α-硫辛酸对大鼠急性胰腺炎的保护作用及其抗氧化机制
王艳红 冯志杰 魏亚宁
·论著·
α-硫辛酸对大鼠急性胰腺炎的保护作用及其抗氧化机制
王艳红 冯志杰 魏亚宁
目的探讨抗氧化剂α-硫辛酸对急性胰腺炎(AP)的治疗作用以及可能的机制。方法3.5%牛磺胆酸钠逆行胰胆管注射制备AP 大鼠模型,数字表法随机分为假手术组、AP组、生理盐水组和α-硫辛酸组,每组30只。α-硫辛酸组于造模后腹腔内注射α-硫辛酸1 mg/kg体重,生理盐水组注射等量生理盐水。分别于术后1、3、6、9、12 h处死大鼠,检测血清淀粉酶、TNF-α、ICAM-1水平,观察胰腺病理改变,测定胰腺组织超氧化物歧化酶(SOD)活力、丙二醛(MDA)含量。结果AP组胰腺水肿、粘连、坏死,腹腔内可见血性腹水。术后6 h AP组血清淀粉酶、TNF-α、ICAM-1水平以及胰腺组织MDA含量分别为(2211.0±547.4)U/L、(174.8±7.9)ng/ml、(49.3±8.0)ng/ml和(32.2±5.9)U/mg prot,较假手术组的(160±23)U/L、(6.5±1.1)mg/ml、(13.9±3.4)mg/ml、(16.2±3.2)U/mg prot明显升高(Plt;0.05);胰腺组织SOD活力为(38.5±9.5)U/mg prot,显著低于假手术组(56.7±6.6)U/mg prot(Plt;0.05)。α-硫辛酸6 h组的血清淀粉酶、TNF-α、ICAM-1水平以及胰腺组织MDA含量分别为(1478±642)U/L、(164.8±6.2)ng/ml、(37.5±3.9)ng/ml和(20.2±8.4)U/mg prot,较AP组显著降低(Plt;0.05);胰腺组织SOD活力为(66.0±8.6)U/mg prot,较AP组显著升高(Plt;0.05)。结论AP发病与氧化应激有关,抗氧化剂α-硫辛酸对AP 大鼠具有较好的治疗作用,其机制可能与抑制TNF-α、ICAM-1活性有关。
胰腺炎; 抗氧化剂; 硫辛酸; 超氧化物歧化酶; 丙二醛
急性胰腺炎(acute pancreatitis,AP)易并发多脏器功能衰竭,危及患者的生命。在AP发病机制中,氧化应激可能发挥着重要作用,并与AP时胰腺外器官损伤有密切的联系[1-2]。α-硫辛酸(α-lipoic acid)作为一种抗氧化剂,具有较强的抗氧化活性。本实验应用α-硫辛酸干预实验性AP大鼠,观察其对AP的抗氧化治疗作用。
材料和方法
一、实验分组及模型制备
健康雄性Wister大鼠120只,由河北医科大学实验动物中心提供(合格证编号:607046),清洁级,体重230~280 g。按数字表法随机分为假手术组、AP组、生理盐水对照(NS)组和α-硫辛酸治疗(α-硫辛酸)组,每组30只。以胰胆管逆行注入3.5%牛磺胆酸钠0.1 ml/100 g体重方法制备AP模型。α-硫辛酸组于造模后腹腔内注射α-硫辛酸1 mg/kg体重;NS组造模后腹腔内注射等量生理盐水;假手术组仅翻动胰腺后关腹。各组于术后1、3、6、9、12 h分批处死大鼠,取血及胰腺组织。
二、观察指标及检测方法
1.血淀粉酶、TNF-α及细胞间黏附分子-1(ICAM-1)测定:血淀粉酶采用全自动生化分析仪检测。血TNF-α及ICAM-1检测采用ELISA方法,测试盒购自上海森雄生物有限公司。
2.胰腺组织学检查:取部分胰腺组织,常规病理检查。
3.胰腺组织超氧化物歧化酶(SOD)活力及丙二醛(MDA)含量测定:取新鲜胰腺组织制成组织匀浆,用化学比色法测定SOD及MDA,测试盒购自南京建成生物工程研究所。
三、统计学分析
结 果
一、大鼠胰腺病理学变化
假手术组胰腺及周围组织大体结构正常。AP组和NS组腹腔内可见血性腹水,胰腺局部出现水肿,与周围组织粘连,表面呈灰褐色并可见坏死灶及皂化斑。α-硫辛酸组较AP组病变明显减轻。光镜下假手术组胰腺及周围组织结构正常;AP组和NS组胰腺及周围组织水肿、出血和坏死;α-硫辛酸组较AP组损害相对减轻(图1)。
二、血清淀粉酶、TNF-α、ICAM-1水平的变化
AP组和NS组血清淀粉酶、TNF-α、ICAM-1水平均较假手术组显著升高(Plt;0.01);α-硫辛酸组则显著低于AP组(Plt;0.01),但仍高于假手术组(Plt;0.01,表1)。
三、胰腺组织SOD活力和MDA含量变化
AP组和NS组于术后3 h起SOD活力明显下降(Plt;0.05或Plt;0.01);α-硫辛酸组术后3 h后SOD活力较AP组明显增加(Plt;0.05或Plt;0.01)。AP组及NS组胰腺组织MDA含量均较同时点假手术组显著升高(Plt;0.01);α-硫辛酸组各时点MDA含量均显著低于AP组(Plt;0.05或Plt;0.01,表1)。
讨 论
在AP的发展过程中,氧自由基(oxygen free radicals,OFRs)及其衍生物通过脂肪酸过氧化作用造成类脂膜破坏及溶酶体膜破坏对胰腺损害起着重要作用。OFRs可以激活补体,促进白细胞黏附、活化和迁移[3],损伤内皮细胞的完整性,增加毛细血管的通透性,造成循环血量的丢失,引起微循环障碍,加重胰腺损伤[4-5]。在AP的炎症应答过程中,致炎因子和氧化应激发挥协同作用,导致炎症的级联扩增[6]。研究发现,在大鼠AP中胰腺损伤伴随着组织中MDA水平增加以及过氧化氢酶、SOD、谷胱甘肽过氧化物酶活力和谷胱甘肽(GSH)水平下降[7]。本实验结果显示,AP组大鼠胰腺组织SOD活力显著降低,而MDA显著升高,表明氧化应激参与AP的发病[8],测定血清氧化应激指标可以反映AP的严重程度[9]。
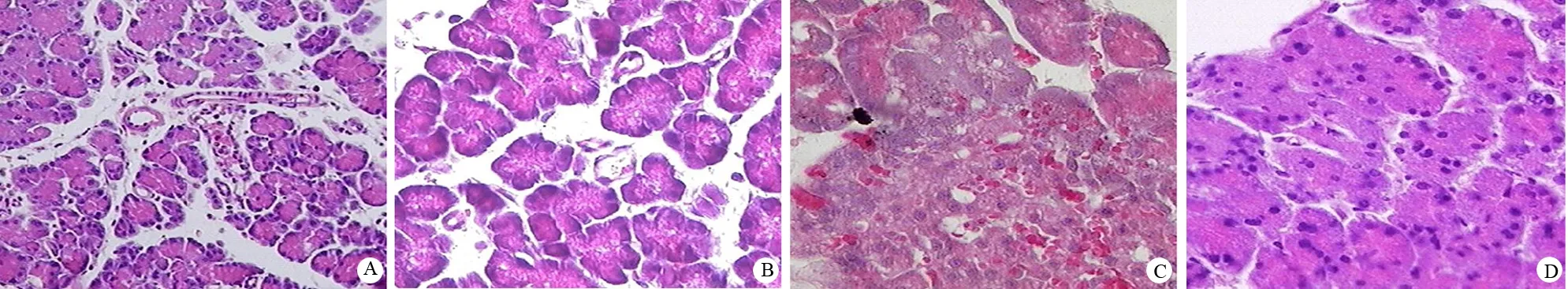
A:假手术组(HE ×200);B:AP组(HE ×200);C:NS组(HE ×400);D:α-硫辛酸组(HE ×400)
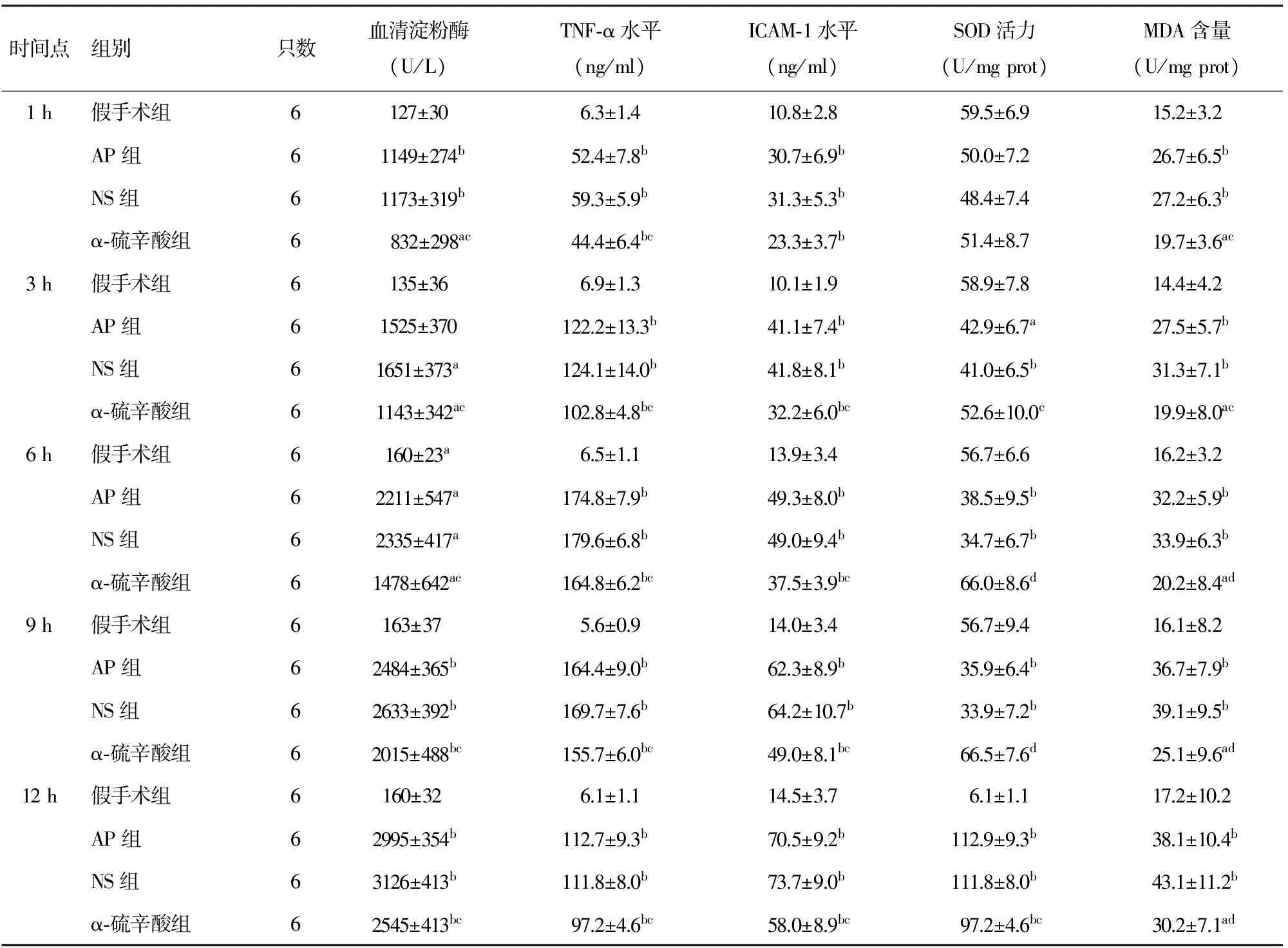
时间点组别只数血清淀粉酶(U/L)TNF⁃α水平(ng/ml)ICAM⁃1水平(ng/ml)SOD活力(U/mgprot)MDA含量(U/mgprot)1h假手术组6127±306.3±1.410.8±2.859.5±6.915.2±3.2AP组61149±274b52.4±7.8b30.7±6.9b50.0±7.226.7±6.5bNS组61173±319b59.3±5.9b31.3±5.3b48.4±7.427.2±6.3bα⁃硫辛酸组6 832±298ac44.4±6.4bc23.3±3.7b51.4±8.719.7±3.6ac3h假手术组6135±366.9±1.310.1±1.958.9±7.814.4±4.2AP组61525±370122.2±13.3b41.1±7.4b42.9±6.7a27.5±5.7bNS组61651±373a124.1±14.0b41.8±8.1b41.0±6.5b31.3±7.1bα⁃硫辛酸组61143±342ac102.8±4.8bc32.2±6.0bc52.6±10.0c19.9±8.0ac6h假手术组6160±23a6.5±1.113.9±3.456.7±6.616.2±3.2AP组62211±547a174.8±7.9b49.3±8.0b38.5±9.5b32.2±5.9bNS组62335±417a179.6±6.8b49.0±9.4b34.7±6.7b33.9±6.3bα⁃硫辛酸组61478±642ac164.8±6.2bc37.5±3.9bc66.0±8.6d20.2±8.4ad9h假手术组6163±37 5.6±0.914.0±3.456.7±9.416.1±8.2AP组62484±365b164.4±9.0b62.3±8.9b35.9±6.4b36.7±7.9bNS组62633±392b169.7±7.6b64.2±10.7b33.9±7.2b39.1±9.5bα⁃硫辛酸组62015±488bc155.7±6.0bc49.0±8.1bc66.5±7.6d25.1±9.6ad12h假手术组6160±32 6.1±1.114.5±3.76.1±1.117.2±10.2AP组62995±354b112.7±9.3b70.5±9.2b112.9±9.3b38.1±10.4bNS组63126±413b111.8±8.0b73.7±9.0b111.8±8.0b43.1±11.2bα⁃硫辛酸组62545±413bc97.2±4.6bc58.0±8.9bc97.2±4.6bc30.2±7.1ad
注:与假手术组比较,aPlt;0.05,bPlt;0.01;与AP组比较,cPlt;0.05,dPlt;0.01
TNF-α是导致AP时胰腺及胰外器官组织损伤的主要细胞因子。TNF-α水平与疾病的严重程度、病死率和预后呈正相关[10]。本实验研究显示,AP组血清TNF-α水平明显升高,在AP发生6 h后达高峰。TNF-α是AP早期增加的细胞因子,能促进炎症部位白细胞聚集和活化,活化的白细胞又可产生大量OFRs,两者形成一个恶性循环[1]。ICAM-1是一种主要表达于内皮细胞表面的蛋白质,能介导白细胞黏附和穿越内皮细胞,促使白细胞通过血管内皮屏障迁移至炎性区域[11]。Frossard等[12]研究发现,AP时存在器官灌注急剧减少及ICAM-1表达增加。本实验结果显示,AP组血清ICAM-1含量呈时间依赖性升高,它的上调是导致AP发生与发展的重要因素之一。
硫辛酸属于维生素B类化合物,在丙酮酸脱氢酶、α-酮戊二酸脱氢酶、氨基己酸脱羧酶等多酶复合体中作为辅酶发挥作用。硫辛酸具有强效的抗氧化作用,可以直接清除OFRs,阻断脂质过氧化,诱导GSH的合成,恢复细胞氧化还原稳态,减少细胞氧化损伤[13]。Park等[14]报道,给予雨蛙肽诱导的AP大鼠腹腔注射α-硫辛酸能够明显减轻血清脂肪酶和淀粉酶水平。本研究显示,给予实验性AP大鼠α-硫辛酸后血清淀粉酶活性明显降低,胰腺病理损伤改善,胰腺组织中MDA含量减少,SOD活力增加,表明α-硫辛酸通过抗氧化作用对AP大鼠起到较好的治疗效果。另外,α-硫辛酸能够降低AP大鼠血清TNF-α和ICAM-1水平,由此推测α-硫辛酸对AP的治疗作用可能也与抑制细胞因子TNF-α、ICAM-1活性有关。
[1] Pereda J,Sabater L,Aparisi L,et al. Interaction between cytokines and oxidative stress in acute pancreatitis.Curr Med Chem,2006,13:2775-2787.
[2] Esrefoglu M,Gul M,Ates B,et al.Antioxidative effect of melatonin,ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats.World J Gastroenterol,2006,12:259-264.
[3] Telek G,Regoly-Merei J,Kovacs GC,et al.The first histological demonstration of pancreatic oxidative stress in human acute pancreatitis.Hepatogastroenterology,2001,48:1252-1258.
[4] Tadao M,Yuji O.Role of free radicals in the development of severe acute pancreatitis.Nippon Rinsho,2004,62:2015-2020.
[5] 刘建生,卫新革,付极, 等.急性胰腺炎与内皮素、一氧化氮和氧自由基关系研究.中国医师杂志,2003,5:28-29.
[6] Pereda J,Sabater L,Aparisi L,et al.Interaction between cytokines and oxidative stress in acute pancreatitis.Curr Med Chem,2006,13:2775-2787.
[7] Esrefoglu M,Gul M,Ates B,et al.Ultrastructural clues for the protective effect of ascorbic acid and N-acetylcysteine against oxidative damage on caerulein-induced pancreatitis.Pancreatology,2006,6:477-485.
[8] Criddle DN,Gillies S,Baumgartner-Wilson HK,et al.Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells.J Biol Chem,2006,281:40485-40492.
[9] Esrefoglu M,Gul M,Ates B,et al.Antioxidative effect of melatonin,ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats.World J Gastroenterol,2006,12:259-264.
[10] Norman J.The role of cytokines in the pathogenesis of acute pancreatitis.Am J Surg,1998,175:76-83.
[11] 万涛,朱冠保.NF-κB、ICAM-1和炎症细胞因子在急性胰腺炎肝损伤中的作用.国外医学·临床生物化学与检验学分册,2005,26:913-915.
[12] Frossard JL,Saluja A,Bhagat L,et al.The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury.Gastroenterology,1999,116:694-701.
[13] Maritim AC,Sanders RA,Watkins JB 3rd.Effects of alpha-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats.J Nutr Biochem,2003,14:288-294.
[14] Park SJ,Seo SW,Choi OS,et al.Alpha-lipoic acid protects against cholecystokinin-induced acute pancreatitis in rats.World J Gastroenterol,2005,11:4883-4885.
2009-03-09)
(本文编辑:屠振兴)
Protectiveeffectandantioxidativemechanismofα-lipoicacidinratswithacutepancreatitis
WANGYan-hong,FENGZhi-jie,WEIYa-ning.
DepartmentofGastroenterology,SecondAffilitedHospital,HeBeiMedicalUniversity,Shijiazhuang050000,China
FENGZhi-jie,Email:zhijiefeng2005@126.com
ObjectiveTo investigate the protective effects of α-lipoic acid in rats with acute pancreatitis (AP) and its potential mechanism.MethodsWistar rats were randomly divided into four groups according to random number table: sham operation (SO) group, AP group, normal saline (NS) group and α-lipoic acid group with 30 rats in each group. AP model was induced by retrograde injection of 3.5% sodium taurocholate into the pancreatobiliary duct. Rats in α-lipoic acid group immediately
α-lipoic acid intra-peritoneal injection at the dose of 1 mg/kg. Rats in NS group received same amount of normal saline. The rats were sacrificed at 1, 3, 6, 9 and 12 h after AP induction. The serum levels of amylase, TNF-α and ICAM-1 were measured. Pancreatic histological changes were observed. The activities of pancreatic SOD and MDA were measured.ResultsIn rats of AP group, optical microscopy showed pancreatic edema, adhesion and necrosis. The serum amylase, TNF-α, ICAM-1 and MDA levels in pancreatic tissue 6h after operation were (2211±547)U/L, (174.8±7.9)ng/ml, (49.3±8.0)ng/ml and (32.2±5.9)U/mg prot, respectively, in AP group; which were significantly increased when compared with those of SO group (Plt;0.05). Pancreatic SOD activity was (38.5±9.5)U/mg prot, which was significantly lower than (56.7±6.7)U/mg prot of SO group
(Plt;0.05). The serum amylase, TNF-α, ICAM-1 and MDA levels in pancreatic tissue 6 h after operation in α-lipoic acid group were (1478±642)U/L, (164.8±6.2)ng/ml, (37.5±3.9)ng/ml and (20.2±8.4)U/mg prot, respectively; which were significantly decreased when compared with those of AP group (Plt;0.05). Pancreatic SOD activity was (66.0±8.6)U/mg prot, which were significantly higher than (38.5±9.5)U/mg prot of AP group (Plt;0.05).ConclusionsThe pathogenesis of AP was associated with oxidative stress, and α-lipoic acid as an antioxidant played a role in the treatment of AP, the possible mechanisms included inhibited production of TNF-α and ICAM-1.
Pancreatitis; Antioxidants; Thioctic acid; Superoxide dismutase; Malondialdehyde
10.3760/cma.j.issn.1674-1935.2009.06.014
050000 石家庄,河北医科大学第二医院消化内科(王艳红,现在河北省邢台市人民医院内镜室工作)
冯志杰,Email: zhijiefeng2005@163.com

