一维链状化合物[Ag(L)]·H2O的合成、晶体结构和荧光性质
邓奕芳 谭雄文 张春华 邝代治 陈满生
(功能金属有机材料湖南省普通高等学校重点实验室,衡阳师范学院化学与材料科学系,衡阳 421008)
研究简报
一维链状化合物[Ag(L)]·H2O的合成、晶体结构和荧光性质
邓奕芳 谭雄文 张春华 邝代治*陈满生*
(功能金属有机材料湖南省普通高等学校重点实验室,衡阳师范学院化学与材料科学系,衡阳 421008)
银配合物;晶体结构;氢键;荧光性质
In recent years,the rational design and synthesis of new extended supramolecular frameworks by covalent and weak intra/intermolecular interactions have brought forth architectures with intriguing structure motifs[1-5].During the past few years,many one-,two-,and threedimensional coordination polymers have been generated from transition metal templates with rigid and flexible pyridyl-containing bidentate or multidentate organic spacers[6-9].However,the control of formation of supramolecular complexes is a fascinating challenge for chemists.Our strategy in this approach is using a new multifunctional organic ligand 4-(isonicotinamido)benzoic acid(HL),which was prepared according to the procedure reported by Puddephatt[10],containing different types of binding sites arranged in an unsymmetrical fashion.In this paper,we report herein the synthesisand X-ray crystal structure of the novel silver complex,[Ag(L)]·H2O 1,which is an infinite 1D chains extended to a 3D supramolecular structures by hydrogen bonds and Ag-O weak interactions.
1 Experimental
1.1 Materials and instruments
All the regents and solvents were used as commercial sources without further purification.Elemental analyses were performed on a Perkin-Elmer 240C analyzer.The IR spectra were recorded on FTIR-8700 spectrophotometer using KBr discs.The luminescent spectra for the solid samples were recorded at room temperature on an Aminco Bowman Series 2 spectrophotometer with xenon arc lamp as the light source.
1.2 Synthesis of the title compound
A methanol solution(5 mL)of AgNO3(0.20 mmol)was mixed under stirring with the solution(5 mL)of HL ligand(0.20 mmol)in the same solvent.Then ammonia was added,the precipitate were dissolved and stirred for another 4 hours.The resulting clear solution was allowed to evaporate slowly at room temperature for three weeks,affording block colorless crystals.The product was collected by filtration,washed with methanol and ether successively,and then dried in air,Yields based on Ag:38%.Molecular formula is C13H11AgN2O4.Elemental analysis(%):C,42.53;H,3.02;N,7.63.Found(%):C,42.57;H,3.10;N,7.58.Main IR bands(cm-1):3423s,1638s,1604s,1413ms,1306ms,1244s,1087s,846ms,725m,624w.
1.3 X-ray crystallography
A block crystal with dimensions of 0.20 mm ×0.18 mm×0.12 mm was selected for the measurement.The diffraction data were collected at 293(2)K on a Bruker Smart ApexⅡCCD diffractometer equipped with a graphite-monochromatized Mo Kα radiation(λ=0.071073 nm).A total of 6120 reflections were collected in the range of 1.99°≤θ≤25.49°by using an ω-scan mode,of which 2 227 were unique with Rint=0.042 9,1 831 were consi-dered to be observed(I>2σ(I))and used in the structu-ralanalysisand refinement.The structure was solved by direct methods and refined on F2by full-matrix least-squares methods with SHELXTL[11].The non-hydrogen atoms were refined anisotropically and hydrogen atoms were localized in their calculation positions and refined by using the riding model.Crystal data and structure refinement parameters are listed in Table 1.
CCDC:762259.
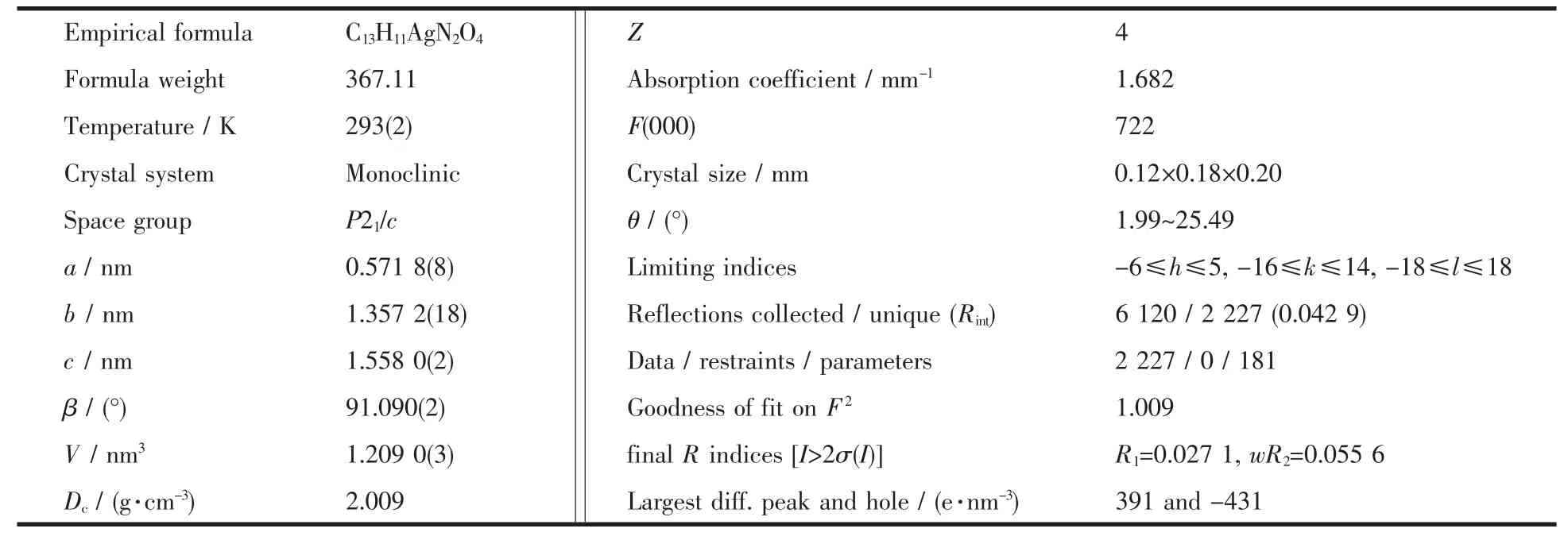
Table 1 Crystal data and structure parameters for the title complex
2 Results and discussion
2.1 Crystal structure of the title complex
The single-crystalX-ray diffraction analysis reveals that complex 1 consists of an infinite 1D Ag-L chains.A perspective view of the coordination unit with the atom-labeling scheme is given in Fig.1,the asymmetric unit of 1 contains one Ag,one L-ligand and one free water molecule.As shown in Fig.1,the Ag1 with linear coordination geometry is twocoordinated by one carboxylate oxygen(O1A)atom and one N(N1)atom from two different L-ligands with Ag1-O1A=0.212 7(18)nm,Ag1-N1=0.214 3(2)nm and the bond angles O1A-Ag1-N1=173.68(8)°,respectively.Then the silver ions are bridged by L-ligands to form a 1D infinite polymeric chain structure,alternately.

Fig.1 Ortep view of the complex with displacement ellipsoids(30%probability)
An interesting feature of this structure is that the 1D chain extended by the Ag1-O1W weak interaction(Ag1-O1W=0.277 8(3)nm)and O1W-H1WB…O2 hydrogen bond interaction to form a 2D layer structure as illustrated in Fig.2,which is different from the alternate Ag chains,2D layer and 3D Ag complexes in the previous reports[12-16].Furthermore,the 2D layers of 1 pack together through O1W-H1WB…O1 as well as N2-H2…O2 hydrogen bonding interactions(Table 2)to generate 3D frameworks.These hydrogen bonds interactions thus appear to stabilize the asymmetry molecular disposition around the Ag centers.

Fig.2 Two-dimensional layer structure of the title complex by Ag1-O1W weak interaction and O(1W)-H(1WB)…O(2)hydrogen bond
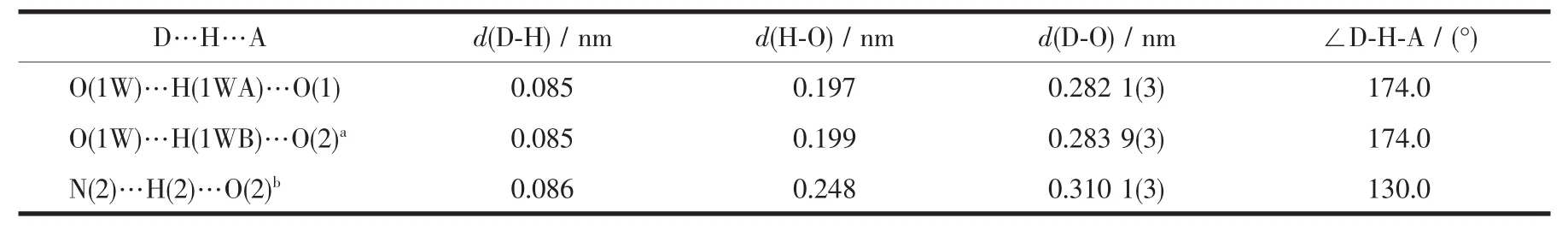
Table 2 Parameters of hydrogen bonds for the complex
2.2 Spectra characteristics
The IR spectrum of the complex exhibits a medium broad band centered at ca.3 423 cm-1,due to the ν(O-H)absorptions of water molecules.One feature of the IR data is the separation between νas(COO-)and νs(COO-),which have often been used to diagnose the coordination modes in the carboxylate ligands.The separation for monodentate carboxylate groups is>200 cm-1,whereas it is <200 cm-1in bidentate groups[17].The separation(Δ)between νas(COO-)and νs(COO-)is 225 cm-1for 1,indicating monodentate coordinating modes for the coordinated carboxylate groups,these IR results are coincident with the crystallographic structural analyses.
In general,inorganic-organic hybrid coordination compounds,especially with d10metal centers,have been investigated for fluorescence properties owing to their potential applications as luminescent materials,such as light-emitting diodes(LEDs)[18-19].Therefore,the photoluminescence properties of 1 and NaL were investigated in the solid state at room temperature.As shown in Fig.3,intense emission bands were observed at 409 nm(λex=380 nm)for NaL ligand,411 nm(λex=380 nm)for 1,respectively.These emissions can be attributed to neither metal-to-ligand charge transfer(MLCT)nor ligand-to-metal charge transfer(LMCT)because the Agions are in d10configuration and difficult to oxidize or to reduce.Therefore the emission observed in 1 is attributed to the π-π*intraligand photoluminescence due to its resemblance to that of NaL ligand,and the enhancement and slight red-shift of 1 compared to that of the NaL ligand probably result from the fact that the coordination of Agions increases the ligand conformational rigidity and thus reduces the loss of energy by thermal vibrational decay[20-21].

Fig.3 Flourscence spectra of the NaL and the title complex
[1]Cheng D P,Khan M A,Houser R P.J.Chem.Soc.Dalton Trans.,2002:4555-4560
[2]Zhang L Y,Liu G F,Zheng S L,et al.Eur.J.Inorg.Chem.,2003:2965-2971
[3]Go Y B,Wang X Q,Anokhina E V,et al.Inorg.Chem.,2004,43:5360-5367
[4]Awaleh M O,Badia A,Brisse F.Cryst.Growth Des.,2005,5:1897-1906
[5]Hong C S,Yoon J H,Lim J H,et al.Eur.J.Inorg.Chem.,2005:4818-4821
[6]Dong Y B,Smith M D,Layland R C,et al.Chem.Mater.,2000,12:1156-1161
[7]Dong Y B,Smith M D,zur Loye H C.Inorg.Chem.,2000,39:4927-4935
[8]Dong Y B,Zhao X,Huang R Q.Inorg.Chem.,2004,43:5603-5612
[9]Ciurtin D M,Dong Y B,Smith M D,et al.Inorg.Chem.,2001,40:2825-2834
[10]Burchell T J,Eisler D J,Puddephatt R J.Inorg.Chem.,2004,43:5550-5557
[11]Sheldrick G M.SHELXTL,Program for the Solution and the Refinement of Crystal Structures,University of Göttingen,Göttingen,Germany,1997.
[12]CHEN Man-Sheng(陈满生),HE Chun-Mei(何春梅),DENG Yi-Fang(邓奕芳),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25:1312-1315
[13]YAN Ze-Qun(严泽群).Chinese J.Struct.Chem.(Jiegou Xuebao),2005,24:315-318
[14]TIAN Ge(田 戈),YUAN Hong-Ming(袁宏明),CHEN Yan(陈 岩),et al.Chem.J.Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2006,27:2405-2407
[15]Liu B,Yuan Q.Inorg.Chem.Commun.,2005,8:1022-1024
[16]Liu Z,Liu P,Chen Y,et al.New J.Chem.,2005,29:474-478
[17]Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds.New York:John Wiley&Sons,1986.
[18]Vogler A,Kunkely H.Coord.Chem.Rev.,2006,250:1622-1626
[19]Wen L L,Li Y Z,Lu Z D,et al.Cryst.Growth Des.,2006,6:530-537
[20]Bunz U H F.Chem.Rev.,2000,100:1605-1644
[21]Yang W Y,Schmider H,Wu Q G,et al.Inorg.Chem.,2000,39:2397-2404
Synthesis,Crystal Structure and Luminescence of a One-Dimensional Chain-Like Complex[Ag(L)]·H2O
DENG Yi-Fang TAN Xiong-Wen ZHANG Chun-Hua KUANG Dai-Zhi*CHEN Man-Sheng*
(Key Laboratory of Functional Organometallic Materials of Hengyang Normal University,College of Hunan Province,Department of chemistry and Materials Science,Hengyang,Hunan 421008)
The title compound,[Ag(L)]·H2O,1,where HL=4-(isonicotinamido)benzoic acid,was synthesized in methanol solution and its crystal structure was determined by X-ray diffraction analysis.The crystal is of monoclinic,space group P21/c with a=0.5718(8)nm,b=1.3572(18)nm,c=1.5580(2)nm,β=91.090(2)°,V=1.2090(3)nm3,Z=4,Dc=2.009 g·cm-3,F(000)=722,Rint=0.0429,R=0.0271,wR=0.0556.In complex 1,the Ag atoms are linearly coordinated by one O atom and one N atom of two ligand molecules.Each L-ligand in turn uses its one carboxylate group and one pyridinyl groups to connect two metal centers,then the one-dimensional(1D)chains is formed.On the other hand,the 1D chains are further connected by O1W-H1WB…O2 hydrogen bonds and Ag-O weak interactions to give a two-dimensional(2D)layer,finally,the 2D net extents to three-dimensional(3D)supramolecular framework by O1W-H1WB…O1 as well as N2-H2…O2 interactions.CCDC:762259.
Agcomplex;crystal structure;hydrogen bond;luminescent property
O614.122
A
1001-4861(2010)05-0909-04
2009-12-14。收修改稿日期:2010-01-12。
湖南省重点学科建设项目,湖南省青年骨干教师基金(2008),衡阳师范学院青年骨干教师基金(2007)和衡阳市科技局资助项目(No.2009KJ29)资助。
*通讯联系人。 E-mail:cmsniu@163.com,hnkdz@yahoo.com.cn
邓奕芳,女,38岁,实验师;研究方向:配位化学。

