Sn-XAg-0.5Cu无铅钎料熔化特性、润湿性及力学性能研究
卫国强,万忠华,赵四勇,张宇鹏,刘凤美
(1华南理工大学机械与汽车工程学院,广州510640;2广州有色金属研究院焊接材料研究所,广州510651)
Sn-XAg-0.5Cu无铅钎料熔化特性、润湿性及力学性能研究
卫国强1,万忠华1,赵四勇2,张宇鹏2,刘凤美2
(1华南理工大学机械与汽车工程学院,广州510640;2广州有色金属研究院焊接材料研究所,广州510651)
应用差示扫描量热(DSC)法、润湿平衡分析技术和拉伸试验,研究了Sn-XAg-0.5Cu(X=0.1~3.0)无铅钎料合金的熔化特性、润湿性能及力学性能。实验结果表明:所有钎料合金的开始熔化温度均为217.3℃;随合金中Ag含量增加,熔化温度区间变窄。钎料的润湿铺展面积和润湿力随Ag含量的增加而先显著增加,而后趋于稳定;对润湿铺展面积和润湿力(或润湿时间)产生显著影响的阈值Ag含量为1.0%(质量分数,下同)。Ag含量在0.5%~3.0%时,随着Ag含量的增加,钎料的断后伸长率逐渐下降,而抗拉强度和0.2%屈服强度均逐渐增加。
Sn-XAg-Cu无铅钎料;熔化特性;润湿性;力学性能
Sn-(3.0~3.9)Ag-(0.5~0.7)Cu(质量分数/%,下同)无铅钎料由于具有较低的共晶温度(固相线温度:Ts=217℃)、较好的综合力学性能和良好的润湿性现已在微电子工业中得到广泛应用[1-3]。但是,该Sn-Ag-Cu系钎料合金在凝固过程中β-Sn存在较大的过冷度,会形成粗大的板条状Ag3Sn初晶(冷却速度越慢,Ag3Sn粗化越严重),同时在随后的服役过程中,板条状Ag3Sn会进一步粗化,导致钎料合金抗冲击及抗跌落(高应变速率条件)性能明显降低;另一方面,高的Ag含量使初生β-Sn含量降低,共晶相含量增加,同样导致更多的粗大的板条状Ag3Sn存在,致使钎料合金强度指标增加,塑性指标降低,从而降低焊点可靠性[4-6]。此外,由于Ag含量高,明显增加了钎料的材料成本。
为提高焊点抗冲击能力及综合力学性能,降低钎焊材料成本,众多学者对降低Sn-Ag-Cu共晶无铅钎料合金Ag含量对熔化温度、力学性能及界面和焊点内的组织演变进行了研究[7-10],同时通过添加第四合金组元来进一步提高该系无铅钎料合金的性能[11-13]。D.W.Suh等人对低Ag的Sn-Ag-Cu钎料合金在高应变速率条件下的断裂性能进行了研究[14],K.S.Lin等人就Ag含量对Sn-Ag-Cu钎料合金焊后时效处理对焊点界面强度的影响进行了研究[16]。但至今为止,Ag含量对Sn-Ag-Cu无铅钎料合金性能影响的系统研究还不多。基于钎料熔化温度、力学性能和润湿性能是评价无铅钎焊材料性能的重要指标,同时为后期开发四元低Ag含量的Sn-Ag-Cu无铅钎料合金提供理论基础,本工作就Ag(0.1%~3.0%)含量对Sn-Ag-Cu无铅钎料合金熔化特性、润湿性能及力学性能的影响进行了系统的研究。
1 实验材料和方法
1.1 实验材料
所用的原材料为 Sn-10Cu中间合金、纯 Sn(99.95%)和纯Ag(99.95%),钎料合金配方为Sn-(X=0.1,0.3,0.5,0.8,1.0,1.5,2.0,2.5,3.0)Ag-0.5Cu,将称好的样品在坩埚中进行熔炼,熔化温度为400℃,熔炼过程中充分搅拌使成分均匀,然后保温30min,最后在钢模中进行浇注。
1.2 熔化特性测试
采用差示扫描量热分析仪(DSC型号:STA-409-PC)测试钎料合金的熔化特性,实验前采用标准纯 In对仪器进行温度校正,试样约为20mg,加热速率为5℃/min,温度范围为 35~300℃,实验采用 ADAMIS7软件来记录各钎料热流随温度的变化规律。
1.3 润湿平衡实验
采用可焊性测试仪(型号:SA T-5100)进行润湿平衡试验,标准试样为纯铜无镀层试片,规格为30mm×5mm×0.3mm,钎料浴温度为250℃(精度:±1℃),浸入深度为2mm,浸入速度为5mm/s,浸入时间为10s,助焊剂为 EF8000(Alpha Metal)。
1.4 润湿铺展实验
用分析天平(精度:±0.1mg)分别称取各种钎料200mg,然后把钎料放置在温度为250℃的油浴中,使其变成球状钎料,自然冷却后,用超声波对钎料球进行清洗待用。铺展实验的铜基板采用20mm×20mm×2mm的紫铜板(纯度为99.95%),实验前用400#砂纸打磨铜板,然后将打磨好的铜板放入稀HCl(5%)中清洗,再用去离子水冲洗,最后用无水乙醇清洗。润湿铺展实验热源为锡浴炉,实验温度为260℃,保温时间40s,助焊剂为 EF8000(Alpha Metal),采用数字式求积仪(QCJ-2A)测量钎料合金的铺展面积。
1.5 室温拉伸力学性能实验
将浇注好的棒料加工成标准拉伸试样,然后在100℃恒温炉中保温1h,以除去残余应力,随炉冷至室温。拉伸试验参照 IPC J STD-004标准,拉伸速度为20.00mm/min。
2 结果与讨论
2.1 Ag含量对熔化特性的影响
图1为Sn-XAg-0.5Cu钎料合金在加热阶段吸热峰处的DSC曲线,从图 1可以看出,当 Ag含量在0.1%~3.0%范围内时,所有钎料合金的起始熔化温度(Ton)均约为217℃(平均:217.3℃),说明 Sn-0.5Cu加少量的Ag,就可使钎料合金的起始熔化温度降为三元共晶反应温度。图2为Ag含量对峰值熔化温度(Tp1,Tp2)和熔程(ΔT=Tp2-Ton)的影响,从图2可以看出,随Ag含量的增加,Tp1逐渐向高温方向漂移,说明参与三元共晶反应的相分数逐渐增加,而Tp2随Ag含量的增加,逐渐移向低温端,从而使Sn-XAg-0.5Cu钎料合金熔程减小。

图1 Sn-XAg-0.5Cu的吸热DSC曲线 (a)X=0.1,0.3,0.5,0.8;(b)X=1.0,1.5,2.0,3.0Fig.1 Endothermic DSC profiles from Sn-XAg-0.5Cu solder alloys(a)X=0.1,0.3,0.5,0.8;(b)X=1.0,1.5,2.0,3.0
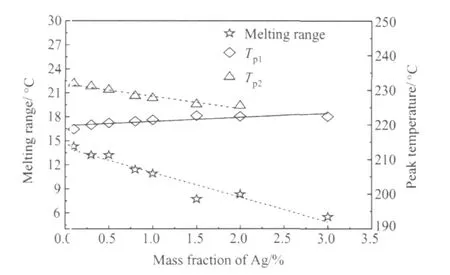
图2 Ag含量对峰值温度和熔程的影响Fig.2 Effect of Ag content on peak temperatures and melting ranges
从以上分析可知,在Sn-0.5Cu合金中加少量的Ag就可降低钎料合金的固相线温度,但Ag含量较少时,参与三元共晶反应的相分数较少,同时钎料合金的熔程相对较大。
2.2 Ag含量对润湿性能的影响
图3为Sn-XAg-0.5Cu钎料合金铺展面积随Ag含量变化曲线,当 Ag含量在0.1%~1.0%时,随着Ag含量的增加,钎料的铺展面积迅速增加,当Ag含量大于1.0%时,随Ag含量的增加,钎料的铺展面积变化不大。图4为Sn-XAg-0.5Cu钎料合金润湿力(包括最大润湿力Fmax和最终力Fend)随Ag含量变化曲线,图5为Sn-XAg-0.5Cu钎料合金润湿时间(包括交零时间T0和最大润湿时间T)随Ag含量变化曲线,当Ag含量大约在0.1%~1.0%时,随Ag含量的增加,钎料合金的润湿力显著增加,润湿时间显著减少,随着Ag含量的进一步增加(从1.0%~3.0%),润湿力和润湿时间几乎不变,说明Ag含量在0.1%~1.0%范围内增加时,Sn-XAg-0.5Cu钎料合金的润湿性能得到显著改善。
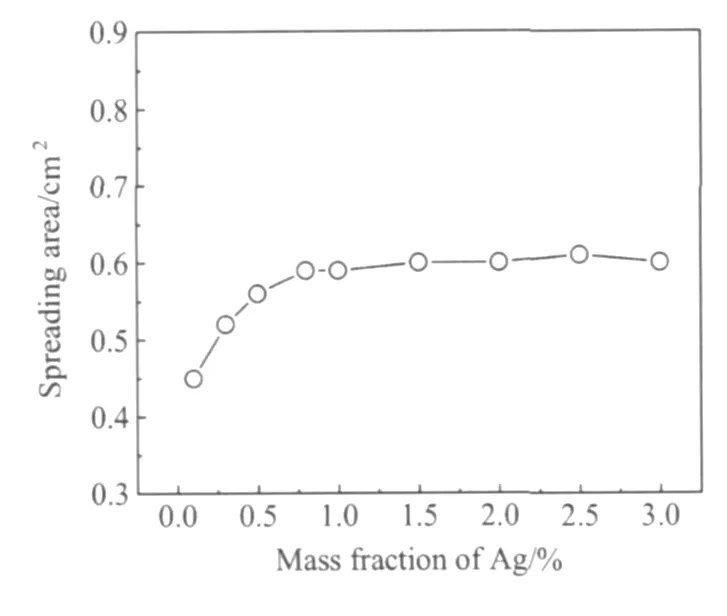
图3 Ag含量对Sn-XAg-0.5Cu钎料铺展面积的影响Fig.3 Effect of Ag content on spreading area of Sn-XAg-0.5Cu solder alloys
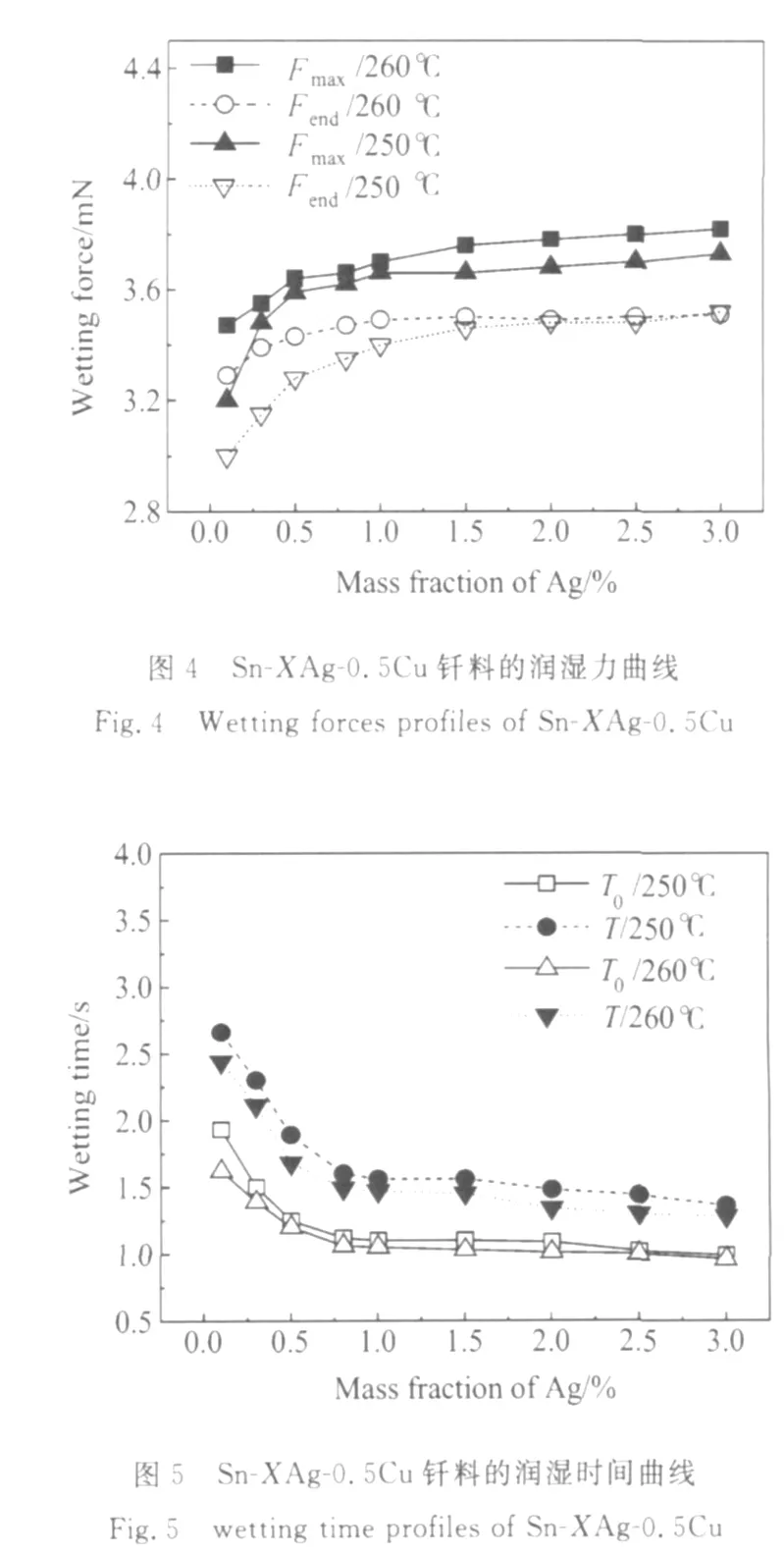
从以上实验数据分析可以看出,在低Ag范围(0.1%~1.0%),随Ag含量的增加,润湿力、润湿时间和铺展面积都显著增加,润湿时间显著减少。如再继续增加Ag含量,则对钎料合金的润湿特性影响不大。
2.3 Ag含量对室温力学性能的影响
图6为钎料合金抗拉强度、0.2%屈服强度和断后伸长率随Ag含量的变化曲线。由图6可以看出,在低Ag范围(0.1%~0.5%),断后伸长率随着Ag含量的增加先下降后上升,而抗拉强度和0.2%屈服强度正好与伸长率呈相反的趋势,这种变化趋势可能和显微组织的变化有关,有待进一步研究。当Ag含量在0.5%~3.0%范围内时,各力学性能呈稳定的变化趋势,即断后伸长率随着Ag含量的增加而下降,抗拉强度和0.2%屈服强度随着Ag含量的增加而逐渐增加。
3 结论
(1)所有钎料合金的固相线温度(Ton)均约217.3℃。随Ag含量的增加,三元共晶反应相的数量增加,钎料合金的熔程(ΔT)减少。
(2)当Ag含量在0.1%~1.0%时,随Ag含量的增加,润湿铺展面积、润湿力显著增加,润湿时间显著减少,随后再增加Ag含量,对润湿性没有显著影响。
(3)当Ag含量在0.1%~0.5%时,力学性能呈先下降后增加趋势;当Ag含量在0.5%~3.0%时,随着Ag含量的增加,钎料的断后伸长率逐渐下降,而抗拉强度和0.2%屈服强度均逐渐增加。
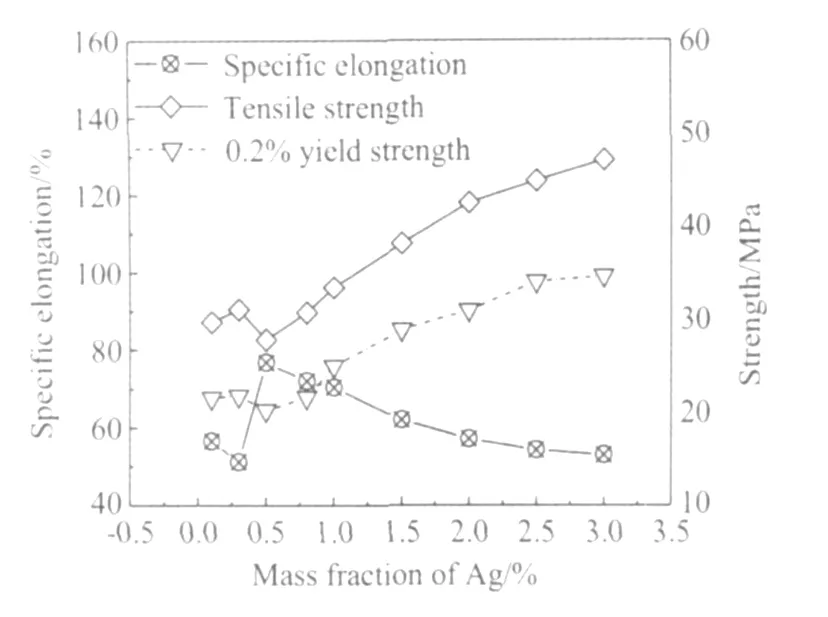
图6 Ag含量对Sn-XAg-0.5Cu钎料拉伸性能的影响Fig.6 Effect of Ag content on tensile properties for Sn-XAg-0.5Cu solders
[1] MOON K W,BOETTINGER W J.Accurately determining eutectic compositions:The Sn-Ag-Cu ternary eutectic[J].JOM,2004,56(4):22-27.
[2] KIM K S,HUH S H,SUGANUMA K.Effects of cooling speed on microstructure and tensile properties of Sn-Ag-Cu alloys[J].Materials Science and Engineering A,2002,333(1-2):106-114.
[3] KIM K S,HUH S H,SUGANUMA K.Effects of intermetallic compounds on properties of Sn-Ag-Cu lead-free soldered joints[J].Journal of Alloys and Compounds,2003,352(1-2):226-236.
[4] SEO S K,KANG S K,AHIH D Y,et al.An investigation of microstructure and microhardness of Sn-Cu and Sn-Ag solders as functions of alloy composition and cooling rate[J].Journal of Electronic Materials,2009,38(2):257-265.
[5] CHANG H W,CHANG K,CHEN J Y.The effect of Ag content on the formation of Ag3Sn plates in Sn-Ag-Cu lead-free solder[J].Journal of Electronic Materials,2006,35(12):2074-2080.
[6] LU H Y,BAL KAN H,NG K Y S.Microstructure evolution of the Sn-Ag-y%Cu interconnect[J].Microelectronics Reliability,2006,46(7):1058-1070.
[7] HUH S H,KIM K S,SUGANUMA K.Effect of Ag addition on the microstructural and mechanical properties of Sn-Cu eutectic solder[J].Materials Transactions-JIM,2001,42(5):739-744.
[8] REID M,PUNCH J,COLLINS M,et al.Effect of Ag content on the microstructure of Sn-Ag-Cu based solder alloys[J].Soldering and Surface Mount Technology,2008,20(4):3-8.
[9] LEE J H,YU A M,KIM J H,et al.Reaction properties and interfacial intermetallics for Sn-xAg-0.5Cu Solders as a function of Ag content[J].Metals and Materials International,2008,14(5):649-654.
[10] LU H Y,BAL KAN H,NG K Y S.Effect of Ag content on the microstructure development ofSn-Ag-Cu interconnects[J].Journal of Materials Science:Materials in Electronics,2006,17(1-2):171-188.
[11] YU A M,LEE C W,KIM M S,et al.The effect of the addition of In on the reaction and mechanical properties of Sn-1.0Ag-0.5Cu solder alloy[J].Metals and Materials International,2007,13(6):517-520.
[12] LIN K S,HUANG H Y,CHOU C P.Interfacial reaction between Sn-1Ag-0.5Cu(Co)solder and Cu substrate with Au/Ni surface finish during reflow reaction[J].Journal of Alloys and Compounds,2009,471(1-2):291-295.
[13] LI G Y,SHI X Q.Effects of bismuth on growth of intermetallic compounds in Sn-Ag-Cu Pb-free solder joints[J].Transactions of Nonferrous Metals Society of China(English Edition),2006,16(s1):739-743.
[14] SUH D W,KIM D W,LIU P L,et al.Effects of Ag content on fracture resistance of Sn-Ag-Cu lead-free solders under highstrain rate conditions[J].Materials Science and Engineering A,2007,460-461(1-2):596-603.
[15] LIN K S,HUANG H Y,CHOU C P.Interfacial reactions and bonding strength of Sn-xAg-0.5Cu/Ni BGA solder joints[J].Journal of Materials Engineering and Performance,2009,18(2):182-189.
Investigations on Melting Property,Wettability and Mechanical Property of Sn-XAg-0.5Cu Lead-free Solder Alloys
WEI Guo-qiang1,WAN Zhong-hua1,ZHAO Si-yong2,ZHANG Yu-peng2,LIU Feng-mei2
(1 School of Mechanical and Automotive Engineering,South China University of Technology,Guangzhou 510640,China;2 Joining Materials Institute,Guangzhou Research Institute of Nonferrous Metals,Guangzhou 510651,China)
The melting property,wettability and mechanical property of Sn-XAg-0.5Cu(X=0.1-3.0)lead-free solder alloys were investigated by differential scanning calorimetry(DSC),wetting balance technique,sessile drop method and tensile test.The DSC revealed that the endothermic onset temperature on heating appeared at about 217.3℃for the all solder alloys.With increasing the Ag content of the solder alloys,the melting temperature ranges of the solders became narrow.It was also shown by the wetting balance and sessile drop experiments that the wettability remarkably increased with the Ag content increased,and then with Ag content farther increased,the wettability were kept constant approximately.The threshold value of the Ag content was 1.0%(mass fraction/%,the same below),which considerably effected on wetting-spreading areas and wetting forces(or wetting time).Meanwhile,when Ag content is between 0.5%and 3.0%,the specific elongation of solders decreased with the Ag content increased,while the tensile strength and 0.2%yield stresses increased,too.
Sn-XAg-Cu lead-free solder;melting property;wettability;mechanical property
TG454
A
1001-4381(2010)10-0053-04
国家自然科学基金(NSFC-广东联合)重点项目(U0734006);广东省科技计划项目(2008A080403008-06);广州市天河区科技计划项目(095G108)
2010-06-20;
2010-07-19
卫国强(1960—),男,在职博士,副教授/高级工程师,主要从事钎焊、电子封装及激光材料加工的研究工作,联系地址:华南理工大学机械与汽车工程学院(510640),E-mail:gqwei@scut.edu.cn

