Hypertensive Nephropathy Treatment by Atorvastatin
——A Study of Anti-Inflammation Therapy for Target Organ Damage of Hypertension
TIAN Dengke,HU Songyou,LING Shuang,CHEN Gangling,LI Yajuan,TANG Ning,LIU Jun,BIAN Ka,2
1.Murad Research Institute for Modernized Chinese Medicine,Shanghai University of Traditional Chinese Medicine;Shanghai 201203,China;
2.Department of Integrative Biology and Pharmacology,Institute of Molecular Medicine,University of Texas Medical School,6431 Fannin,Houston,TX 77030,USA
Hypertensive Nephropathy Treatment by Atorvastatin
——A Study of Anti-Inflammation Therapy for Target Organ Damage of Hypertension
TIAN Dengke1,HU Songyou1,LING Shuang1,CHEN Gangling1,LI Yajuan1,TANG Ning1,LIU Jun1,BIAN Ka1,2
1.Murad Research Institute for Modernized Chinese Medicine,Shanghai University of Traditional Chinese Medicine;Shanghai 201203,China;
2.Department of Integrative Biology and Pharmacology,Institute of Molecular Medicine,University of Texas Medical School,6431 Fannin,Houston,TX 77030,USA
This work was supported by grants from Key Projectof Ministry of Science and Technology(2006BAI11B08-03),E-Institutes of NitricOxide and Inflammatory Medicine of Shanghai Municipal Education Commission (E-04010),Key Project of Shanghai Committee of Science&Technology(08430711300,08DZ1972104)
This study was designed to investigate the effect of atorvastatin(ATO)on inflammatory damage of kidney from spontaneously hypertensive rats (SHR),and to explore preventive and therapeutic usage of ATO on hypertensive nephropathy. Male SHR of 4weeksold were divided into SHR model groupandSHR+ATO group(8 mg/kg).Age-matched Wistar-Kyoto ratswere used asnormal control.All ratswere killed at 12 wk of age.Enzyme linked immunosorbent assay(ELISA)was used to determine plasma and renal angiotensin II(AngII)contents.Renal inflammatory statuswasevaluated by the following parameters:inducible nitric oxide synthase (iNOS); intercellular adhesion molecule-1(ICAM-1);plasma and renal tissue nitrite(NO2-)concentration;histological changes of glomeruli and tubuleinterstitum of the kidney.Renal function was evaluated by urinary excretion of total protein.The results showed that ATO treatment decreasedrenal AngⅡconcentrationsignificantly.ATOinhibitedrenal inflammationasreflectedthrough reduced protein expression of iNOS and ICAM-1,decreased NO2-concentration in plasma and renal tissue.The ATO significantly improved renal function of hypertensive rats.This study indicates that ATO is a potent suppressor of renal inflammatory damage in SHR,which may serve the base for therapeutic utility of ATO in hypertensive nephropathy.
Atorvastatin;Spontaneously hypertensive rats;Inflammation;Hypertensive nephropathy
0 Introduction
Spontaneously hypertensiverats(SHR) have been widely used asa primary hypertension animal model,and multiple renal structural and functionalalterations mark the feature of hypertensive nephropathy[1~17]. Previous studies of our research center and other labs indicated that inflammatory related pathological changes have been involved in hypertensive nephropathy[2,7,11,17~19].Thus,we hypothesize that anti-inflammatory therapeutic strategy may manage hypertensive renal damage.
Atorvastatin(ATO,lipitor)is a member of the drug class known as statins,which has been effectively used for lowering blood cholesterol. Recently,ithas been reported that ATO hasprotective effect on renal injury related to hypertension.Cheng[8]indicated that without interfering with blood pressure,ATO reduced Th1 cytokine production.Little is known,however,about the mechanisms underlying the renal protective effects of ATO.
The presentstudy was designed to explore the effect of ATO on renal inflammatory damage and functional change in SHR.With thisstudy,we expect to clarify the protective mechanisms and potential clinical benefits of ATO on hypertensive renal disease.
1 Materials and Methods
1.1 Animals
Theanimal experimental procedureswerein accor dance with the Animal Use and Care guidelines of th e Experimental Animal Centerof Shanghai University of Traditional Chinese Medicine.Male SHR (4 wk of age)and WKY rats(4 wk of age)were purchased from Shang hai Experimental Animal Center of Chinese Academy of Sciences.
1.2 Materials
ATO were purchased from PfizerPharmaceuticals Limited(USA).Instrument for noninvasive blood pressure determination was purchased from Shanghai Alcott Biotech (China). Commercially available angiotensin II(Ang II)enzymelinked immunosorbent assay(ELISA)kits were obtained from BioDev Tech(China).Antibodies for rat inducible nitric oxide synthase(iNOS)and intercellular adhesion molecule-1(ICAM-1) were obtained from Santa Cruz Biotechnology Inc(USA).Computerized gel imaging analysis system was obtained from Shanghai Tannon(China).Coomassie Brilliant Blue was purchased from Sigma-Aldrich(St.Lous,MO).Multifunctional microplate reader SpectraMax 190 wasobtainedfrom Molecular Devices(USA).All chemicals were of analytical grade.
1.3 Experimental Groups and Treatment
SHR were divided into two groups:SHR model group(n=9),and SHR+ATO(n=9).ATO powder was dissolved in purified water(0.8 mg/mL),and daily administrated through intragastric gavage(8 mg/kg).Drinking water(10 ml/kg)was used for SHR model group.The treatments were carried out through the week 4 to week 12 of the age of SHR.In the whole experimental process WKY rats(n=9)were used as the age-matched control of SHR and drinking water(10 ml/kg)was used for sham treatment.
1.4 Sample Preparation
24 h urine samples were collected on the day before ratswere killed by cervical dislocation. Urine samples were lowerspeed centrifuged and supernatants were separated and stored at-20℃ until assayed for urinary concentration of total protein.
Blood samples were obtained at12 wk ofage through cardiac puncture and collected in centrifuge tubes that contained 50 μl EDTA (0.30 mol/L),25 μl dimercaprol(0.32 mol/L),50 μl 8-hydroxyquinoline sulfate(0.34 mol/L).Plasma was separated and stored at-20℃for further assays.
Kidneys were immediately removed after scarification of the rats.One kidney was fixed with 4%paraformaldehyde for histological analysis,and another was frozen in liquid nitrogen for further protein assays.For total protein extraction,about 200 mg kidney tissueswerehomogenized in cell lysis buffer containing protein inhibitors cocktail(pH 7.4),then centrifuged for 15 min at 4℃.Concentration of total protein was determined by Lowry assay.
1.5 Plasma and Kidney AngII Determination
Ang II concentration was detected using ELISA kit according to the manufacturer's instructions.
1.6 Examination of Renal Inflammation
iNOS and ICAM-1 protein expression was analyzed by Western-blot.50 μg total protein wasseparated in sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE),protein was transferred to nitrocellulose membrane,and then incubated with primary antibodies of iNOS and ICAM-1(1∶200)overnight and second antibodiesconjugated with horseradish peroxidasefor 1 h.The determination was performed with enhanced chemiluminescene by exposure to Kodak X-Omat BT Films.
Griess reaction was used for the detection of renal tissue and serum NO2-concentration.100 μl of test solutions was added to 96-well flat-bottomed plates containing 100 μl/well of Griess reagent(1%sulfanilamide,0.1%naphtylethylenediamine dihydrochloride,and 5%phosphoric acid).After 10 min at room temperature the absorbance of each well was measured at 540 nm and the NO2-concentration was determined from a sodium nitrite standard curve.
1.7 Analysis of Renal Pathological Parameters
HE andPeriodicacidsilver metheramine(PASM)stained kidney sections were evaluated by using a semiquantitative histological score (score 0-3; normalto severe)for glomerulosclerosis,interstitial fibrosis,tubular atrophy,and arteriolar hyalinosis(maximal score=12)as previously described by Nakamura[13].
1.8 Evaluation of Renal Function
Urinary concentration of total protein was measured by Coomassie Brilliant Blue method.
1.9 Statistical Analysis
Data were expressed as mean±standard deviation(SD).Findings were analyzed by the SPSS11.0 software package for the one-way analysis of variance(ANOVA).The P value of less than 0.05 was considered statistically significant.
2 Results
2.1 Plasma and Kidney Ang II
In the whole experimental procedure ATO treatment failed to prevent the development of hypertension in SHR(data not shown). Plasma Ang II concentrationswere similar between WKY and SHR at 12 wk of age(P>0.05).ATO treatment showed a tendency to increase plasma Ang II levels in the SHR group.However,these changeswere not statistically significant(P>0.05). In contrast,kidney tissue levels of Ang II were significantly increased in SHR at12 wk ofage compared with age-matched WKY rats(P<0.05). After8 weeksof treatment,kidney Ang II levels were markedly decreased by ATO(P<0.05)(Table 1).
Table 1 Effect of ATO on plasma and kidney Ang II of SHR(±S)表1 ATO对SHR血浆和肾组织AngII含量的影响(±S)

Table 1 Effect of ATO on plasma and kidney Ang II of SHR(±S)表1 ATO对SHR血浆和肾组织AngII含量的影响(±S)
▲P<0.05 vs WKY control group(SHR model group only);●P<0.05 vs SHR model group;SHR of SHR+ATO group were daily administrated ATO (8 mg/kg) intragastrically during weeks4 through 12,SHR of SHR model group and WKY rats were daily administrated isovolumic drinking water.After plasma and renal tissue homogenateswere harvested,ELISA kit was used for the examination of AngII concentration;n=8与WKY正常对照组比较,▲P<0.05(仅高血压模型组);与高血压模型组比较,●P<0.05;ATO治疗组(8 mg/kg)每日灌胃给药ATO 1次,高血压模型组的SHR和WKY大鼠每日给予等量饮用水;给药8周后收集血浆样品和肾组织匀浆液,采用ELISA试剂盒测定AngII含量;n=8
Group Ang II Plasma(pg/mL) Kidey(pg/mg tissue)WKY control group 435.2±154.8 18.09±7.92 SHR model group 474.9±96.9 30.70±10.94▲SHR+ATO group 520.2±106.8 23.87±7.18●
2.2 Renal Protein Expression of iNOS and ICAM-1
Renal protein expression of iNOS and ICAM-1 were significantly increased in SHR at 12 wk of age compared with age-matched WKY rats(P<0.05). Treatmentof ATO inhibited the expression ofthese inflammatory related biomarkers(P<0.05)(Figure 1).
2.3 Plasma and Renal Tissue NO2-Concentration
Compared with WKY rats,plasma and renal tissue levels of NO2-in SHR changed in parallel with protein expression of iNOS.ATO decreased NO2-concentration in SHR after 8 weeks of treatment(P<0.05)(Table 2).
2.4 PathologicalScores of Glomeruli and Tubuleinterstitium
Pathological scoresof glomeruli and tubule-interstitum were significantly increased in SHR at 12 wk of age compared with age-matched WKY rats(P<0.05).After 8 weeks of treatment,ATO markedly decreased the pathological scores of glomeruli and tubule-interstitum in SHR(P<0.05)(Figure 2).
2.5 Urinary Excretion of Total Protein
Urinary excretion oftotal protein was significantly increased in SHR at12 wk ofage compared with age-matched WKY rats (P<0.05). ATO and HMP decreased urinary excretion of total protein after 8 weeks of treatment(P<0.05)(Table 2).
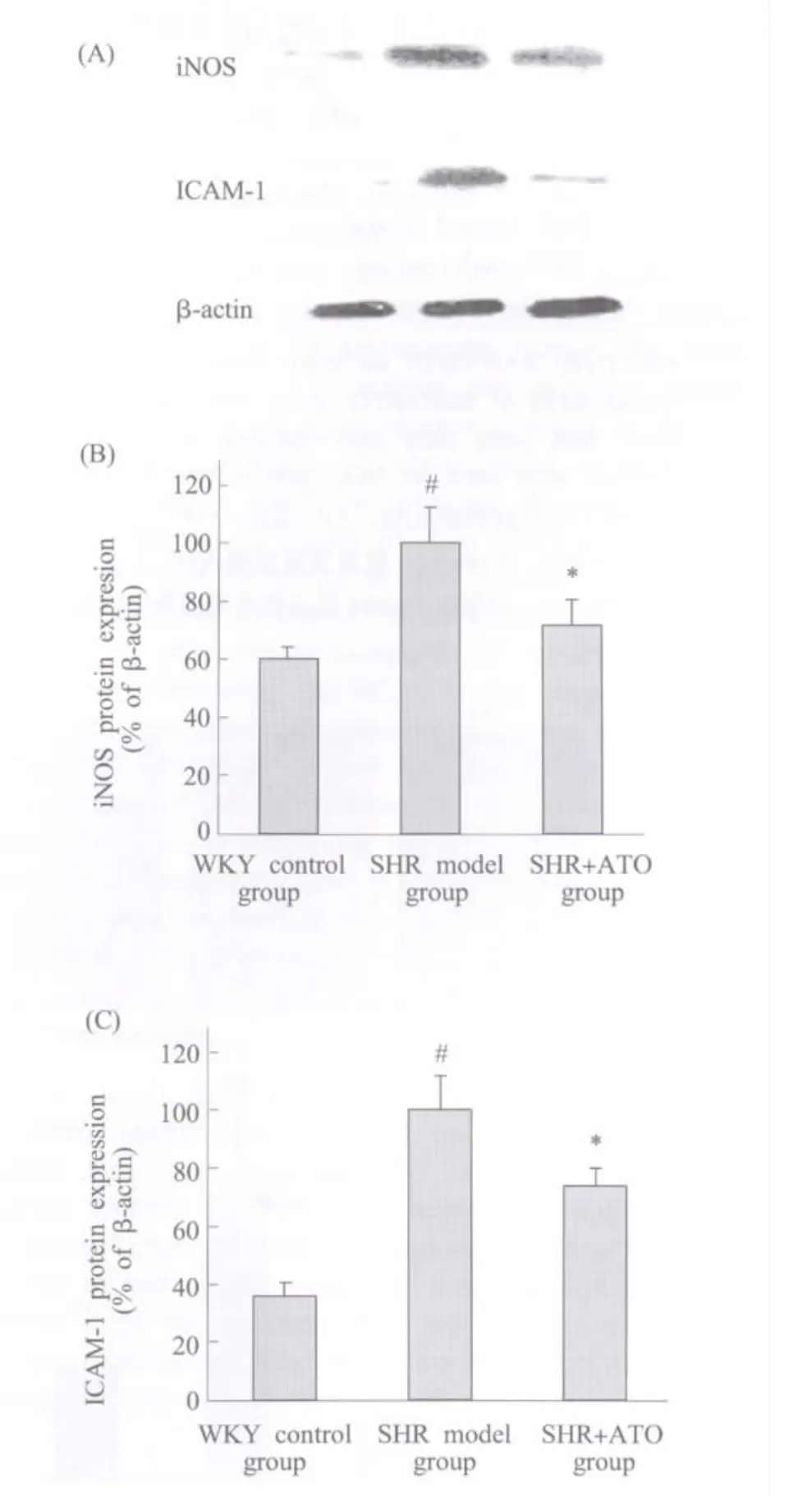
Fig.1 Effect of ATO on iNOS and ICAM-1 protein expression in SHR kidney Total protein was extracted from kidney forWestern-blotanalysis to determine iNOS and ICAM-1 protein expressions.(A)Bands from left side corresponding to the samples from WKY,SHR,and SHR+ATO respectively.The statistics analysis of the Western blot for iNOS(B)and ICAM-1(C)were presented in bar graph.#P<0.05 vs WKY control group(SHR model group only);*P<0.05 vs SHR model group;n≥3图1 ATO对SHR肾脏iNOS和ICAM-1蛋白表达的影响 提取肾脏组织总蛋白,以Western-blot法测定iNOS和ICAM-1的蛋白表达。(A)大鼠肾组织总蛋白样品的相应Westen-blot条带;(B)和(C)分别为iNOS和ICAM-1的统计分析图。与WKY正常对照组比较,#P<0.05(仅高血压模型组);与高血压模型组比较,*P<0.05;n≥3
Table 2 Effect of ATO on plasma and renal tissue NO2-and urinary total protein concentration of SHR(±S)表2 ATO对SHR血浆、肾组织NO2-和尿蛋白含量的影响(±S)
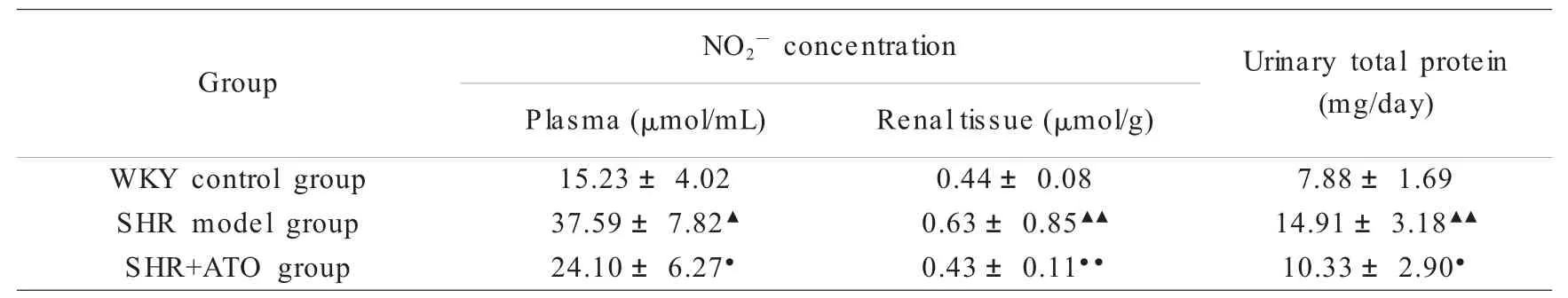
Table 2 Effect of ATO on plasma and renal tissue NO2-and urinary total protein concentration of SHR(±S)表2 ATO对SHR血浆、肾组织NO2-和尿蛋白含量的影响(±S)
▲P<0.05,▲▲P<0.01 vs WKY control group(SHR model group only);●P<0.05,●●P<0.01 vs SHR model group;SHR of SHR+ATO group was daily administrated ATO(8 mg/kg)intragastrically,SHR model group and WKY ratswere daily administrated isovolumic drinking water.Griessreaction and Coomassie Brilliant Blue method were used for NO2-and urinary total protein evaluation respectively;n=9与WKY正常对照组比较,▲P<0.05,▲▲P<0.01(仅高血压模型组);与高血压模型组比较,●P<0.05,●●P<0.01;ATO治疗组 (8 mg/kg)每日灌胃给药ATO 1次,高血压模型组的SHR和WKY大鼠每日给予等量饮用水;给药8周后处理动物,分别以Griess反应和考马斯亮蓝法测定NO2-和尿蛋白含量;n=9
Urinary total protein(mg/day)WKY control group 15.23±4.02 0.44±0.08 7.88±1.69 SHR model group 37.59±7.82▲0.63±0.85▲▲14.91±3.18▲▲SHR+ATO group 24.10±6.27●0.43±0.11●●10.33±2.90●Group NO2-concentration Plasma(μmol/mL) Renal tissue(μmol/g)
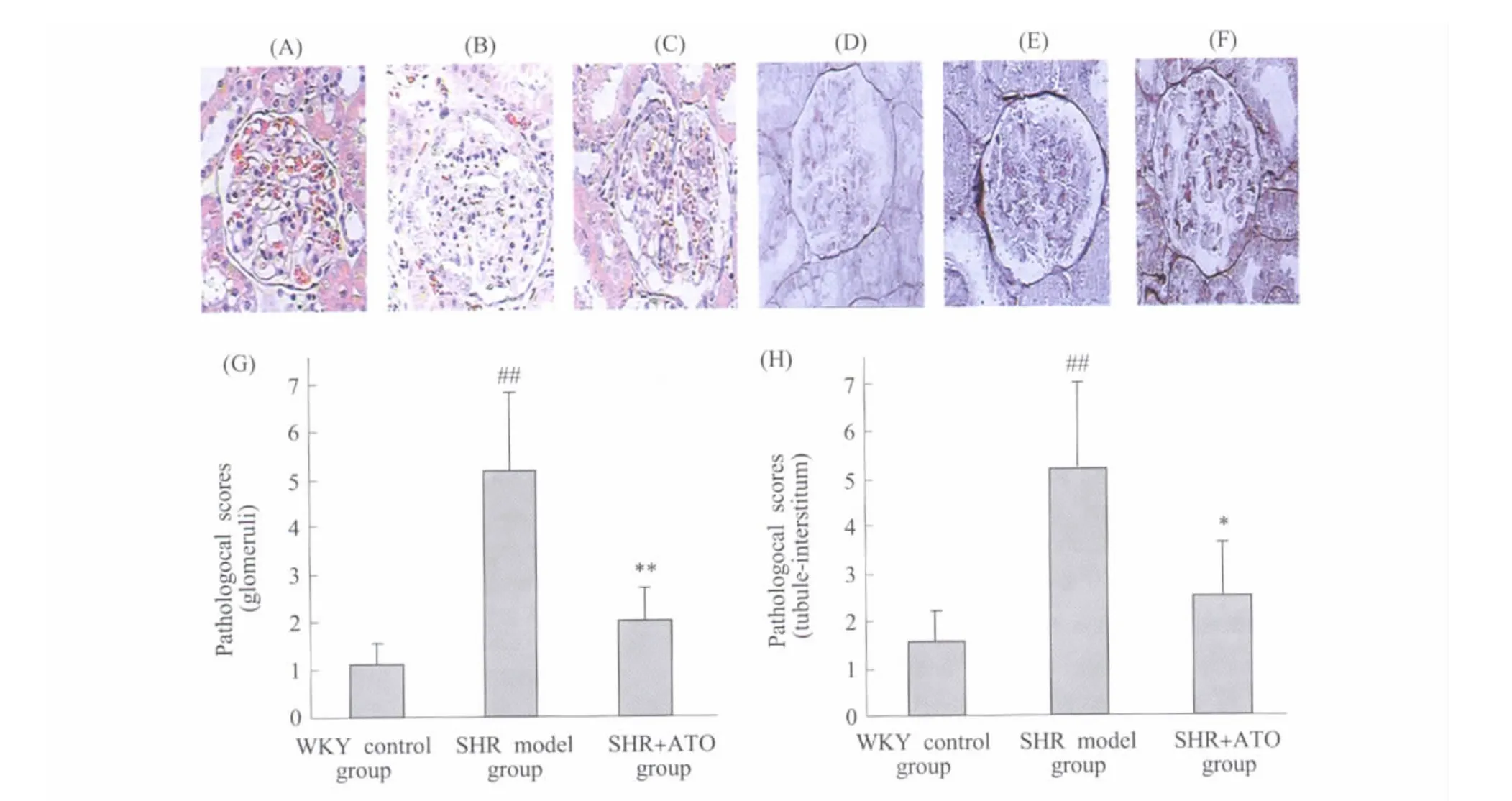
Fig.2 Effect of ATO on pathological scores of glomeruli and tubule-interstitum in SHR Kidney samples were harvested at 12 wk of age and fixed in 4% paraformaldehyde.The pathological scores were obtained by an pathological image analysis system with HE(A~C)and PASM(D~F)stained kidney tissue sections.(A)and(D):refer to WKY control group;(B)and(E):refer to SHR model group;C and F:refer to SHR+ATO group;The statistics analysis of pathological scores for glomerular(G)and tuble-interstitial(H)were presented respectively.#P<0.05,##P<0.01 vs WKY control group(SHR model group only);*P<0.05,**P<0.01 vs SHR model group;n=6图2 ATO对SHR肾小球和小管-间质病理积分的影响 ATO给药8周后收集肾脏组织以4%多聚甲醛固定,进行 HE(A-C)和PASM(D-F)染色后,以病理图像分析系统测定病变积分。A和D:WKY正常对照组;B和E:高血压模型组;C和F:ATO治疗组;G和H分别为肾小球和小管-间质病变积分的统计分析图。与WKY正常对照组比较,##P<0.01(仅高血压模型组);与高血压模型组比较,*P<0.05,**P<0.01;n=6
3 Discussion
The pathological changes due to chronic inflammation are evidential in hypertensive renal disease.Interstitial fibrosis,proteinuria and decreased creatinine clearance rate manifest severe renal disorders of SHR.Our previous study indicated an increased oxidative stress and up-regulated inflammatory cytokines are presented inSHR kidney[2,17].A role of inflammatory reactionin hypertensive nephropathy is further suggested by the study of nuclear transcription factor-κB (NF-κB) inhibitor which prevented the developmentofhypertension and renal injury[10].To add more evidence,chronic treatment with p38 MAPK inhibitor delayed the onset of renal dysfunction characterized by increased total protein and albumin excretion of SHR[14].In the present study,we have evaluated the effectofATO on inflammatory related kidney damage in SHR to furtherappeal our proposed conceptthatinflammation holds the key of target organ damage of hypertension which should serve as primary target for therapeutic strategy.Our choosing of atorvastatin is due to the following considerations:1)ATO is not an anti-hypertensive drug;2)ATO exerts cardiovasculareffectthrough anti-inflammatory mechanisms;3)ATO isthe top-selling prescription medicine in the world and available immediately for therapeutic use.Our data here defines the optimal treatment of hypertension through an anti-inflammation pathway of end-organ protection.
Previous studies indicated a complex causative network involving Ang II and inflammatory cytokines in triggering renal damage in hypertension such as increasing mesangial cell proliferation,contracting afferent and efferent glomerular arteriole,and deceasing glomerular filtration rate[16]. Also,ithas been reported thatAng II stimulated the synthesis of Type I,IV collagen and fibronectin[15],thus increasing the extracellular matrix(ECM)deposition.On theother side,olmesartan,an Ang II type-1(AT1)receptor blocker,decreased proteinuria,renal immune cells infiltration and morphology alterations in SHR by inhibition of kidney but not serum levels of Ang II[12].In thisstudy(Table 1) we observed similar result that ATO significantly inhibited kidney Ang II which imply a key role of inflammation in regulation of kidney level Ang II.
Inflammatory cytokineshavebeen crucially involved in the pathogenesis of hypertensive renal damage.Our current study(Figure 1)focused on iNOS and ICAM and showed that ATO decreased expression of the inflammatory molecules.The production of unphysiological higher concentration of nitricoxide (NO) by iNOS hasbeen considered as a pro-inflammatory mediator and implicated in several inflammatory diseases[3~6]. Inflammatory cytokines,including interleukin-1β (IL-1β),tumor necrosis factor-α (TNF-α),interferon-γ (IFN-γ) and bacterial lipopolysaccharide (LPS) can induce the expression of iNOS in monocyte/macrophages,neutrophil granulocytes and many other cell types.The over-produced NO will react with superoxide anion(O2·-)to form peroxynitrite(ONOO-),which can react with tyrosine residue to form nitrotyrosin and cause tissue injury including renal damage[3~6]. In current study plasma and renal tissue NO2-concentration was evaluated as the consequence of over expressed iNOS which could be inhibited by ATO treatment(Table 2).Up-regulation of ICAM-1 in tubular epithelial cells was observed in kidney diseases with inflammatory reaction[17,18]which actively participate progress of fibrosis in glomeruli and tubule-interstitum[1,9].Our study(Figure 1)indicated that the ICAM-1 expression was significantly inhibited by ATO treatmentand suggests an action mechanism of ATO in preserving renal function and reducing interstitial fibrosis.
Hypertensive nephropathy marks with increased urinary excretion of total protein,which ismostly attributed to increased permeability of glomerularfiltration membrane,damage ofelectrostatic barrierand renal tubular reabsorption. Our current and previous studies have detected that renal pathological changes in SHR at 12 wk of age including:1)accumulation of mesangial matrix,thickening and focal sclerosis of capillary basement membrane;2)cloudy swelling and irregular basement membrane thickening of renal tubules;3)interstitial inflammatory cell infiltration,accumulation of ECM and fibrosis;4)increased concentration of urinary β2-microglobulin concentration (β2-MG). In the presentstudy,ATO effectively reversed pathological scores of glomeruli and tubule-interstitum in SHR and strongly demonstrated the efficacy of anti-inflammation therapy on hypertensive renal injury(Figure 2).The restoration of normal renal function(measured by urinary protein excretion)by ATO further prove our concept of anti-inflammation therapy for hypertensive target organ damage.
4 Conclusions
In conclusion,this study demonstrates thatATO inhibited inflammatory statusof the hypertensive kidney which was associated with a marked restoration of kidney function in SHR.It isworth to noting that the protective effect of ATO on hypertensive renal diseases is not related to the change of plasma AngII but renal tissue AngII concentration.We propose that anti-inflammation mechanism may servethebasefor therapeutic usage of ATO in hypertensive nephropathy.
1. Arrizabalaga P,Sole M,Abellana R,de las Cuevas X,Soler J,Pascual J,Ascaso C. Tubular and interstitial expression of ICAM-1 as a marker of renal injury in IgA nephropathy.American journal of nephrology,2003,23:121~128
2. Bian K,Davis K,Kuret J,Binder L,Murad F.Nitrotyrosine formation with endotoxin-induced kidney injury detected by immunohistochemistry.Am J Physiol,1999,277:F33~40
3. Bian K,Doursout MF,Murad F.Vascular system:role of nitric oxide in cardiovascular diseases. J Clin Hypertens(Greenwich),2008,10:304~310
4. Bian K,Gao Z,Weisbrodt N,Murad F.The nature of heme/iron-induced protein tyrosine nitration.Proc Natl Acad Sci USA,2003,100:5712~5717
5.Bian K,Harari Y,Zhong M,Lai M,Castro G,Weisbrodt N,Murad F.Down-regulation of inducible nitric-oxide synthase(NOS-2)during parasite-induced gut inflammation:a path to identify a selective NOS-2 inhibitor. Mol Pharmacol,2001,59:939~947
6. Bian K,Murad F. Nitric oxide (NO)--biogeneration,regulation,and relevance to human diseases.Front Biosci,2003,8:d264~278
7.Cerasola G,Guarneri M,Cottone S.Inflammation,oxidative stress and kidney function in arterial hypertension.G Ital Nefrol,2009,26(Suppl 46):8~13
8. Cheng X,Ding Y,Xia C,Tang T,Yu X,Xie J,Liao M,Yao R,Chen Y,Wang M,Liao YH.Atorvastatin modulates Th1/Th2 response in patients with chronic heart failure.Journal of cardiac failure,2009,15:158~162
9.Chow FY,Nikolic-Paterson DJ,Ozols E,Atkins RC,Tesch GH.Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice.J Am Soc Nephrol,2005,16:1711~1722
10.Elks CM,Mariappan N,Haque M,Guggilam A,Majid DS,Francis J.Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. American journalof physiology,2009,296:F298~305
11.Johns EJ.Inflammation:the underlying foe in renovascular hypertension?Journal of hypertension,2009,27:1964~1965
12. Koike H,Sada T,Mizuno M. In vitro and in vivo pharmacology of olmesartan medoxomil,an angiotensin II type AT1 receptor antagonist.J Hypertens Suppl,2001,19:S3~14
13.NakamuraS,Ishibashi-UedaH,Suzuki C,NakataH,Yoshihara F,Nakahama H,Kawano Y. Renal artery stenosisand renal parenchymal damage in patientswith abdominal aortic aneurysm proven by autopsy.Kidney&blood pressure research,2009,32:11~16
14.Nerurkar SS,Olzinski AR,Frazier KS,Mirabile RC,O'Brien SP,Jing J,Rajagopalan D,Yue TL,Willette RN.P38 MAPK inhibitors suppress biomarkers of hypertension end-organ damage,osteopontin and plasminogen activator inhibitor-1.Biomarkers,2007,12:87~112
15.Sharma R,Sharma M,Reddy S,Savin VJ,Nagaria AM,Wiegmann TB.Chronically increased intrarenal angiotensin II causes nephropathy in an animal model oftype 2 diabetes.Front Biosci,2006,11:968~976
16.Skrbic R,Igic R.Seven decades of angiotensin(1939-2009).Peptides,2009,30:1945~1950
17.Sun L,Gao YH,Tian DK,Zheng JP,Zhu CY,Ke Y,Bian K. Inflammation of different tissues in spontaneously hypertensive rats.Sheng Li Xue Bao,2006,58:318~323
18.Sun L,Ke Y,Zhu CY,Tang N,Tian DK,Gao YH,Zheng JP,Bian K. Inflammatory reaction versus endogenous peroxisome proliferator-activated receptorsexpression,reexploring secondary organ complicationsof spontaneously hypertensive rats. Chinese medical journal,2008,121:2305~2311
19.Wang Y,Wang DH.Aggravated renal inflammatory responses in TRPV1 gene knockout mice subjected to DOCA-salt hypertension.American journal of physiology,2009,297:F1550~1559
阿托伐他汀对高血压肾脏并发症炎性损伤的治疗作用
田登科1,胡送友1,凌 霜1,陈刚领1,李亚娟1,唐 宁1,刘 俊1,卞 卡1,2
1.上海中医药大学穆拉德中药现代化研究中心,上海 201203;
2.美国德克萨斯大学休斯顿医学院综合生物及药理学系,德克萨斯大学分子医学研究所,休斯顿 TX 77030
2010-03-10;接受日期:2010-03-31
国家科技部“十一·五”支撑计划 (2006BAI11B08-03);上海市教委高校一氧化氮与炎症医学E-研究院计划(E-04010);上海市科委基础研究重点项目(08430711300,08DZ1972104)
卞卡,电话:(021)51322535,传真:(021)51322446,E-mail:Ka.Bian@uth.tmc.edu
通过考察阿托伐他汀(atorvastatin,ATO)对自发性高血压大鼠(spontaneously hypertensive rats,SHR)肾脏炎性损害的影响,探讨了ATO对高血压肾脏并发症的防治作用。将4周龄SHR分为高血压模型组和ATO治疗组(8 mg/kg),以同周龄的Wistar-Kyoto大鼠为正常对照。灌胃给药8周后,采用酶联免疫法(enzyme linked immunosorbent assay)测定血浆和肾组织血管紧张素Ⅱ(angiotensin,AngⅡ)含量;测定诱导性一氧化氮合酶(inducible nitric oxide synthase,iNOS)及细胞间粘附分子 -1(intercellular adhesion molecule-1,ICAM-1)的蛋白表达和亚硝酸阴离子(nitrite,NO2-)含量,以评价肾脏炎症状态;以苏木素伊红(hematoxylin and eosin)和过碘酸六胺银染色(periodic acid-silver metheramine)染色示SHR肾小球和肾间质形态学病变,并以尿蛋白含量为指标衡量肾脏功能。结果显示,ATO给药后显著降低了SHR肾组织AngⅡ含量;肾组织iNOS和ICAM-1的蛋白表达及NO2-含量明显降低,同时伴随着SHR肾脏病理形态的改善和肾功能的提高。该研究表明,ATO可显著改善SHR肾脏炎性损害,ATO可能在高血压肾脏并发症的防治方面有一定的疗效。
阿托伐他汀;自发性高血压大鼠;炎症;高血压肾病
R544.1;R285.5
Mar 10,2010 Accepted:Mar 31,2010
BIAN Ka,Tel:+86(21)51322535,Fax:+86(21)51322446,
E-mail:Ka.Bian@uth.tmc.edu

