hours and the luciferase activity was measured with the Dual Luciferase Reporter Assay System Kit (Promega).The data were presented as relative luciferase activity after normalizing the Firefly values to Renilla.
Statistical analysis
The quantitative data were expressed as means±SD.Inter-group comparisons were tested byt-test.APvalue lower than 0.05 was considered statistically significant.
RESULTS
Positive correlation between NF-E2p45 and AHSP expression in DS19 cells
NF-E2p45 mRNA level decreased about 80% (P<0.01) and the protein level was correspondingly reduced in DS19shp45 cells,indicating the high efficiency of NF-E2p45 knockdown in this cell line (Fig.1).Loss of NF-E2p45 led to great reduction of α-globin expression at both mRNA (about 20%remaining,P<0.01) and protein levels.The mRNA expression of AHSP was reduced by 35% (P<0.01) after NF-E2p45 knockdown,and the decrease in AHSP protein level was more than 50% as shown by the Western blot result.The expression of Gpx1 (known as a non-NF-E2p45 target) remained unchanged in DS19shp45 cells,excluding the possibility of off-target effect.
AHSP as a direct downstream target of NF-E2
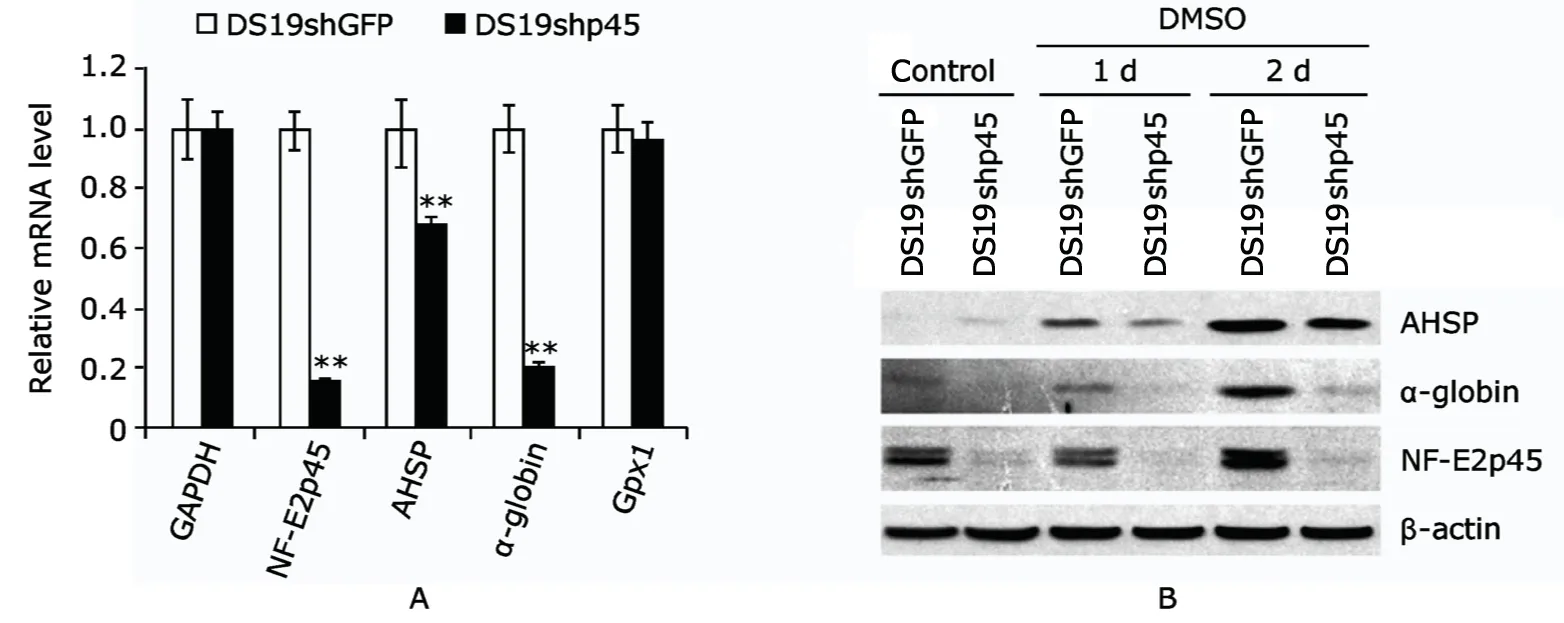
Figure 1.Real-time polymerase chain reaction (A) and Western blot results (B) of gene expression in DS19shGFP (green fluorescent protein) and DS19shp45 cells show that NF-E2p45 knockdown by RNAi leads to reduction of AHSP expression in DS19 cells.The possibility of off-target effects was excluded based on the unchanged Gpx1 expression.DMSO was used to increase baseline expression of AHSP and α-globin in DS19 cells.
ChIP results showed obvious high NF-E2p18 occupancy across the AHSP locus,which was not changed significantly after DMSO induction (Fig.2A).Significant increase of NF-E2p45 binding after DMSO induction was observed at locations 04-07 compared with the occupancy in uninduced cells (Fig.2B).These results confirm strong NFE2 binding at AHSP gene locus and suggest the involvement of NF-E2 in AHSP regulation during erythroid differentiation.
To further investigate whether NF-E2 could transcriptionally activate the AHSP promoter,luciferase reporter assay was performed.Although to a lesser extent than GATA-1 (about 4-fold) and EKLF (4-fold to 5-fold) did,NF-E2p45 obviously activated the AHSP promoter (about 3-fold) compared with the pcDNA4 group (Fig.2C).The pGL3-basic reporter without promoter fragment was affected by neither transcription factors nor empty vector pcDNA4.
H3K4 trimethylation across the AHSP gene affected by NF-E2p45
In ChIP assay,we observed broad acetylation of histone H3 and H4 across the promoter and protein coding region of AHSP,which did not change significantly with NF-E2p45 knockdown (Figs.3A,B).This finding indicates that some other regulators might compensate the function of NFE2p45 in this process.Of particular interest,H3K4 trimethylation was extensively decreased at most detected regions of AHSP gene in DS19shp45 cells (Fig.3C,P<0.05 at locations 06-08 compared with the results in DS19shGFP cells).These results suggested that NF-E2p45 was necessary for H3K4 trimethylation across the AHSP gene.

Figure 2.NF-E2 binds to AHSP gene locus and transactivates its transcription.Chromatin immunoprecipitation (ChIP) was performed to analyze NF-E2 occupancy across AHSP gene in DS19 cells before and after induction with 2% DMSO for 4 days.Occupancy of NF-E2p18 (A) and NF-E2p45 (B) were detected across the AHSP gene locus (results of primers 09 and 11 are not available).IgG was employed as negative control.Primer locations are indicated as 01-11.Signals are quantified using the standard curve of the relevant input DNA.Luciferase assay results (C) demonstrate the activation of AHSP promoter by NF-E2p45 in HeLa cells.1.1K,0.7K,and 0.4K are three truncated AHSP promoter fragments.GATA-1 and EKLF serve as positive controls,and pcDNA4 is negative control.

Figure 3.ChIP shows selective effect of NF-E2p45 on histone H3 acetylation (A),histone H4 acetylation (B),and histone H3 lysine 4(H3K4) trimethylation (C) across the AHSP gene locus in DS19shGFP and DS19shp45 cells.Signals are quantified using the standard curve of the relevant input DNA.acH3:acetylated histone H3;acH4:acetylated histone H4;H3K4me3:trimethylated H3K4.
Cooperation between NF-E2p45 and GATA-1 in regulating AHSP gene expression
ChIP assay revealed a significant peak in the binding of GATA-1 at the AHSP promoter (location 05) in estradiol-induced G1E-ER4 cells (Fig.4A).With the restoration of GATA-1 transcriptional activity,NF-E2p45 binding at AHSP gene was observed as expected (Fig.4B).ChIP with anti-GATA-1 antibody revealed that GATA-1 occupancy at the AHSP promoter as well as at the β-major promoter and β-globin HS2 region was all reduced with silenced NF-E2p45 expression (Fig.4C,allP<0.05 compared with the results in DS19shGFP cells).Taken together,these results support our hypothesis that NF-E2p45 cooperates with GATA-1 in the regulation of AHSP expression.
DISCUSSION
AHSP is a potential target for the gene therapy for β-thalassemia because it binds free α-globin specifically and prohibits the latter from destroying cellular components as a potent oxidant.The effect of NF-E2,a lineage-restricted transcription factor,on AHSP expression has not been investigated,although NF-E2 is prominently associated with globin gene regulation.In the present study,we demonstrated that AHSP expression was highly dependent on NF-E2.NF-E2 binds to AHSP gene,and interplays with GATA-1 and other chromatin modulators in regulating AHSP gene expression.
NF-E2p18 has dual-function– it switches the regulatory complex on the target genes from a repressive pattern to an activating one by exchanging its partners from Bach1 to NF-E2p45 or Nrf2.22,23In our study,NF-E2p18 was found binding to AHSP chromatin irrespective of DMSO induction,whereas the activating subunit NF-E2p45 started to bind only after induction,supporting the partner-switching effect of NF-E2p18 at the AHSP gene locus during erythroid differentiation of DS19 cells.
Co-occupancy of GATA-1 and NF-E2 has been demonstrated to occur extensively.20,21Our results showed the reciprocal effects of their bindings to the AHSP gene locus–NF-E2p45 deficiency caused reduction in GATA-1 binding,and restored GATA-1 activity led to increase in NF-E2p45 binding.A previous study proved that GATA-1 is critical for NF-E2 binding to the regions lacking consensus NF-E2 binding sites but not for its strong binding to the typical ones like HS2.21This is consistent with our findings that no canonical NF-E2 binding site exists at the AHSP gene locus.
Besides interacting with classical transcription factor,NF-E2 associates with multiple chromatin modulators and participates in forming co-regulatory complexes.We observed a global decrease of H3K4 trimethylation across the AHSP gene in the DS19 cells with silenced NF-E2p45.This result suggests the role of NF-E2 in the induction of H3K4 trimethylation,one of the potential mechanisms underlying the AHSP regulation by NF-E2.
In summary,the results of this study support that NF-E2 is a novel regulator of AHSP expression.NF-E2 may be involved in keeping the hemoglobin balance not only by affecting α-and β-globin gene expression directly but also by maintaining appropriate AHSP production.These findings provide further insights into the regulatory network of AHSP expression,as well as new possibilities for β-tha-lassemia treatment.

Figure 4.NF-E2 cooperates with GATA-1 in AHSP gene regulation.In G1E-ER4 cells before and after 24-hour induction with 25 nmol/L estradiol,ChIP was performed to analyze GATA-1 occupancy (A) and NF-E2p45 occupancy (B) across the AHSP gene locus.Signals are quantified using the standard curve of the relevant input DNA and normalized to IgG.The recruitment of GATA-1/NF-E2p45 at the β-H1 promoter serves as negative control,and that at the β-major promoter is positive control.ChIP was performed in DS19shGFP and DS19shp45 cells to detect GATA-1 recruitments at the AHSP gene locus (C),location 05 was singled out based on the results presented in Figure 4A.The recruitments of GATA-1 at the β-major promoter and β-globin locus control region hypersensitive site (HS) 2 were analyzed as controls.G1E-ER4-E2-:G1E-ER4 cells before estradiol induction;G1E-ER4-E2+:G1E-ER4 cells after estradiol induction;βH1:β-H1 promoter;βmaj:β-major promoter.
ACKNOWLEDGEMENT
We thank Dr.Huan Gong,Dr.Yu-sheng Wei,Dr.Duo-nan Yu,Dr.Jing Jiang,Dr.Eugene Khandros,Janine D’Souza,and Dr.Shefali Parikh for critical reading of this manuscript.
1.Shinar E,Rachmilewitz EA.Oxidative denaturation of red blood cells in thalassemia.Semin Hematol 1990;27:70-82.
2.Kihm AJ,Kong Y,Hong W,et al.An abundant erythroid protein that stabilizes free alpha-haemoglobin.Nature 2002;417:758-63.
3.Gell D,Kong Y,Eaton SA,et al.Biophysical characterization of the alpha-globin binding protein alpha-hemoglobin stabilizing protein.J Biol Chem 2002;277:40602-9.
4.Kong Y,Zhou S,Kihm AJ,et al.Loss of alpha-hemoglobin-stabilizing protein impairs erythropoiesis and exacerbates beta-thalassemia.J Clin Invest 2004;114:1457-66.
5.Yu X,Kong Y,Dore LC,et al.An erythroid chaperone that facilitates folding of alpha-globin subunits for hemoglobin synthesis.J Clin Invest 2007;117:1856-65.
6.Andrews NC.The NF-E2 transcription factor.Int J Biochem Cell Biol 1998;30:429-32.
7.Kotkow KJ,Orkin SH.Complexity of the erythroid transcription factor NF-E2 as revealed by gene targeting of the mouse p18 NF-E2 locus.Proc Natl Acad Sci U S A 1996;93:3514-8.
8.Shivdasani RA,Orkin SH.Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2.Proc Natl Acad Sci U S A 1995;92:8690-4.
9.Lu SJ,Rowan S,Bani MR,et al.Retroviral integration within the Fli-2 locus results in inactivation of the erythroid transcription factor NF-E2 in Friend erythroleukemias:evidence that NF-E2 is essential for globin expression.Proc Natl Acad Sci U S A 1994;91:8398-402.
10.Forsberg EC,Downs KM,Bresnick EH.Direct interaction of NF-E2 with hypersensitive site 2 of the beta-globin locus control region in living cells.Blood 2000;96:334-9.
11.Strauss EC,Andrews NC,Higgs DR,et al.In vivofootprinting of the human alpha-globin locus upstream regulatory element by guanine and adenine ligation-mediated polymerase chain reaction.Mol Cell Biol 1992;12:2135-42.
12.Demers C,Chaturvedi CP,Ranish JA,et al.Activator-mediated recruitment of the MLL2 methyltransferase complex to the beta-globin locus.Mol Cell 2007;27:573-84.
13.Gallagher PG,Liem RI,Wong E,et al.GATA-1 and Oct-1 are required for expression of the human alpha-hemoglobin-stabilizing protein gene.J Biol Chem 2005;280:39016-23.
14.Pilon AM,Nilson DG,Zhou D,et al.Alterations in expression and chromatin configuration of the alpha hemoglobin-stabilizing protein gene in erythroid Kruppellike factor-deficient mice.Mol Cell Biol 2006;26:4368-77.
15.Keys JR,Tallack MR,Hodge DJ,et al.Genomic organisation and regulation of murine alpha haemoglobin stabilising protein by erythroid Kruppel-like factor.Br J Haematol 2007;136:150-7.
16.Ohta Y,Tanaka M,Terada M,et al.Erythroid cell differentiation:murine erythroleukemia cell variant with unique pattern of induction by polar compounds.Proc Natl Acad Sci U S A 1976;73:1232-6.
17.Tsang AP,Visvader JE,Turner CA,et al.FOG,a multitype zinc finger protein,acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation.Cell 1997;90:109-19.
18.Wu M,Mergia A.Packaging cell lines for simian foamy virus type 1 vectors.J Virol 1999;73:4498-501.
19.Letting DL,Rakowski C,Weiss MJ,et al.Formation of a tissue-specific histone acetylation pattern by the hematopoietic transcription factor GATA-1.Mol Cell Biol 2003;23:1334-40.
20.Steiner LA,Maksimova Y,Schulz V,et al.Chromatin architecture and transcription factor binding regulate expression of erythrocyte membrane protein genes.Mol Cell Biol 2009;29:5399-412.
21.Johnson KD,Grass JA,Boyer ME,et al.Cooperative activities of hematopoietic regulators recruit RNA polymerase II to a tissue-specific chromatin domain.Proc Natl Acad Sci U S A 2002;99:11760-5.
22.Igarashi K,Hoshino H,Muto A,et al.Multivalent DNA binding complex generated by small Maf and Bach1 as a possible biochemical basis for beta-globin locus control region complex.J Biol Chem 1998;273:11783-90.
23.Sun J,Brand M,Zenke Y,et al.Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network.Proc Natl Acad Sci U S A 2004;101:1461-6.
 Chinese Medical Sciences Journal2010年4期
Chinese Medical Sciences Journal2010年4期
- Chinese Medical Sciences Journal的其它文章
- Epigenetic Repression of SATB1 by Polycomb Group Protein EZH2 in Epithelial Cells△
- Clinicopathological Features of Non-familial Colorectal Cancer with High-frequency Microsatellite Instability△
- Thoraco-abdominal Aorta Revascularization through a Retroperitoneal Approach
- Endothelial Nitric Oxide Synthase Gene Polymorphisms Associated with Susceptibility to High Altitude Pulmonary Edema in Chinese Railway Construction Workers at Qinghai-Tibet over 4 500 Meters above Sea Level△
- Regulation of Acyl-coenzyme A:Cholesterol Acyltransferase 2 Expression by Saturated Fatty Acids△
- Ellagic Acid-induced Hypercoagulable State in Animals:a Potentially Useful Animal Hypercoagulable Model for Evaluation of Anticoagulants
