纳米二氧化钛吸入对小鼠肺部和血清生化指标的影响
何娉婷 陶 杰 张焱焱 汤育欣 王月勤
(南京航空航天大学材料科学与技术学院,南京,210016,中国)
INTRODUCTION
TiO2is widely used as a white pigment in the production of paper,paints,plastics and food colorant[1]. Recently,people have also found increasing applications of ultrafine TiO2(<100 nm)in otherindustrial products[2-4],with a potential ofwidespread exposure in workers involved in the processes of its manufacture and application.Rationally the widespread application of nano-products would arise the concerns about the nano-TiO2 risk on human being health because of their size and high reactive surface,totally differing from their bulk materials[5-9].Coarse and fine(> 100 nm)TiO2is generally viewed as biologically inert and nontoxic in both human and animals[10-12]. However, D H Garabrant,et al[13]reported that 50% of workers exposed to TiO2 suffered from respiratory symptoms, accompanied by impairment of pulmonary function.Following inhaled pigmentgrade TiO2particles result in benign lung tumors in rats[14-16].In this regard,the results of 90d and 2 a inhalation studies with anatase/rutile ultrafine-TiO2 orrutile fine-TiO2 particles(average primary particle sizes—25 and— 300 nm,respectively) demonstrate that inhaled ultrafine anatase/rutile TiO2 particles produce a lot of pulmonary inflammation,fibrosis,or lung tumors in rats[17-19]. Moreover, the work of J Ferin,et al[20],K Donaldson,et al[21],and G Oberdorster,et al[5,22-23]showed that ultrafine particles produced severerinflammatory and particle translocation effects on rats when compared with fine-sized particles ofsimilar chemical composition at equivalent doses or mass concentrations. However,seldom investigation on the effect of inhaled nano-TiO2particles on serum biochemical indexes is reported.The effect of nano-TiO2on serum biochemical indexes and acute lung toxicity in mice through inhalation is studied in this paper.
1 METHODS
1.1 Animals
Mice used in the paper are specific pathogenfree animals from the Comparative Medical Center of Yangzhou University(Yangzhou,China).The mice are approximately four weeks old at the start of the study(with mean weight of 18—22 g).
1.2 Particle type
An ultrafine particle-type is defined as a particle of average primary size approximating 100 nm or less and exhibiting a property that is uniquely different from that of its bulk counterpart. The ultrafine TiO2particle(—99.5% TiO2)used in this study is obtained from Huzheng Nano Technology Co.,Ltd.(Shanghai,China),which is composed of TiO2nanoparticles with an average primary particle size smaller than 10 nm.
Nano-TiO2 sample is characterized to identify its size,surface area and crystal form in its dry native state. The data are obtained through three separate methods: transmission electron microscope(TEM),BET surface area analysis and X-ray diffraction. TEM measurements(Hitach-800)are taken on the sample in ethanol solution.BET(Micromeritics ASAP 2010),to measure specific surface area,is taken on the sample in its dry state on the nitrogen.The X-ray powder diffraction(XRD)pattern isobtained from D8-Advance X-ray diffractometer with CuKa radiation(λ=0.154 18 nm)with the voltage of 40 kV and the current of 30 mA in the region 10—90°with a step size of 0.04°.
1.3 Inhalation
The focus points of the study are the time course intensity of serum enzyme activities and histopathological evaluation of lung tissue.
Groups of mice(eight mice per group per time point),approximately 30 d old,are exposed to test atmospheres(1 500 mg/m3)3 h/d for 7,14 and 28 d.Another eight mice are exposed to indoor air only and served as controls.
Mice are exposed to TiO2aerosols in a 0.24 m3inhalation chamber constructed of organic glass.There is a hole on the cover,through which TiO2 particles are introduced into the chamber.In addition,the chamber is divided into two parts with a wire mesh,and mice are placed on the wire mesh while a fan below the mesh disperses the particles to enhance their inhalation.
1.4 Clinical chemistry
The animals are fastened overnight and all mice are killed by exsanguinations following sodium pentobarbital injection,and blood samples are collected from the orbital plexus.
The blood for clinical chemistry studies is collected into tubes containing no anticoagulant,allowed to clot,and centrifuged to obtain serum.An Olympus AU2700 Automatic Analyzer is used to measure the following parameters: lactate dehydrogenase(LDH),alanine aminotransferase(ALT),aspartate aminotransferase(AST),alkaline phosphatase(ALP), creatine kinase(CK),glucose(Glu), blood urea nitrogen(BUN),creatinine(Cr),total bile acid(TBA),and angiotensin converting enzyme(ACE).
1.5 Morphology
The lungs of mice exposed to TiO2particles or indoor air are fixed with 10% formalin solution for tissue pathologicalexamination. Sagittal sections of the lungs are made with a razor blade.Tissue blocks are dissected from left,right upper,and right lower regions of the lung and are subsequently prepared for examination by light microscopy(paraffin embedded,sectioned,and hematoxylin-eosin stained).
1.6 Statistical analyses
For analysis,each of the experimental values is compared with their corresponding control valuesforeach time point. The differences between the groupsare examined using the standard one-way analysis of variance(ANOV A).SPSS for Windows 13.0 software package is used.P<0.05 indicates statistical significance.
2 RESULTS
2.1 Particle type
A TEM image of nano-TiO2 after suspending in ethanol and sonicating for a period of time is shownin Fig.1.As can be seen that the ultrafine TiO2 from Huzheng Corporation possesses an average particle size of—15 nm in diameter and BET surface area of—73 m2/g.XRD pattern of the nanophase TiO2is shown in Fig.2.The five major peaks locate at 25.2,37.9,48.1,55.1,and 62.8°corresponding to crystal planes of anatase,respectively.The another three major peaks locate at 27.4,36.1,and 54.3° corresponding to crystalplanes of rutile,respectively.So,most of the power are anatase,but a very small amount of them is rutile.
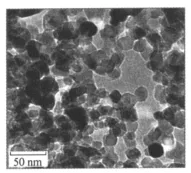
Fig.1 TEM of nano-TiO2particles
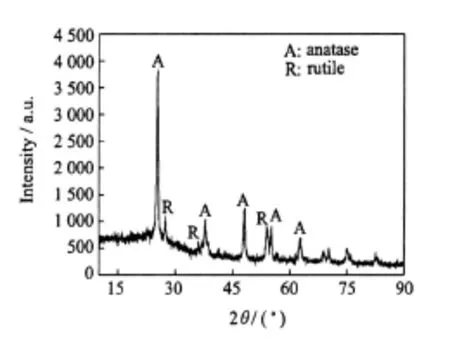
Fig.2 XRD pattern of nano-TiO2particles
2.2 Clinical chemistry
LDH activities in mice exposed to ultrafine TiO2nanoparticles in all experimental groups are significantly differentfrom those of control groups(P<0.05).ALT activity and the BUN level on the 28 d exposure as well as the Cr levels on the 14 and 28 d exposure are greatly higher than those of control groups(P<0.05).There are no obvious changes observed in other serum biochemicalindexes among the controland experimental groups(Table 1).
2.3 Lung morphology
Histopathological analysis of lung tissues reveals no significant morphological differences among the mice on the first 7 d exposure to TiO2nanoparticleswhen compared with indoorair exposed controls(Fig.3,with a magnification of 40).This micrograph illustrates that just few TiO2nanoparticles could be found(arrows).The fusion of pulmonary alveoli(see ellipses in Fig.4(a),with a magnification of 10)along with some occasional tissue thickening(see arrows in Fig.4(b),with a magnification of 40)is usually noticed,and most of the lung tissue sections demonstrate particle-laden macrophages at bronchoalveolar junctions following inhalation of particles.Also it is easily found that many TiO2nanoparticles accumulate in the interstitium of pulmonary alveoli(see arrows in Fig.4(c),with a magnification of 40).
Table 1 Statistically significant clinical chemistry parameters
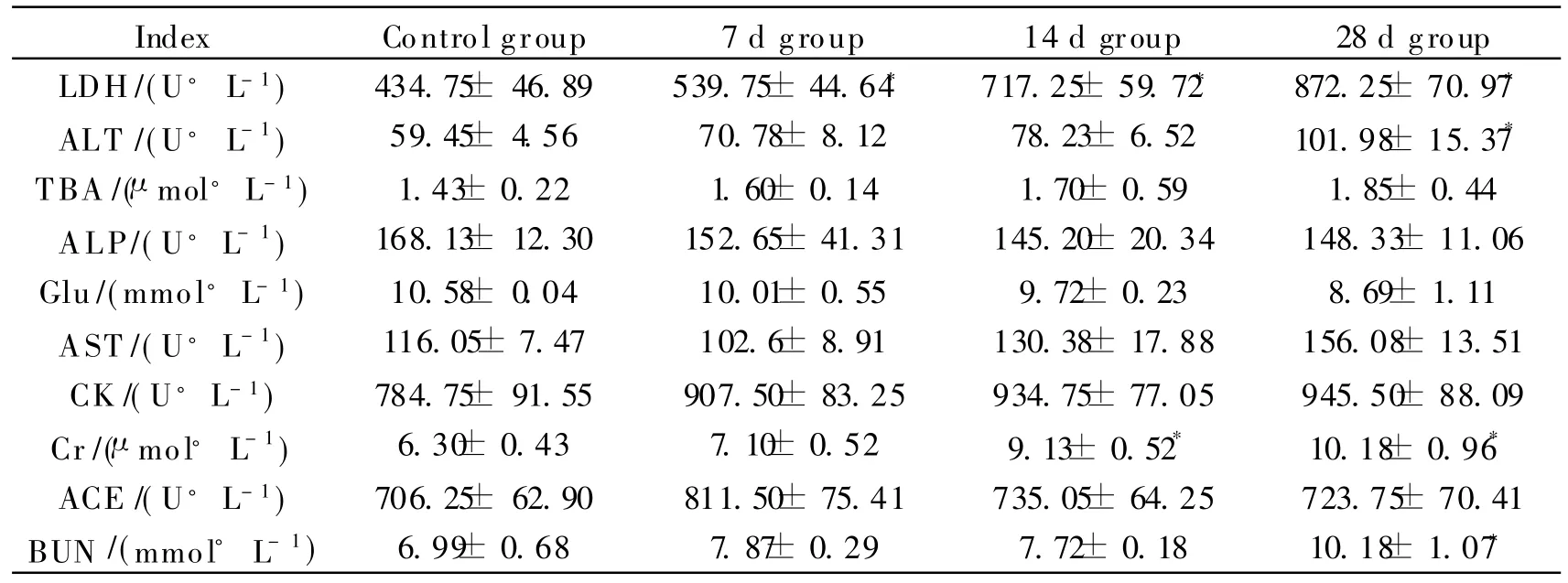
Table 1 Statistically significant clinical chemistry parameters
*P<0.05,as compared with the controls.
?
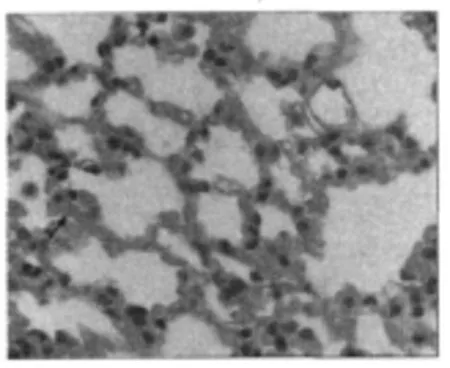
Fig.3 Light micrograph of lung tissue from a mouse exposed to nano-TiO2for 7 d
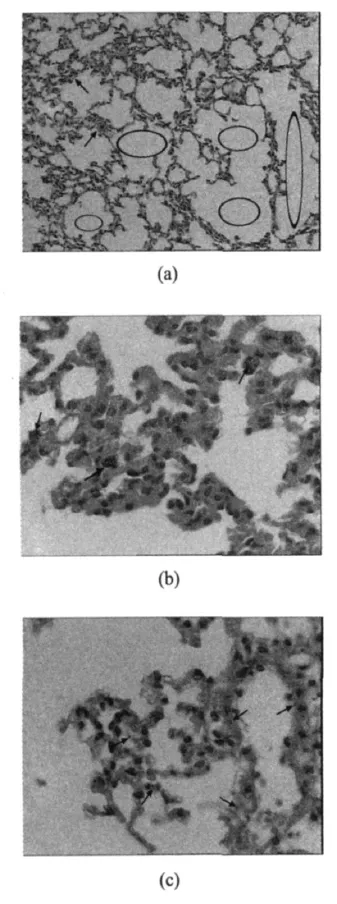
Fig.4 Light micrographs of lung tissue from a mouse exposed to nano-TiO2for28d
3 DISCUSSION
Earlier inhalation studies on TiO2ultrafine particles[24-25]focus on pulmonary inflammation by analyses of bronchoalveolar lavage(BAL)fluid.In the study,the effects of inhaled TiO2 on serum biochemical indexes are investigated.
LDH,a kind of intracellular enzyme,is used as an indicator of cell and organ injuries.The LDH activities in all experimental groups are significantly differentcompared with control groups(P<0.05),suggesting that nano-TiO2 can cause injury to cells or/and organ.
AL T is widely distributed and found in liver,heartand otherorgans. Elevation in AL T indicates damage to the liver and heart. High levels are detected only after 28 d exposure,indicating that any damage to these organs can suffer longer exposure.
Cr and BUN levels reflect kidney function.Both of the two indexes greatly increase during the 28 d,suggesting that long time(i.e,28 d)inhalation ofTiO2 aerosols probably has an impact on the kidney.
In previous inhalation studies, mice are allowed to recover from exposure for various periods,for example,24 h,1 week,1 and 3 months[17,24-26]. In this study, mice are killed immediately after indicated exposure to TiO2 aerosols.The results show that the values of LDH,ALT,BUN,and Cr increase after 28 d exposure,indicating that inhalation ofTiO2 aerosols can cause damage to the cell,heart,liver,and other organs.Histological sections find that inhaled TiO2 ultrafine particles can gain access to the interstitium of pulmonary alveoli in mice exposed for 28 d(Fig.4(c)).
It should be noted that the doses(i.e.,250 and 1 500 mg/m3)of inhalation and the sizes of the used particles in these studies are also different from in other available research findings.
There is much evidence to suggestthat instilled nano-TiO2 particles could cause pulmonary inflammation and cytotoxicity. A number of studies(inhalation and instillation)suggest that ultrafine particles produce increased inflammatory and particle translocation effects in rats[20-23]. Particle number and surface area indices appear to play important roles in the development of ultrafine particle lung toxicity[21,23]. V H Grassian,et al[26]reported that mice exposed to 2—5 nm TiO2nanoparticles showed a moderate but significant inflammatory response among animals at week 0,1,or 2 after exposure that released by week 3 post-exposure.These manufactured nanoparticles, with the highest commercially available surface area and smallest particle size for TiO2,do not show particularly toxic effects on this inhalation study.In contrast, this murine model demonstrates robust inflammatory responses upon inhalation of grain dust[27-28]or endotoxin[29]with a large number of total cells,neutrophils,or IL-6or TNF-αlevel in BAL fluid.It should be noted,with short-term inhalation exposures in rats to iron nanomaterials,inflammatory responses are also minimal[30].The results are in conflict with the notion that inflammatory response is expected to be high with large surface area powders that are composed of some of the smallest nanoparticles.A surface area dependence and correlation are observed in instillation studies[23]. However,a recent instillation study involving rats shows that the surface area for TiO2nanodots and nanorods is nota significantfactorin inflammatory response. D B Warheit, et al[31]tested the nanoparticle versus fine size hypothesis of pulmonary toxicity,i.e.,particle size and surface area strongly influenced toxicity.Groups of rats are exposed via intratracheal instillation to(1)fine-TiO2particles in the rutile phase,(2)nano-TiO2rods(100%anatase,—200 nm×40 nm),or(3)nano-TiO2dots(100% anatase,10— 20 nm)at doses of 1 mg/kg or 5 mg/kg body weight. The results demonstrate thatTiO2 particle-types produce transient pulmonary inflammatory responses,without corresponding adverse lung tissue effects. Moreover, no significant differences are measured in the inflammatory or cell injury responses among any of the groups at any postexposure time periods.Also,D B Warheit,et al[32]reported that there were differential responses related to surface properties. Three forms of ultrafine-TiO2particles(1)two different rutile-TiO2 particles(uf-1 or uf-2),(2)R-100 rutile-type,fine-sized TiO2(F-1),and(3)80/20 anatase/rutile P25 ultrafine-TiO2(uf-3) are used. The results demonstrate that the lung toxicity of anatase/rutile uf-3 should not be viewed as representative for all ultrafine-TiO2 particle-types.It can be concluded that the crystal structures of TiO2 particles are probably concerned with the toxicity.As a result,whether the surface area and particle size will play important roles in the development of ultrafine particle lung toxicity still needs further study.And the crystal structures also will be considered.
4 CONCLUSION
The results demonstrate that mice exposed to TiO2nanoparticles(15 nm in diameter and BET surface area of 0.73 m2/g)have little response after 7 d inhalation exposure.As the exposure time prolonged,several serum indexes including LDH,ALT,BUN and Cr are elevated compared with those of the control group. Many TiO2 nanoparticles accumulate in the interstitium of pulmonary alveoliafter28 d exposure. In general, these studies indicate that prolonged exposure to inhaled TiO2 nanoparticlescan induce some tissue damage in the mice.
[1] Sayes C M,Wahi R,Kurian P A,et al.Correlating nanoscale titanium structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells[J].Toxicol Sci,2006,92(1):174-185.
[2] Lee K P, TrochimowiczH J,ReinhardtC F.Pulmonary response of rats exposed to titanium dioxide(TiO2)by inhalation for two years[J].Toxicol Appl Pharmacol,1985,79(2):179-192.
[3] Lomer M C,Thompson R P,Powell J J.Fine and ultrafine particles ofthe diet: influence on the mucosal immune response and association with Crohn’s disease[J].P Nutr Soc,2002,61(1):123-130.
[4] Gelis C,Girard S,Mavon A,et al.Assessment of the skin photoprotective capacities of an organomineral broad-spectrum sunblock on two ex vivo skin models[J].Photodermatol Photo,2003,19(5):242-253.
[5] Oberdö rster G, Oberdö rster E, Oberdö rster J.Nanotoxicology: an emergingdiscipline evolving from studies of ultrafine particles[J]. Environ Health Perspect,2005,113(7):823-839.
[6] Dingman J. Nanotechnology: its impact on food safety[J].J Environ Health,2008,70(6):47-50.
[7] Li Shiqiang, Zhu Rongrong , Zhu Hong,et al.Nanotoxicity of TiO2nanoparticles to erythrocyte in vitro[J].Food and Chemical Toxicology,2008,46(12):3626-3631.
[8] Liu Shichang, Xu Lanju, Zhang Tao, etal.Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells[J].Toxicology,2010,267(1):172-177.
[9] Tamara Galloway,Ceri Lewis,Ida Dolciotti,et al.Sublethaltoxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete[J].Environmental Pollution,2010,158(5):1748-1755.
[10]Bernard B K,Osheroff M R,Hofmann A,et al.Toxicology and carcinogenesis studies ofdietary titanium dioxide-coated mica in male and female Fischer 344 rats[J].J Toxicol Env Heal,1990,29(4):417-429.
[11]Chen J L,Fayerweather W E.Epidemiologic study of workers exposed to titanium dioxide[J].J Occup Med,1988,30(12):937-942.
[12]Hart G A,Hesterberg T W.In vitro toxicity of respirable-size particles of diatomaceous earth and crystalline silica compared with asbestos and titanium dioxide[J].J Occup Env M ed,1998,40(1):29-42.
[13]GarabrantD H, Fine L J, Oliver C, etal.Abnormalities of pulmonary function and pleural disease among titanium metal production workers[J].Scandinavian Journal of Work Env Heal,1987,13(1):47-51.
[14]HextP M. Currentperspectives on particulate induced pulmonary tumours[J].Hum Rxp Toxicol,1994,13(10):700-715.
[15]ILSI Risk Science Institute.The relevance of the rat lung response to particle overload for human risk assessment:a workshop consensus report[J].Inhal Toxicol,2000,12(1):1-17.
[16]Wang Jiangxue,Zhou Guoqiang,Chen Chunying,et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration [J].Toxicology Letters,2007,168(2):176-185.
[17]Bermudez E,Mangum J B,Asgharian B,et al.Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles[J].Toxicol Sci,2002,70(1):86-97.
[18]Liao Chung-Min,Chiang Yu-Hui,Chio Chia-Pin.Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles[J].Science of the Total Environment,2008,407(1):165-177.
[19]Heinrich U,FuhstR,Rittinghausen S,et al.Chronic inhalation exposure of Wistar rats and two different strains of mice to diesel engine exhaust,carbon black,and titanium dioxide [J]. Inhal Toxicol,1995,7(4):533-556.
[20]Ferin J,Oberdorster G,Penney D P.Pulmonary retention of ultrafine and fine particles in rats[J].Am J Respir Cell Mol Biol,1992,6(5):535-542.
[21]Donaldson K,Stone V,Clouter A,et al.Ultrafine particles[J].J Occup Env M ed,2001,58(3):211-216.
[22]Oberdorster G,Finkelstein J N,Johnston C,et al.Acute pulmonary effects of ultrafine particles in rats and mice[J].Res Rep Health Eff Inst,2000,96:5-74.
[23]Oberdorster G,Maynard A,Donaldson K,et al.Principles for characterizingthepotential human health effects from exposure to nanomaterial:elements of a screening strategy[J].Particle and Fibre Toxicol,2005,2(8):1-35.
[24]Bermudez E,Mangum J B,WongB A,et al.Pulmonary responses of mice,rats,and hamsters to subchronic inhalation of ultrafine titanium dioxide particles[J].Toxicol Sci,2004,77(2):347-357.
[25]WarheitD B,Brock W J, Lee K P,et al.Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact ofsurface treatments on particle toxicity[J].Toxicol Sci,2005,88(2):514-524.
[26]Grassian V H,O’Shaughnessy P T,Adamcakova-Dodd A,et al.Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of2 to5nm[J].Environ Health Persp,2007,115(3):397-402.
[27]Jagielo P J,Thorne P S,Kern J A,et al.Role of endotoxin in grain dust-induced lung inflammation in mice[J].Am J Physiol,1996,270(6):1052-1059.
[28]Mueller-Anneling L J,O’Neill M E,Thorne P S.Biomonitoring for assessment of organic dustinduced lung inflammation[J].Eur Respir J,2006,27(6):1096-1101.
[29]Thorne P S,McCray P B,Howe T S,et al.Early-onset inflammatory responses in vivo to adenoviral vectors in the presence or absence of lipopolysaccharide-induced inflammation[J].Am J Respir Cell Mol Biol,1999,20(6):1155-1164.
[30]Zhou Y M,ZhongC Y,KennedyI M,et al.Pulmonary responses of acute exposure to ultrafine iron particles in healthy adult rats[J]. Environ Toxicol,2003,18(4):227-235.
[31]Warheit D B,Webb T R,Sayes C M,et al.Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats:toxicity is not dependent upon particle size and surface area[J].Toxicol Sci,2006,91(1):227-236.
[32]WarheitD B,Webb T R,Reed K L,et al.Pulmonary toxicity study in rats with three forms of ultrafine-TiO2particles: differential responses related to surface properties[J].Toxicology,2007,230(1):90-104.
——材料科学与工程
 Transactions of Nanjing University of Aeronautics and Astronautics2010年4期
Transactions of Nanjing University of Aeronautics and Astronautics2010年4期
- Transactions of Nanjing University of Aeronautics and Astronautics的其它文章
- 机身整体壁板在轴压载荷下后屈曲计算及结构优化
- 微型叶轮机械联合试验台设计
- 飞机除冰液对高性能混凝土抗冻性的影响
- 染料敏化太阳能电池用聚偏氟乙烯 /丙烯酸酯互穿网络基凝胶聚合物电解质的制备与性能表征
- 航班进场调度的改进捕食搜索算法
