Regulation of Acyl-coenzyme A:Cholesterol Acyltransferase 2 Expression by Saturated Fatty Acids△
Zhu-qin Zhang,Hou-zao Chen,Rui-feng Yang,Ran Zhang,Yu-yan Jia,Yang Xi,De-pei Liu*,and Chih-chuan Liang
National Laboratory of Medical Molecular Biology,Institute of Basic Medical Science,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100005,China
ACYL-coenzyme A∶cholesterol acyltransferase(ACAT) is an important enzyme family involved in cholesterol metabolism.ACAT converts cholesterol and fatty acyl coenzyme A to cholesterol esters,which are then assembled into very low density lipoprotein (VLDL) or stored in lipid droplet.ACAT family has two members,ACAT1 and ACAT2,the latter being expressed mainly in liver and intestine.Liver ACAT2 catalyzes formation of cholesterol esters,which are assembled into VLDL together with ApoB and secreted into blood.1-3Overexpression of ACAT2 in cells increases cholesterol ester synthesis and ApoB secretion.4,5In contrast,liver-specific downregulation of ACAT2 in mice leads to reduced packaging of cholesterol into ApoB-containing lipoproteins.6ACAT2 deficiency in mice also compromises ApoB secretion and leads to a dramatic decrease of VLDL cholesterol content.7Thus ACAT2 and the regulation of its expression are significant in ApoB secretion and VLDL cholesterol level.
Saturated fatty acids (SFAs) have been regarded as“bad”for its deteriorating effects in increasing low density lipoprotein (LDL) cholesterol and decreasing high density lipoprotein (HDL) cholesterol,8-10in which the former effect is known to be the result of reduced uptake of LDL from plasma.11SFAs were also reported to stimulate ApoB secretion and to increase plasma VLDL cholesterol in the presence of high cholesterol level.12-14The mechanism for SFA regulation of ApoB and VLDL metabolism,however,is still not clear.Given the importance of ACAT2 in ApoB and VLDL metabolism,we speculate that one possible mechanism for SFAs might be to regulate ACAT2,therefore we performed this study to verify this hypothesis.
MATERIALS AND METHODS
Cells and cell cultures
Human liver carcinoma cells HepG2 and HuH7 obtained from American Type Culture Collection were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS).Mouse primary hepatocytes(mPH) were isolated from male C57 mice with two-step collagenase perfusion method.15The perfusion was performed through inferior vena cava.After examined by means of trypan blue exclusion,the cells with more than 80% viability were used in the following experiments.
Fatty acid preparation and cell treatment
To observe whether ACAT2 expression was regulated by SFAs,palmitic acid (PA) and oleic acid (OA) were used,in which the former is the most abundant saturated fatty acid in plasma,and the latter is the most widely distributed and most plentiful unsaturated fatty acid in nature,used as control.16-18Plasma free fatty acids (FFAs) concentration was suggested to be 0.25-3.0 mmol/L.19FFA solutions were prepared as previously described.20,21Briefly,PA at 100 mmol/L (Sigma-Aldrich,St.Louis,MO,USA) and OA at 100 mmol/L (Sigma-Aldrich) were prepared in 0.1 mol/L NaOH at 70°C and diluted with bovine serum albumin (BSA)solution,producing 5 mmol/L FFA/5% BSA mixture.The cells were washed twice with phosphate-buffered saline(PBS) before treatment.The 5mmol/L FFA/5% BSA mixture was mixed with serum-free culture medium at different ratios to reach the needed final concentrations of FFA,to be specific,at the ratio of 1∶4 to the concentration of 1 mmol/L,1∶24 to the concentration of 0.2 mmol/L,and 1∶9 to the concentration of 0.5 mmol/L.In the assay indicated in Figure 1,the cells were treated with BSA,1 mmol/L OA,or 1 mmol/L PA for 14 hours.In Figure 2A,cells were treated with BSA,0.2 mmol/L,0.5 mmol/L,or 1 mmol/L PA for 14 hours.In Figture 2B,cells were treated with 1 mmol/L PA for 0,6,12,and 18 hours.
SFA diet in mice
Three-month-old male C57/BL6 mice (Vital River Laboratory Animal Technology Co.Ltd.,Beijing,China) were subjected to experiments.In initial experiment,16 mice were divided into two groups according to body weight(n=8) and were given SFA or control diet for 3 months.Mice were sacrificed after 3 months and livers were isolated for reverse transcription-polymerase chain reaction(RT-PCR).In another experiment,21 mice were divided into 7 groups according to body weight (n=3),and given SFA diet for 0,1,2,3,5,7,and 14 days,respectively.SFA diets of all the 7 groups were stopped at the same end point,and control diet was given before the start of SFA diet,which was different among the groups.All the mice were sacrificed at the same end point to isolate livers for RT-PCR.
Diet recipes were determined according to a previous report.22Recipe for SFA diet∶hydrogenated coconut oil(144 g/kg),corn oil (17 g/kg),corn starch (123 g/kg),sucrose (182 g/kg),and cellulose (146 g/kg).Recipe for control diet∶hydrogenated coconut oil (33 g/kg),corn oil(21 g/kg),corn starch (245 g/kg),sucrose (292 g/kg),and cellulose (12 g/kg).The ingredients contained in both diets include casein (206 g/kg),maltodextrin (100 g/kg),cholesterol (2.0 g/kg),methionine (3.0 g/kg),vitamin mix (10 g/kg),choline bitartrate (2.3 g/kg),ethoxyquin(0.037 g/kg),mineral mix (37 g/kg),and calcium phosphate (4.2 g/kg).The hydrogenated coconut oil (Fuji Oil,Shanghai,China) used in the experiments included caprylic acid (8.63%),undecylic acid (6.7%),lauric acid (49.47%),myristic acid (16.82%),palmitic acid (7.83%),stearic acid(9.97%),oleic acid (0.23%),and others (0.35%).
RNA isolation,reverse transcription,and real-time PCR
RNAs were extracted from HepG2,HuH7,mouse primary hepatocytes,and mouse livers with TRIzol (Invitrogen,Carlsbad,CA,USA) according to the manufacturer’s instructions,and reversely transcribed to cDNA as template for PCR.Real-time PCR was performed with an iQ5 thermal cycler (Bio-Rad Laboratories,Hercules,CA,USA) to detect ACAT expression level.The primers used are as follows∶human ACAT2,5’-TCTATCCTGCATGCCACGTTG-3’ (forward),5’-AGTTCCACCAGTCCCGGTAGAA-3’ (reverse);human ACAT1 (negative control),5’-TTAACTCCATCTTGCCAGGTGTG-3’ (forward),5’-TGTCACCAAAGCGTAACATCTCA-3’ (reverse);human GAPDH (internal control),5’-GCCTCAAGATCATCAGCAATGC-3’ (forward),5’-TCTTCTGGGTGGCAGTGATGG-3’ (reverse);mouse ACAT2,5’-CGCTGCGTGCTGGTCTTT-3’ (forward),5’-ATGCCCTTTCCTCCTCTGACA-3’ (reverse);mouse actin (internal control),5’-CCTTCCTTCTTGGGTATGGAATCAG-3’ (forward),5’-AGCACTGTGTTGGCATAGAGGT-3’ (reverse).
Statistical analysis
All statistics analyses were performed with GraphPad Prism 5.0 (GraphPad Software,La Jolla,CA,USA).Data were expressed as means±SEM.Differences among groups were tested with one-way analysis of variance (ANOVA).Comparisons between two groups were performed with unpairedt-test.The results were presented as means±SEM.APlever under 0.05 was considered statistically significant.
RESULTS
PA treatment induced ACAT2 expression in hepatic cells
The results of real-time PCR showed that in HepG2 cells,1 mmol/L PA treatment for 14 hours induced a significant increase in ACAT2 expression,while OA treatment caused no significant change (Fig.1A,P<0.01).Similar results were also observed in HuH7 cell line and mPH treated with 1 mmol/L PA or 1 mmol/L OA for 14 hours (Fig.1A,bothP<0.01).We also observed ACAT1 expression in response to PA treatment since it is also expressed in liver.In contrast to ACAT2,ACAT1 expression level nearly remained constant after PA treatment (Fig.1B).ACAT1 and ACAT2 are two isoforms of ACAT gene family.SFA regulation of ACAT2,but not ACAT1,suggested that ACAT2 and ACAT1 have different regulation patterns.It is possible that ACAT1 is expressed constitutively whereas ACAT2 expression may be induced by SFA.
PA treatments led to dose-and time-dependent increases of ACAT2 expression
Treatment with PA at different concentrations in HepG2 cells resulted in a concentration-dependent increase of ACAT2 expression.Although PA at 0.2 mmol/L did not induce much change in ACAT2 expression after 14-hour treatment,a significant increase in ACAT2 expression was observed in HepG2 cell treated with 0.5 mmol/L PA (P<0.05) and an even more dramatic one with 1 mmol/L PA(Fig.2A,P<0.01).To investigate the existence of timedependent effect of PA,we treated HepG2 cells with 1 mmol/L PA for different time periods ranging from 0 to 18 hours.There was a sustained and gradual rise of ACAT2 expression along with the extension of treatment duration(Fig.2B,allP<0.05).Taken together,these results suggested that PA treatment has a dose-and time-dependent effect in inducing the increase of ACAT2 expression.
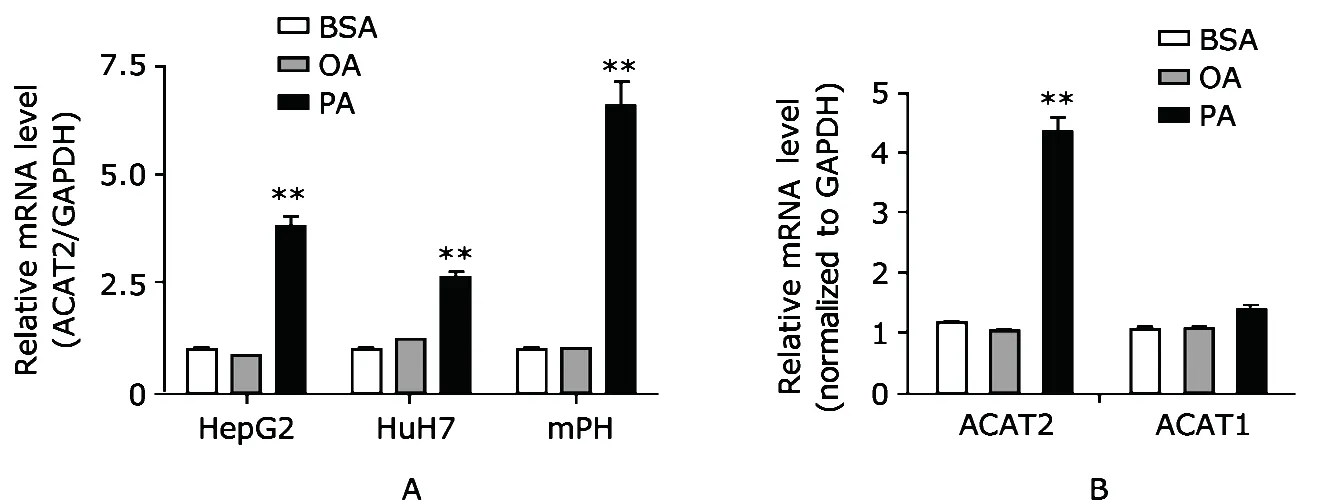
Figure 1.Palmitic acid (PA) treatment in hepatic cells HepG2,HuH7,and mouse primary hepatocytes (mPH) induces ACAT2 expression as confirmed by the results of real-time polymerase chain reaction (PCR).The three types of cells were treated with bull serum albumin (BSA) (as control),1 mmol/L oleic acid (OA),or 1 mmol/L PA for 14 hours in a serum-free medium (A).In contrast to ACAT2,ACAT1 expression is not promoted by PA treatment in HepG2 cells (B).
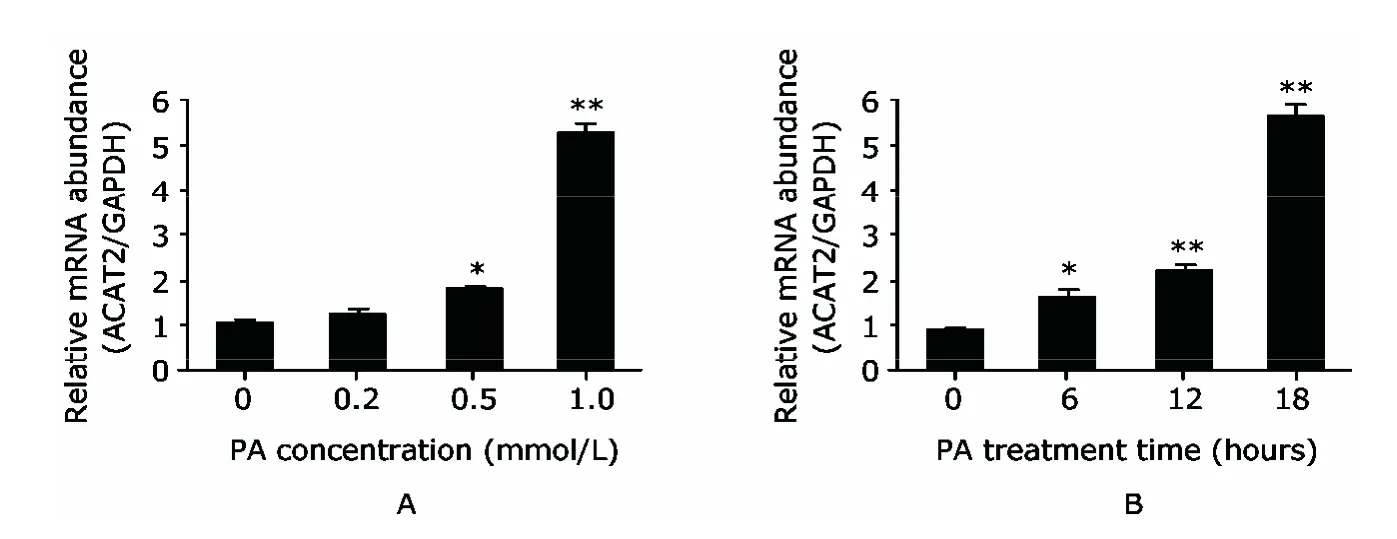
Figure 2.Dose-and time-dependent increases of ACAT2 expression induced by PA treatment in HepG2 cells.
SFA diets induced transient increase of ACAT2 expression in mouse livers
To observe the effect of SFAs on ACAT2 expressionin vivo,we fed mice with diets containing hydrogenated coconut oil which are enriched with SFAs.Between the groups fed with SFA diet or control diet respectively for three months,no difference was found in ACAT2 expression in their livers.In the following experiment feeding 7 groups of mice with SFA diet for different periods,a peak of ACAT2 expression was noticed in those fed with SFA diet for two days (P<0.05 compared with the level in 0-day group).When mice were fed with SFA diet for more than two days,ACAT2 expression began to decline from the highest level until back to the normal level in 14-day group (Fig.3).These results showed that SFA caused a short-term and transient increase of ACAT2 expression in mouse livers.*
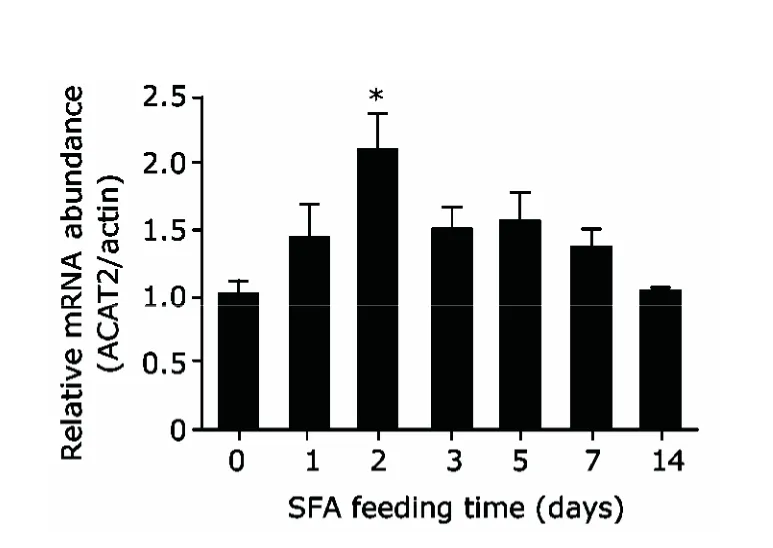
Figure 3.Saturated fatty acid (SFA) diet induces short-term and transient increases of ACAT2 expression in livers of C57/BL6 mice.Mice divided into seven groups (n=3)were given SFA diet for time periods ranging from 0 to 14 days.SFA diet was started at different time points among the groups to ensure the some end point.Control diet was given before the initiation of SFA diet.ACAT2 expression level in the livers of those mice was analyzed with real-time PCR.
DISCUSSION
In this study,we found that PA,the most abundant saturated fatty acid in plasma,induced ACAT2 expression in HepG2,HuH7,and mPH.The degree of the elevation of ACAT2 expression was found rising as the dose of PA increased or the treatment duration extended.In addition,diets containing a large proportion of SFAs induced a transient increase of ACAT2 expression in mouse liver.Therefore,our results showed that SFA might increase liver ACAT2 expression.
There are few reports about regulation of ACAT2 expression by SFA.It was found that in hamsters,hepatic ACAT activity was significantly higher in response to PA and stearic acid treatment.However,there was no significant difference in hepatic ACAT-2 expression levels.23In our experiments,we found that SFA feeding for a long time lead to no change of ACAT2 expression.In contrast,ACAT2 expression showed a significant increase in response to short-term SFA treatment.The results suggest that the regulation of ACAT2 expression may be dependent on feeding period.
In cell experiments,ACAT2 expression was induced by 0.5-1 mmol/L PA treatment for 14 hours or by 1 mmol/L PA treatment for 6-18 hours.Though PA treatments with this intensity was reported to cause cell apoptosis,24the concentration we used was within the range of plasma FFAs,0.25-3.0 mmol/L.19We also observed increased ACAT2 expression by PA treatment in different hepatic cells,including HepG2,HuH7,and mPH.Based on the findings,we suggested that the effect of PA on ACAT2 expression really existed,and this effect might coexist with,but different from,its apoptotic effect.
In animal experiments,we fed the experimental mice with hydrogenated coconut oil as SFA source,which is a major type of oil often used in animal diets.Another major type is palm oil,containing a high proportion of palmitic acid,yet also large quantities of unsaturated fatty acids including oleic acid and linoleic acid.25In contrast,hydrogenated coconut oil is primarily composed of SFA,making it more suitable than palm oil for our study.Although its major component is not palmitic acid,hydrogenated coconut oil presents a mixture of SFAs,and the impact of hydrogenated coconutoil on ACAT2 expression could be concluded as the effect of SFA.
In HepG2 cells,we observed that PA treatment led to a sustained and gradual increase of ACAT2 expression,but hydrogenated coconut oil feeding caused only a short-term rise of ACAT2 expression in mouse liver,with a peak in those fed with SFA diet for two days.This discrepancy was associated with different treatments,reflecting the difference in strength and duration of the impact of different treatments on ACAT2 expression.The results of our study suggest that PA might have a strong and long effect on ACAT2 expression in hepatic cells,whereas hydrogenated coconut oil might have a weak and short influence on ACAT2 expression in liverin vivo.
ACAT2 is an important enzyme in lipid metabolism,the overexpression of which stimulates cholesterol ester synthesis and ApoB secretion.4,5Given the changes observed in ACAT2 expression in our study,we inferred that PA and hydrogenated coconut oil might regulate cholesterol ester synthesis,ApoB secretion,and VLDL cholesterol ester through acting on ACAT2.Further work will be necessary to demonstrate detailed mechanism in the regulatory process of ACAT2 expression by SFA and to determine the possible role of SFA in regulating cholesterol ester biosynthesis and ApoB secretion.
ACKNOWLEDGEMENT
We thank Lei Li,Lu Lu,Guo-wei Zhao,Bei-bei Mao,Huan Gong,Zhen-ya Li,Li Li,Hui-na Zhang,and Shuang Zhou for their technical assistance in this study.
1.Chang TY,Li BL,Chang CC,et al.Acyl-coenzyme A∶cholesterol acyltransferases.Am J Physiol Endocrinol Metab 2009;297∶E1-9.
2.Chang TY,Chang CC,Lin S,et al.Roles of acyl-coenzyme A∶cholesterol acyltransferase-1 and -2.Curr Opin Lipidol 2001;12∶289-96.
3.Joyce C,Skinner K,Anderson RA,et al.Acyl-coenzyme A∶cholesteryl acyltransferase 2.Curr Opin Lipidol 1999;10∶89-95.
4.Liang JJ,Oelkers P,Guo C,et al.Overexpression of human diacylglycerol acyltransferase 1,acyl-coa∶cholesterol acyltransferase 1,or acyl-CoA∶cholesterol acyltransferase 2 stimulates secretion of apolipoprotein B-containing lipoproteins in McA-RH7777 cells.J Biol Chem 2004;279∶44938-44.
5.Temel RE,Hou L,Rudel LL,et al.ACAT2 stimulates cholesteryl ester secretion in ApoB-containing lipoproteins.J Lipid Res 2007;48∶1618-27.
6.Bell TA 3rd,Brown JM,Graham MJ,et al.Liver-specific inhibition of acyl-coenzyme a∶cholesterol acyltransferase 2 with antisense oligonucleotides limits atherosclerosis development in apolipoprotein B100-only low-density lipoprotein receptor-/-mice.Arterioscler Thromb Vasc Biol 2006;26∶1814-20.
7.Lee RG,Shah R,Sawyer JK,et al.ACAT2 contributes cholesteryl esters to newly secreted VLDL,whereas LCAT adds cholesteryl ester to LDL in mice.J Lipid Res 2005;46∶1205-12.
8.Abbey M,Noakes M,Belling GB,et al.Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low-density-lipoprotein cholesterol.Am J Clin Nutr 1994;59∶995-9.
9.Babayan VK.Plasma cholesterol responsiveness to saturated fatty acids.Am J Clin Nutr 1988;48∶1520-2.
10.Mattson FH,Grundy SM.Comparison of effects of dietary saturated,monounsaturated,and polyunsaturated fatty acids on plasma lipids and lipoproteins in man.J Lipid Res 1985;26∶194-202.
11.Horton JD,Cuthbert JA,Spady DK.Dietary fatty acids regulate hepatic low density lipoprotein (LDL) transport by altering LDL receptor protein and mRNA levels.J Clin Invest 1993;92∶743-9.
12.Arrol S,Mackness MI,Durrington PN.The effects of fatty acids on apolipoprotein B secretion by human hepatoma cells (HEP G2).Atherosclerosis 2000;150∶255-64.
13.Kummrow E,Hussain MM,Pan M,et al.Myristic acid increases dense lipoprotein secretion by inhibiting ApoB degradation and triglyceride recruitment.J Lipid Res 2002;43∶2155-63.
14.Vallim T,Salter AM.Regulation of hepatic gene expression by saturated fatty acids.Prostaglandins Leukot Essent Fatty Acids 2010;82∶211-8.
15.Klaunig JE,Goldblatt PJ,Hinton DE,et al.Mouse liver cell culture.I.hepatocyte isolation.In Vitro 1981;17∶913-25.
16.Risé P,Eligini S,Ghezzi S,et al.Fatty acid composition of plasma,blood cells and whole blood∶relevance for the assessment of the fatty acid status in humans.Prostaglandins Leukot Essent Fatty Acids 2007;76∶363-9.
17.Cater NB,Denke MA.Behenic acid is a cholesterol-raising saturated fatty acid in humans.Am J Clin Nutr 2001;73∶41-4.
18.Rhead MM,Eglinton G,Draffan GH,et al.Conversion of oleic acid to saturated fatty acids in severn estuary sediments.Nature 1971;232∶327-30.
19.Hamilton JA,Kamp F.How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes 1999;48∶2255-69.
20.Karaskov E,Scott C,Zhang L,et al.Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress,which may contribute to INS-1 pancreatic beta-cell apoptosis.Endocrinology 2006;147∶3398-407.
21.Cousin SP,Hügl SR,Wrede CE,et al.Free fatty acidinduced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1.Endocrinology 2001;142∶229-40.
22.Merkel M,Velez-Carrasco W,Hudgins LC,et al.Compared with saturated fatty acids,dietary monounsaturated fatty acids and carbohydrates increase atherosclerosis and VLDL cholesterol levels in LDL receptor-deficient,but not apolipoprotein E-deficient mice.Proc Natl Acad Sci U S A 2001;98∶13294-9.
23.Lee JY.Regulation of gene expression by dietary fatty acids in cholesterol metabolism.ETD collection for University of Nebraska -Lincoln,Paper AAI3045524.Available from http∶//digitalcommons.unl.edu/dissertations/AAI3045524.
24.Das SK,Chu WS,Mondal AK,et al.Effect of pioglitazone treatment on endoplasmic reticulum stress response in human adipose and in palmitate-induced stress in human liver and adipose cell lines.Am J Physiol Endocrinol Metab 2008;295∶E393-400.
25.Cottrell RC.Introduction∶nutritional aspects of palm oil.Am J Clin Nutr 1991;53∶989S-1009S.
 Chinese Medical Sciences Journal2010年4期
Chinese Medical Sciences Journal2010年4期
- Chinese Medical Sciences Journal的其它文章
- Endothelial Nitric Oxide Synthase Gene Polymorphisms Associated with Susceptibility to High Altitude Pulmonary Edema in Chinese Railway Construction Workers at Qinghai-Tibet over 4 500 Meters above Sea Level△
- Thoraco-abdominal Aorta Revascularization through a Retroperitoneal Approach
- Clinicopathological Features of Non-familial Colorectal Cancer with High-frequency Microsatellite Instability△
- Epigenetic Repression of SATB1 by Polycomb Group Protein EZH2 in Epithelial Cells△
- NF-E2:a Novel Regulator of Alpha-hemoglobin Stabilizing Protein Gene Expression△
- Expression of Cyclooxygenase-2 and Its Relationship with Mismatch Repair and Microsatellite Instability in Hereditary Nonpolyposis Colorectal Cancer△
