Antagomir Dependent MicroRNA-205 Reduction Enhances Adhesion Ability of Human Corneal Epithelial Keratinocytes△
Jun Li,Hua Bai,Yong Zhu,Xiao-yan Wang,Fang Wang,Jun-wu Zhang,Robert M.Lavker,and Jia Yu*
1Department of Biochemistry,Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100005,China
2Department of Cardiology,Chongqing First-aid Medical Center,Chongqing 400014,China
3Department of Ophthalmology,The Military General Hospital of Beijing PLA,Beijing 100700,China
4Department of Dermatology,Feinberg School of Medicine,Northwestern University,Chicago 60611,USA
MICRORNAs (miRNAs) are small,18-to 25-nucleotide,non-coding RNAs.They exhibit as wide a variety of developmental and tissue distribution patterns as the more commonly encountered mRNA-coding proteins.1Although the exact function of most miRNAs remains unknown,these endogenous silencing RNAs have been shown to play important roles in development and differentiation,cellular stress response,stem cell regulation,and cancer.
Keratinocyte motility is essential for maintaining epidermal homeostasis and re-epithelialization in wound healing.2,3Much has been learned about the regulation of keratinocyte motility,including the roles that cell-matrix interactions and actin cytoskeleton play in this process.This keratinocyte motility machinery is tightly regulated by both extrinsic and intrinsic factors.For example,soluble growth factors and extracellular matrix (ECM) actviacell surface receptors and integrins,respectively,to trigger a number of intracellular signaling pathways that commonly converge on the regulation of Rho family GTPases,reorganization of actin cytoskeleton,and cell adhesion.At the same time,the basic activation state of these various signaling pathways likely determines the ability of keratinocytes to respond to environmental cures and direct migration.To date,most of the previous researches have focused on the genes and proteins involved in the extrinsic regulation of keratinocyte motility machinery;however,it is now becoming appreciated that miRNAs play a significant role in determining the intrinsic potential of cells to move.Most of our knowledge concerning how miRNAs regulate movement is in the context of cancer,where attention has been focused on the regulatory roles these molecules play in invasion and metastasis.4-6Much less is known about how miRNAs regulate adhesion and migration of normal cells.
MiR-205 is the second most abundant miRNA in corneal epithelial cells,and is expressed in the basal,suprabasal,and superficial layers of the cornea,as well as in limbal and conjunctival epithelia.7It has been reported that miR-205 regulates SH2-containing inositol 5-phosphatase 2 (SHIP2)to modulate Akt signaling in keratinocytes.8Moreover,SHIP2 and phosphoinositide 3-kinase (PI3K)/Akt pathway have also been implicated in regulation of actin cytoskeleton and cell migration,9-12although more studies are focused on the upstream regulation of Akt signaling by phosphatase and tensin homolog (PTEN),the related lipid phosphatase and tumor suppressor.13-15Therefore,we designed this research to investigate the effects of miR-205 in adhesion of normal human corneal epithelial keratinocytes (NHCEKs).
MATERIALS AND METHODS
Cell culture
NHCEKs were obtained from the Department of Ophthalmology,the Military General Hospital of Beijing PLA and cultured in CnT50 medium with 10% fetal bovine serum(FBS) (CellnTech,Bern,Switzerland) on collagen IV coated plates (BD Biosciences,San Jose,CA,USA) at 37°C in 5%CO2.
Antagomirs and treatment
Antagomirs are cholesterol-linked single-stranded RNAs that are complementary to a specific miRNA and cause depletion of that miRNA.The antagomirs used in the present study were synthesized by Dharmacon Inc (Lafayette,CO,USA).Sequences were 5’-mCsmAsmGsmAmCmUmCm-CmGmGmUmGmGmAmAmUmGmAmAsmGsmGsmAChol-3’(antago-205),and 5’-mGsmGsmCsmAmUmUmCmAmCm-CmGmCmGmUmGmCmCsmUsmUsm-AChol-3’ (irrelevant antago).The“m”in the sequences represents 2’-Omethyl-modified oligonucleotide,the subscript“s”represents a phosphorothioate linkage,and“Chol”represents linked cholesterol.NHCEKs were grown in CnT50 medium at a confluence of 70% and were then treated with antagomir-containing or irrelevant-antagomir-containing CnT50 medium for 72 hours at a final concentration of 1 000 pmol/mL.The medium was changed every 24 hours.After 72-hour cultivation,the cells were harvested for Northern blot,Western blot,and immunohistochemical staining.
Northern and Western blots
The sequence of miR-205 probe used in Northern blot was 5’-CAGACUCCGGUGGAAUGAAGGA-3’ and U6snRNA was used as an RNA loading control (Invitrogen,Carlsbad,CA,USA).The antibodies used for Western blot included phosphorylated paxillin (p-Pax,tyrosine 118),Pax (Cell Signaling Technology,Danvers,MA,USA),phosphorylated focal adhesion kinase (p-FAK,tyrosine 576/577),and FAK(Santa Cruz Biotechnology,Santa Cruz,CA,USA) for the function of FAK and Pax in focal contacts.16-18α-tubulin(Invitrogen) was used as a loading control.
Cell adhesion assay
It is well accepted that adhesion plays a central role in cell motility;therefore,we investigated the influence of modulating miR-205 on NHCEKs adhesion.NHCEKs were seeded onto 6-well plates (5×105cells per well) the day before transfection.The cells (about 70% confluent) were treated with antago-205 or antago-124 (an irrelevant antagomir and neuronal-specific miRNA) at a final concentration of 1 000 pmol/mL.All treatments were carried out in triplicate using DharmaFECT 2 (Dharmacon).After 72 hours,NHCEKs were harvested in phosphate buffered saline (PBS)with ethylene diamine tetraacetic acid (EDTA),resuspended in CnT50 medium,and seeded onto collagen-coated 6-well plates (BD Biosciences).After incubation for 1 hour at 37°C,the cells were washed for three times with PBS.Adherent cells were fixed with 10% formalin and stained with methylene blue.The adherent NHCEKs were visualized and quantified using a Zeiss Axiovert light microscope (Carl Zeiss,Göttingen,Germany) and image analysis software Axiovision 4.7.
Immunohistochemistry and light microscopy
NHCEK cultures grown on glass coverslips were fixed with 4% paraformaldehyde at room temperature for 20 minutes.After washed with PBS,the cells were blocked and permeabilized in PBS containing 2.5% goat serum and 0.1%Triton X-100 at room temperature for 90 minutes.Filamentous actin (F-actin),an element involved in cell migration,cell adhesion to ECM,and focal contacts,19was detected by incubating the coverslips with rhodamineconjugated phalloidin (1:50,Sigma-Aldrich,Deisenhofen,Germany) at room temperature for 2 hours.For p-Pax,Pax,p-FAK,and FAK staining,antibodies were incubated at a ration of 1:25 at 4°C overnight.Alexa Fluor 488 goat anti-rabbit IgG (1:500,Invitrogen) was incubated at room temperature for 1 hour.Alexa Fluor antibodies against rabbit IgG were used as a negative control.Cells were viewed and photographed with a Zeiss UV LSM 510 confocal microscope.
Statistical analysis
Student’st-test (two-tailed) was performed to analyze the numeric data.APvalue less than 0.05 was considered significant.
RESULTS
Antago-205 downregulates endogenous miR-205 in NHCEKs
We conducted the miRNA loss-of-function study using antago-205,an antagomir to miR-205.The results of Northern blot indicated that endogenous miR-205 was markedly reduced by 72-hour’s treatment with antago-205,whereas antago-124 did not show such effect (Fig.1).
MiR-205 inhibition enhances cell adhesion
Cell adhesion assay found that the adherence rate of NHCEKs treated with irrelevant antagomir was about 40%lower than that of antago-205-treated NHCEKs (0.53±0.08vs.0.88±0.06,P<0.01).These findings indicated that the acceleration of keratinocyte migration induced by miR-205 might be partly due to miR-205-mediated reduction of NHCEK adhesion to ECM.
MiR-205 suppression augments focal contacts
NHCEKs treated with antago-205 showed elevated levels of p-FAK and p-Pax compared with those treated with irrelevant antagomir,whereas no changes were observed in total levels of the two proteins (Fig.2).Such an increase in focal contacts after miR-205 inhibition is consistent with the enhancement of cell adhesion.It is obvious that antago-205-enhanced attachment leads to a reduction in the migratory ability of NHCEKs.
MiR-205 suppression downregulates F-actin
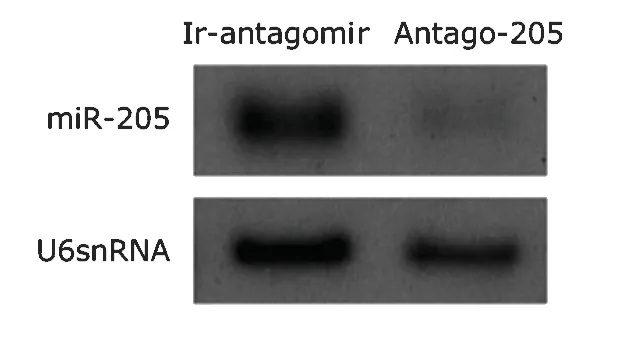
Figure 1.Northern blot analysis of microRNA-205 (miR-205) in normal human corneal epithelial keratinocytes(NHCEKs) treated with antago-205 or an irrelevant antagomir (ir-antagomir).U6snRNA is set as a loading control.
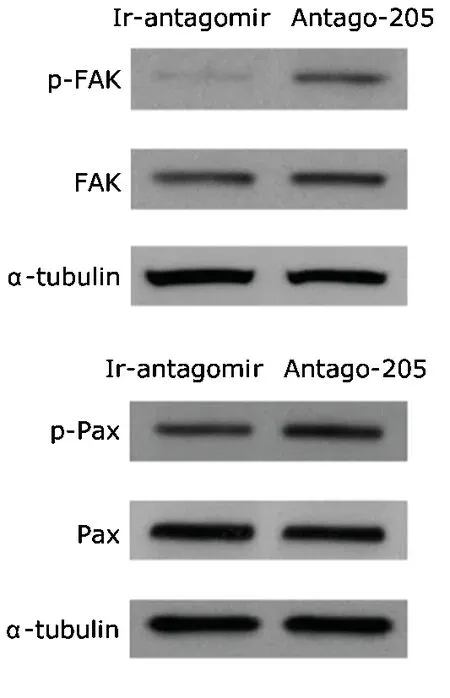
Figure 2.Western blot analysis of focal adhesion kinase (FAK),phosphorylated FAK (p-FAK),paxillin (Pax),and phosphorylated paxillin (p-Pax) in NHCEKs treated with antago-205 or an ir-antagomir.α-tubulin serves as a loading control.
Immunostaining with phallodin showed a marked diminution of F-actin in antago-205-treted NHCEKs compared with the control (Figs.3,4).Meanwhile,we used immunofluorescence staining to measure the changes of p-FAK and p-Pax,both being biomarkers of focal contacts,after the down-regulation of miR-205 in NHCEKs.As shown in Figures 3 and 4,the reduction of miR-205 resulted in a considerable increase in p-FAK and p-Pax,whereas no obvious change was noted in the total levels of FAK or paxillin.These findings suggested the effect of miR-205 in maintaining F-actin organization.
DISCUSSION
The corneal epithelium is unique in that it exhibits distinct as well as overlapping expressions of miR-205 and miR-184.7MiR-205 is expressed in a wide variety of epithelial tissues.In stratified squamous epithelia such as the epidermis and the anterior ocular surface,miR-205 is detected in the basal,suprabasal,and superficial layers.7This broad epithelial expression has led to the idea that miR-205 may be important for establishment of epithelial cell lineages during early development and maintaining epithelial homeostasis in adult.8,20Despite such a potentially fundamental role in epithelial biology,most knowledge on miR-205 comes from investigations on cancer in which this miRNA has been proposed to function either as an“oncomir”or a tumor suppressor,depending on the type of cancer.21-29Low-density lipoprotein receptor-related protein 1,ErbB3/HER3,and vascular endothelial growth factor-A have been shown to be targets of miR-205 in previous studies using transformed cell lines.21,22,24It was also found that miR-205 represses SHIP2 in primary cultures of normal human epidermal keratinocytes (NHEKs)and NHCEKs.8SHIP2 is a ubiquitous lipid phosphatase that dephosphorylates phosphatidylinositol 3,4,5-triphosphate(PIP3),a critical second messenger in several cell signaling pathways including Akt pathway.30-32Our previous study indicated that miR-205 enhanced the Akt signaling pathwayviaSHIP2 suppression,leading to improved cell survival.8Interestingly,SHIP2 and PI3K/Akt pathway have also been implicated in regulating actin cytoskeleton and cell migration.9-12

Figure 3.Immunofluorescent analysis of FAK,p-FAK and immunohistochemistry of filamentous actin in NHCEKs treated with antago-205 or an irrelevant antagomir.× 63

Figure 4.Immunofluorescent analysis of paxillin,p-Pax and immunohistochemistry of filamentous actin in NHCEKs treated with antago-205 or an irrelevant antagomir.× 63
Cell adhesion and migration is vital for the homeostasis of self-renewing tissues such as the epidermis and the epithelia of the ocular anterior surface.They are also key components of re-epithelialization,since this process is initiated by keratinocyte movement at the edges of a wound.The events necessary for successful cell movement include reorganization of cytoskeleton,cell polarization,assembly and disassembly of cell-cell contacts,and interactions between the migrating cell and ECM.In the present study,we confirmed the role of miR-205 as a negative regulator of NHCEK adhesion.Down-regulation of miR-205 could accelerate p-FAK and p-Pax to increase cell-substrate adhesion and alter F-actin organization.It is well established that actin dynamics plays a major role in cell adhesion and migration,and that the changes in actin during this process are highly regulated by a series of actin-associated proteins.33Therefore,it is not surprising that the suppression of F-actin in NHCEKs following downregulation of miR-205 would alter levels of p-FAK and p-Pax.
1.Landgraf P,Rusu M,Sheridan R,et al.A mammalian microRNA expression atlas based on small RNA library sequencing.Cell 2007;129:1401-14.
2.Lu L,Reinach PS,Kao WW.Corneal epithelial wound healing.Exp Biol Med 2001;226:653-64.
3.Raja,Sivamani K,Garcia MS,et al.Wound re-epithelialization:modulating keratinocyte migration in wound healing.Front Biosci 2007;12:2849-68.
4.Ma L,Teruya-Feldstein J,Weinberg RA.Tumour invasion and metastasis initiated by microRNA-10b in breast cancer.Nature 2007;449:682-8.
5.Tavazoie SF,Alarcón C,Oskarsson T,et al.Endogenous human microRNAs that suppress breast cancer metastasis.Nature 2008;451:147-52.
6.Valastyan S,Reinhardt F,Benaich N,et al.A pleiotropically acting microRNA,miR-31,inhibits breast cancer metastasis.Cell 2009;137:1032-46.
7.Ryan DG,Oliveira-Fernandes M,Lavker RM.MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity.Mol Vis 2006;12:1175-84.
8.Yu J,Ryan DG,Getsios S,et al.MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia.PNAS 2008;105:19300-5.
9.Qian Y,Corum L,Meng Q,et al.PI3K induced actin filament remodeling through Akt and p70S6K1:implication of essential role in cell migration.Am J Physiol Cell Physiol 2004;286:C153-63.
10.Dyson JM,O’Malley CJ,Becanovic J,et al.The SH2-containing inositol polyphosphate 5-phosphatase,SHIP-2,binds filamin and regulates submembraneous actin.J Cell Biol 2001;155:1065-79.
11.Prasad NK,Decker SJ.SH2-containing 5’-inositol phosphatase,SHIP2,regulates cytoskeleton organization and ligand-dependent down-regulation of the epidermal growth factor receptor.J Biol Chem 2005;280:13129-36.
12.Vandermoere F,El Yazidi-Belkoura I,Demont Y,et al.Proteomics exploration reveals that actin is a signaling target of the kinase Akt.Mol Cell Proteomics 2007;6:114-24.
13.Leslie NR,Downes CP.PTEN function:how normal cells control it and tumour cells lose it.Biochem J 2004;382:1-11.
14.Lim MA,Yang L,Zheng Y,et al.Roles of PDK-1 and PKN in regulating cell migration and cortical actin formation of PTEN-knockout cells.Oncogene 2004;23:9348-58.
15.Salmena L,Carracedo A,Pandolfi PP.Tenets of PTEN tumor suppression.Cell 2008;133:403-14.
16.Yurko MA,O’Toole EA,Woodley DT.Phosphorylation of focal adhesion kinase (pp125(FAK)) is increased in human keratinocytes induced to migrate by extracellular matrices.J Cell Physiol 2001;188:24-32.
17.Trinkaus-Randall V,Kewalramani R,Payne J,et al.Calcium signaling induced by adhesion mediates protein tyrosine phosphorylation and is independent of pHi.J Cell Physiol 2000;184:385-99.
18.Shi Q,Boettiger D.A novel mode for integrin-mediated signaling:tethering is required for phosphorylation of FAK Y397.Mol Biol Cell 2003;14:4306-15.
19.Ridley AJ,Schwartz MA,Burridge K,et al.Cell migration:integrating signals from front to back.Science 2003;302:1704-9.
20.Gregory PA,Bert AG,Paterson EL,et al.The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1.Nat Cell Biol 2008;10:593-601.
21.Wu H,Zhu S,Mo YY.Suppression of cell growth and invasion by miR-205 in breast cancer.Cell Res 2009;19:439-48.
22.Iorio MV,Casalini P,Piovan C,et al.microRNA-205 regulates HER3 in human breast cancer.Cancer Res 2009;69:2195-200.
23.Gandellini P,Folini M,Longoni N,et al.miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon.Cancer Res 2009:69:2287-95.
24.Song H,Bu G.MicroRNA-205 inhibits tumor cell migration through down-regulating the expression of the LDL receptor-related protein 1.Biochem Biophys Res Commun 2009;388:400-5.
25.Iorio MV,Ferracin M,Liu CG,et al.MicroRNA gene expression deregulation in human breast cancer.Cancer Res 2005;65:7065-70.
26.Iorio MV,Visone R,Di Leva G,et al.MicroRNA signatures in human ovarian cancer.Cancer Res 2007;67:8699-707.
27.Jiang J,Lee EJ,Gusev Y,et al.Real-time expression profiling of microRNA precursors in human cancer cell lines.Nucleic Acids Res 2005;33:5394-403.
28.Tran N,McLean T,Zhang X,et al.MicroRNA expression profiles in head and neck cancer cell lines.Biochem Biophys Res Commun 2007;358:12-7.
29.Lebanony D,Benjamin H,Gilad S,et al.Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma.J Clin Oncol 2009;27:2030-7.
30.Aman MJ,Lamkin TD,Okada H,et al.The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells.J Biol Chem 1998;273:33922-8.
31.Cantley LC.The phosphoinositide 3-kinase pathway.Science 2002;296:1655-7.
32.Hawkins PT,Anderson KE,Davidson K,et al.Signaling through Class I PI3Ks in mammalian cells.Biochem Soc Trans 2006;34:647-62.
33.Le Clainche C,Carlier MF.Regulation of actin assembly associated with protrusion and adhesion in cell migration.Physiol Rev 2008;88:489-513.
 Chinese Medical Sciences Journal2010年2期
Chinese Medical Sciences Journal2010年2期
- Chinese Medical Sciences Journal的其它文章
- Liquid Chromatography-tandem Mass Spectrometry for Analysis of Acylcarnitines in Dried Blood Specimens Collected at Autopsy from Neonatal Intensive Care Unit
- Pure Mucinous Carcinoma of the Breast:a Clinicopathologic Analysis with 56 Patients
- D-Tyr-tRNATyr Deacylase,a New Role in Alzheimer’sassociated Disease in SAMP8 Mice△
- Nectin-like Molecule 1 Inhibits the Migration and Invasion of U251 Glioma Cells by Regulating the Expression of An Extracellular Matrix Protein Osteopontin△
- A Second Protein Marker of Caveolae:Caveolin-2△
- Gaussia Luciferase Reporter Assay for Assessment of Gene Delivery Systems in Vivo△
