Adsorption Behavior of Methyl Orange onto Modified UltrafineCoal Powder*
LIU Zhuannian (刘转年), ZHOU Anning (周安宁), WANG Guirong (王贵荣) and ZHAO Xiaoguang (赵晓光)
Adsorption Behavior of Methyl Orange onto Modified UltrafineCoal Powder*
LIU Zhuannian (刘转年)1,**, ZHOU Anning (周安宁)2, WANG Guirong (王贵荣)1and ZHAO Xiaoguang (赵晓光)1
1College of Geology and Environment, Xi’an University of Science & Technology, Xi’an 710054, China2College of Chemistry and Chemical Engineering, Xi’an University of Science & Technology, Xi’an 710054, China
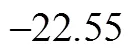
ultrafine coal powder, methyl orange, adsorption, wastewater treatment
1 INTRODUCTION
Dye is used in many industries such as food, paper, carpets, rubbers, plastics, cosmetics, and textiles. The discharge of colored wastewater from these industries causes many significant environmental problems. Methyl orange is a representative contamination in industrial wastewater and shows poor biodegradability. Many physical-chemical methods have been tested to remove the dye from aqueous solutions, and adsorption is considered superior to other techniques.
Activated carbon is one of the most widely used adsorbents for removing dye from aqueous solutions [1], but it is limited due to the high operation costs. Many low cost adsorbents are used to remove dye from aqueous solutions, such as coal fly ash [2], sepiolite [3], montmorillonite [4], clay [5], agricultural waste [6], unburned carbon [7] and low-rank coal [8]. Coal is an efficient natural adsorbent with special porous structure, plentiful functional groups such as carboxylic acids and phenolic hydroxyl. Many researchers believe that natural coal is a promising material for the removal of various organic compounds from aqueous solutions [9]. However, the adsorption capacity of natural coal is not good enough to use in industry. Activation of coal is an efficient way to improve its adsorption capacity. The most common methods for coal activation include heat treatment and/or treatment with chemical reagent, such as alkali metal hydroxides [10], oxides [11], hydrogen peroxide [12] and other chemical reagents [13]. These methods enhance the adsorptive properties of coal by increasing the surface functionality and/or increasing the porosity of coal. Some study on natural coal showed that the contribution of closed pores in total porosity in most cases exceeded 60% [14]. The investigation on physical structure and combustion properties for superfine pulverized coal particles of eight Chinese coals showed that the particle specific surface area (SSA) and pore volume increased as coal particle size decreased [15]. The adsorption properties of aniline and Ni2+on coal powder with different particle size showed that the adsorption capacity of aniline and Ni2+exponentially increased with the decreasing mean diameter of Shenfu coal powder. The adsorption ability of ultrafine coal powder was much better than that of normal size coal powder [16, 17].
Part of the closed pores in coal will be opened when the coal is ground. The specific surface area of coal powder will increase obviously as the particle size decreases [18]. Thus the adsorption capacity of coal powder will increase greatly, especially as the particle size of coal powder is about nanometer grade. Coal holds inorganic mineral matters which are transformed into ash by chemical reaction occurring in the combustion of coal. The inorganic minerals in coal, such as quartz, carbonate (CaCO3, MgCO3), pyrite (FeS2) and FeS, can be removed by washing with acid solution such as HCl, HF, or their mixed solution. When ultrafine coal powder is treated by hydrochloric acid solution, the mineral matters except silica mineral in coal will interact with the acid, then are dissolved, and new pores are produced and the specific surface area and pore volume will increase, so that the adsorption capacity of coal powder will be enhanced.
In this study ultrafine coal powder, with particle size near nanometer grade by pulverization, and modified ultrafine coal powder are used to remove methyl orange from aqueous solutions. The objective is to investigate the mechanism and behavior of methyl orange adsorption onto ultrafine coal powder and modified ultrafine coal powder to develop a new and cheap adsorbent for the removal of dye from wastewater.
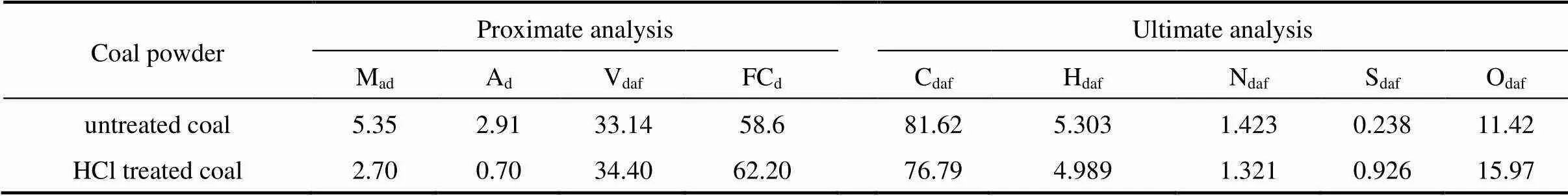
Table 1 The proximate and ultimate analyses of untreated and HCl treated Shenfu coal (%, by mass)
Note: ad—air dried basis; d—dry basis; daf—dry ash free basis; M—moisture; A—ash; V—volatile matter; FC—fixed carbon; S—total sulfur.

Table 2 Specific surface area and pore distribution of Shenfu coal
2 MATERIALS AND METHODS
2.1 Materials
The coal used in the experiment was obtained from Shenfu coalfield in Shaanxi province, China. The pore volume and Brunauer-Emmett-Teller (BET) specific surface area of the coal were determined on Sorptomatic 1990 (Thermo Quest, gas: N2). Raw coal was pulverized by a planetary high energy ball miller (Model QH-4H, China) for 5 h (250 r·min-1) and the ultrafine coal powder (UCP) was obtained. The laser granularity of UCP was analyzed on a laser grainulometric analyzer LS-POP (III) (China). The transmission electron microscopy (TEM) analysis of UCP was performed on JEM-100SX transmission electron microscope (Japan). The modified ultrafine coal powder (MUCP) was obtained by immersing UCP into 1mol·L-1HCl solution for 3 h followed by washing with distilled water till the pH value of the washing liquid was 7.0, and then dried in an oven at 353 K overnight. All the reagents used were of analytical grade. Methyl orange was used as the adsorbate . The chemical formula of methyl orange is C14H14N3NaO3S, and its structure is shown in Fig. 1.
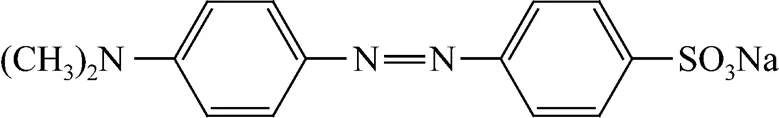
Figure 1 Structure of methyl orange
2.2 Kinetic procedure
Batch studies were conducted in a temperature- controlled water bath shaker using 50 ml (100 mg·L-1) of adsorbate solution and a fixed adsorbent dosage of 0.50 g. The samples at different time intervals (10 min, 30 min, 1 h, 2 h, 3 h and 4 h) were taken and filtered, and the filtrate was analyzed for the concentration of methyl orange left using a spectrophotometer.
2.3 Equilibrium experiments
The study on isotherms was conducted in batch mode to determine the adsorption of methyl orange on MUCP. In these experiments, 50 ml of methyl orange solutions with known initial concentration were shaken with a certain amount of adsorbent at 303, 313, and 323 K for 3 h using a temperature-controlled water bath shaker. At the end of a predetermined time, samples were filtered and the filtrate was analyzed for residual methyl orange. The effects of various parameters, such as the solution pH and the concentration of methyl orange in the solution, were studied. The pH value of the solution was adjusted with 0.1 mol·L-1HCl or 0.1 mol·L-1NaOH.
3 RESULTS AND DISCUSSION
3.1 Characteristics
The ultimate and proximate analyses of untreated and treated Shenfu coal are listed in Table 1. The pore structure of raw Shenfu coal is summarized in Table 2. As seen from Table 2, the pores are mainly mesopores with a diameter between 2.0 and 53 nm. The specific surface area of raw coal is 8.2018 m2·g-1by BET method. The particle size distribution of UCP used in this study is given in Table 3. The TEM analysis of UCP is presented in Fig. 2. The particle size of UCP is so small that the diameter of coal particle approaches nanometer, and coal particles conglutinate each other severely.

Table 3 Particle size distribution of UCP
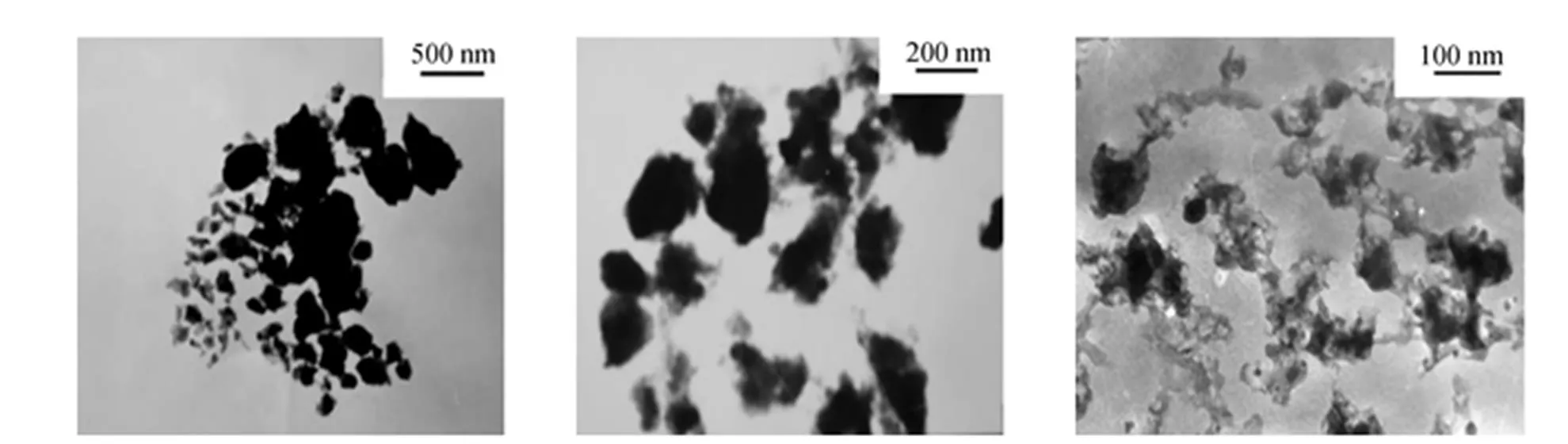
Figure 2 TEM analysis of UCP
3.2 Kinetic studies
Figure 3 illustrates the adsorption of methyl orange onto UCP and MUCP. The adsorption capacity of methyl orange onto MUCP is much better than that of UCP, which indicates that the chemical modification for UCP with HCl solution is effective. Some of mineral matters in coal are dissolved when ultrafine coal powder is treated by HCl solution, new pores are created, and the pore volume and the specific surface area increase. The amount of methyl orange uptake,q(mg·g-1), increases with contact time on both UCP and MUCP. Methyl orange uptake is rapid in the first 30 min, then proceeds at a slower rate, and finally reaches equilibrium when contact time is 240 min. Similar results have been obtained for the sorption of methyl orange on carbon bottom ash and de-oiled soya [19].

Figure 3 Effect of contact time on adsorption amount of methyl orange onto UCP and MUCP
□ UCP;○ MUCP
The behavior of the methyl orange adsorption is analyzed using the Lagergren first-order kinetic model, pseudo second-order kinetic model and intraparticle diffusion [20].
A linear form of the Lagergren first-order model expression is:

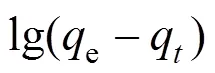
Figure 4 Lagergren plots for methyl orange adsorption onto UCP and MUCP
□ UCP;○ MUCP
The kinetic data are further analyzed using the pseudo second-order kinetics expressed as:
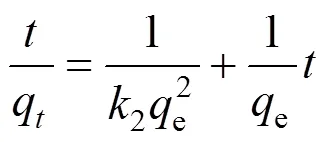
where2(g·mg-1·min-1) is the rate constant of pseudo second-order adsorption. If the second-order kinetics is applicable, the plot of/qshould give a linear relationship and it is not needed to know any parameter beforehand (Fig. 5).
Figure 5 Pseudo second-order plots for methyl orange adsorption onto UCP and MUCP
□ UCP;○ MUCP
e,cis the adsorption amount calculated from Eqs. (1) and (2).e,c,1,2and the corresponding linear regression correlation coefficient2values are shown in Table 4. The regression correlation coefficient of methyl orange onto UCP for the Lagergren first-order kinetic model (0.999) is greater than that for the pseudo second-order kinetic model (0.997). Thus, the adsorption of methyl orange onto UCP obeys the Lagergren first-order rate kinetics better. The regression correlation coefficient of methyl orange onto MUCP for the pseudo second-order kinetic model (0.999) is higher than that for the first-order kinetic model (0.918), indicting the applicability of this kinetic equation and the second-order nature of the adsorption process of methyl orange onto MUCP.
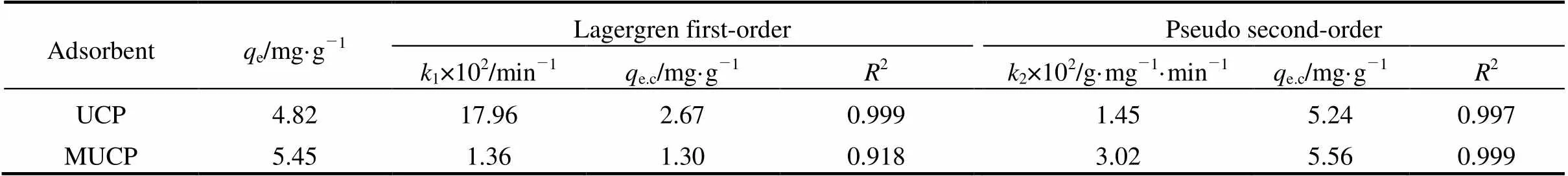
Table 4 Lagergren first-order and pseudo second-order adsorption rate constants of methyl orangeadsorption onto UCP and MUCP
The adsorbate species are most probably transported from the bulk of the solution into the solid phase with an intraparticle diffusion process, which is often the rate-limiting step in many adsorption processes. The possibility of intraparticle diffusion is explored by using the intraparticle diffusion model [21],
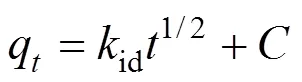
whereis the intercept andidis the intraparticle diffusion rate constant. Plot ofq1/2is shown in Fig. 6. The valuesid,and the corresponding linear regression correlation coefficient2are given in Table 5. The intraparticle rate constants calculated from Fig. 6 are 0.176 and 0.117 mg·g-1·min-1/2for UCP and MUCP respectively. The intraparticle diffusion rate constant of methyl orange adsorption onto MUCP (0.117 mg·g-1·min-1/2) is smaller than that onto UCP (0.176 mg·g-1·min-1/2).
Figure 6 Intraparticle diffusion plots for methyl orange onto UCP and MUCP
□ UCP;○ MUCP
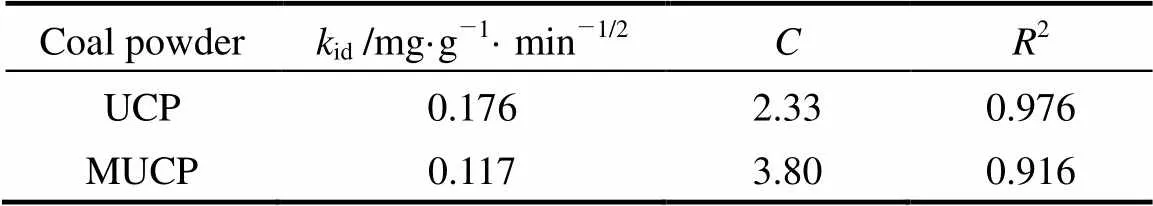
Table 5 The intraparticle diffusion rate constants of methyl orange adsorption onto UCP and MUCP
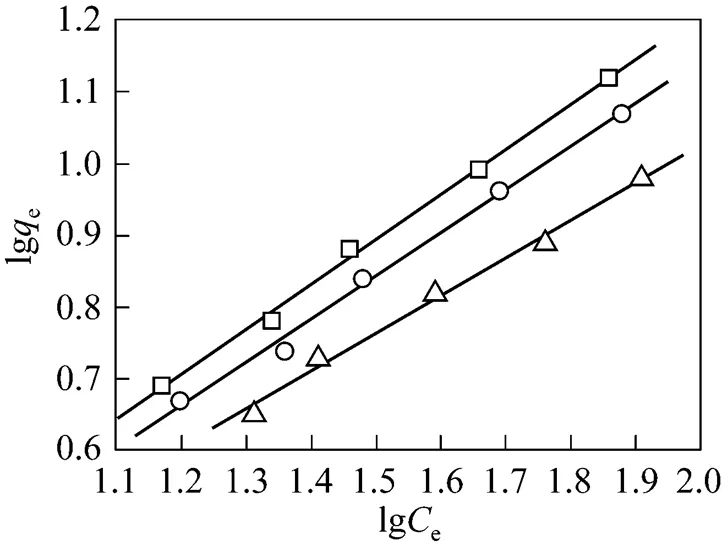
Figure 7 Freundlich isotherms for methyl orange adsorption onto MUCP at different temperature
□ 303 K;○ 313 K;△ 323 K

Figure 8 Langmuir isotherms for methyl orange adsorption onto MUCP at different temperature
□ 303 K;○ 313 K;△ 323 K
3.3 Isotherm studies
The Langmuir isotherm and Freundlich isotherm models are used to study the adsorption equilibrium. The linear forms of Langmuir and Freundlich models are [22]
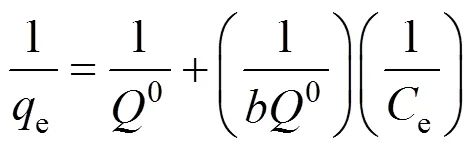
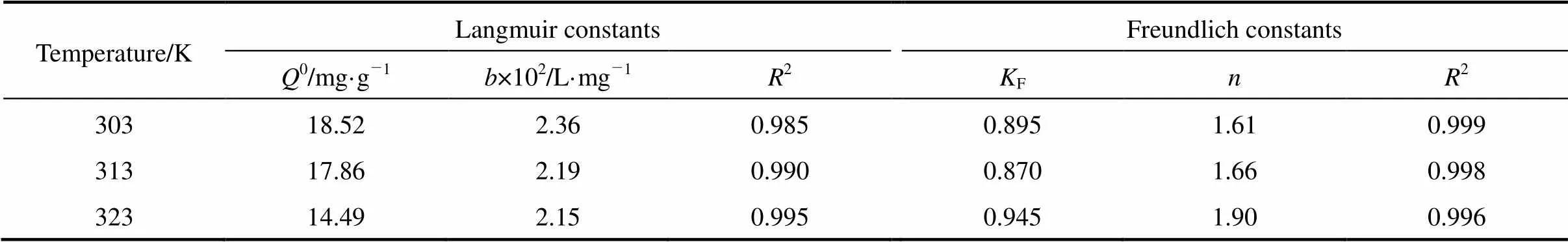
Table 6 Langmuir and Freundlich constants for methyl orange adsorption onto MUCP
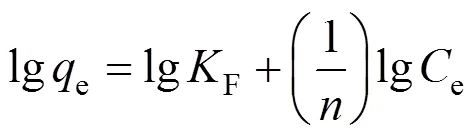
whereeis the equilibrium concentration of the solute (mg·L-1),0is the amount of solute adsorbed per unit mass of adsorbent in forming a complete monolayer on the surface (mg·g-1),is a constant related to the affinity of binding sites (L·mg-1),Fandare Frendlich constants related to adsorption capacity and adsorption intensity, respectively.0andwere calculated from the slope and intercept of the straight lines of the plot of 1/e1/e,Fandcan be determined from the linear plot of lgelge.
Figures 7 and 8 are the Freundlich and Langmuir adsorption isotherms of methyl orange adsorption on MUCP at 303, 313, and 323 K. The Langmuir and Freundlich constants for methyl orange adsorption onto MUCP are presented in Table 6. The regression correlation coefficients of Freundlich model (2) at 303 K, 313 K and 323 K are 0.999, 0.998 and 0.996 respectively, indicating a very good mathematical fit.Fis a Freundlich constant that shows the adsorption capacity of an adsorbent, and the strength of the relationship between adsorbate and adsorbent. The valuesFof MUCP for methyl orange at 303, 313, and 323 K are 0.895, 0.870 and 0.945, respectively. In all cases the exponent 1<<10 shows beneficial adsorption [23]. Freundlich exponentbetween 1.61 and 1.90 in this study indicates favorable adsorption.
As seen from Table 6, good regression correlation coefficients (0.985, 0.990 and 0.995) are obtained for all the temperatures studied, suggesting that Langmuir isotherm model is applicable to the methyl orange adsorption equilibrium by MUCP. The Langmuir monolayer adsorption capacityis 2.36×10-2, 2.19×10-2and 2.15×10-2L·mg-1at 303 K, 313 K and 323 K, respectively. The Langmuir adsorption intensity0is 18.52, 17.86 and 14.49 mg·g-1at 303 K, 313 K and 323 K, respectively. The values of0decrease as temperature increases, confirming the exothermic nature of adsorption process. This result is in accord with the result of methyl orange on the bottom ash and de-oiled soya [19].
The characteristics of a Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor or equilibrium parameter,L, which is defined as

3.4 Estimation of thermodynamic parameters
The free energy of adsorption,D0, can be related to the equilibrium constant(L·mol-1), corresponding to the reciprocal of the Langmuir constant[21],

whereis the gas universal constant (8.314 J·mol-1·K-1) andis the absolute temperature. The changes in enthalpy,D0, and entropy,D0, can be estimated by
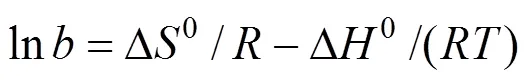
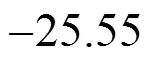
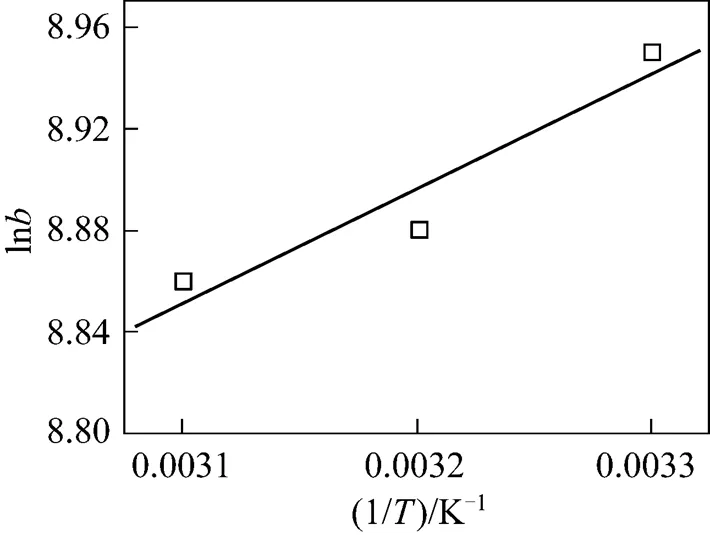
Figure 9 Plot of ln. 1/for methyl orange adsorption onto MUCP

Table 7 Thermodynamic parameters for adsorption of methyl orange onto MUCP
3.5 Effect of pH
The pH value of the aqueous medium has profound influence on the adsorption of methyl orange onto coal powder. The effect of pH on adsorption of methyl orange was studied in 50 ml of 100 mg·L-1methyl orange solution, in which the dosage of coal powder was 0.50 g. The pH value of solution was adjusted by 0.1 mol·L-1HCl or 0.1 mol·L-1NaOH. The adsorbed amount of methyl orange at pH values in the range of 6-12 is depicted in Fig. 10. In the study, the pH value should be higher than the color change pH range of 3.1-4.4. It is evident that the adsorption behavior of methyl orange on MUCP and UCP is similar. The adsorbed amount of methyl orange on MUCP and UCP increase gradually from 4.13 and 3.34 mg·g-1to 6.09 and 5.85 mg·g-1as pH increases from 6 to 11. The adsorbed amount drops rapidly when pH is higher than 11.

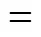
□ UCP;○ MUCP

3.6 Effect of methyl orange concentration
In order to investigate the effect of initial concentration on the adsorption, 0.50 g coal powder was add to 50 ml of 50, 100, 200 and 400 mg·L-1methyl orange solutions separately and shaken for 3 h at 25°C, then taken out and filtered. The effect of concentration on the adsorption of methyl orange on UCP and MUCP is presented in Fig. 11. The adsorbed amount for methyl orange increases with the initial concentration. The same conclusion has been obtained for methyl orange to adsorb on the bottom ash and de-oiled soya [19]. The increase in adsorbed amount with relation to dye ion is probably due to the high driving force for mass transfer.
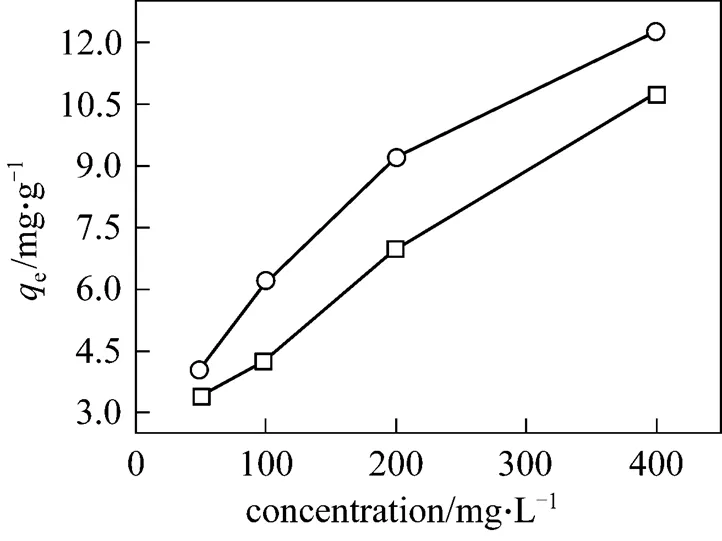
□ UCP;○ MUCP
3.7 Effect of adsorbent dosage
Adsorbent dosage is an important parameter at a given initial concentration of the adsorbate. 0.1, 0.3, 0.5, 0.7, 0.9, and 1.2 g coal powder was added to 50 ml of 100 mg·L-1methyl orange solution separately, keeping all other experimental conditions constant. Fig. 12 shows that as the adsorbent dosage increases, the amount adsorbed per unit mass of the adsorbent decreases. This is due to the increase in total surface area of coal powder [26].

Figure 12 Effect of adsorbent dosage on adsorption amount of methyl orange onto UCP and MUCP
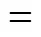
□ UCP;○ MUCP
4 Conclusions
UCP and MUCP were used to remove methyl orange from aqueous solutions. The mechanism and behavior of methyl orange adsorption onto UCP and MUCP were studied. The results show that the adsorption capacity of methyl orange onto MUCP is much better than that of UCP. Adsorption isotherms of methyl orange on MUCP at 303, 313, and 313 K follow the Freundlich model and Langmuir model. The exponent is 1<<10, indicating favorable adsorption. The adsorption kinetics of methyl orange onto UCP and MUCP fits the Lagergren first-order kinetic model and the pseudo second-order kinetic model, respectively. Negative free energy change of adsorption (D0), negative enthalpy change (D0) and positive entropy change (D0) indicate that the adsorption is a spontaneous and exothermic process.
1 El Qada, E.N., Allena, S.J., Walker, G.M., “Adsorption of basic dyes from aqueous solution onto activated carbons”,..., 135 (1), 174-184 (2008).
2 Lin, J.X., Zhan, S.L., Fang, M.H., Qian, X.Q., Yang, H., “Adsorption of basic dye from aqueous solution onto fly ash”,..., 87 (1), 193-200 (2008).
3 Özdemir, Y., Doğan, M., Alkan, M., “Adsorption of cationic dyes from aqueous solutions by sepiolite”,..., 96 (1-3), 419-427 (2006).
4 Teng, M.Y., Lin, S.H., “Removal of methyl orange dye from water onto raw and acid activated montmorillonite in fixed beds”,, 201 (1-3), 71-81 (2006).
5 Gürses, A., Doğar, Ç., Yalçın, M., Açıkyıldız, M., Bayrak, R., Karaca, S., “The adsorption kinetics of the cationic dye, methylene blue, onto clay”,..., 131 (1-3), 217-228 (2006).
6 Hameed, B.H., Krishni, R.R., Sata, S.A., “A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions”,..., 162 (1), 305-311 (2009).
7 Wang, S., Li, H., “Dye adsorption on unburned carbon: Kinetics and equilibrium”,..., B126 (1-3), 71-77 (2005).
8 Janoš, P., Michálek, P., Turek, L., “Sorption of ionic dyes onto untreated low-rank coal—oxihumolite: A kinetic study”,., 74 (2), 363-370 (2007).
9 Tarasevich, Y.I., “Porous structure and adsorption properties of natural porous coal”,., 176 (2-3) ,267-272 (2001).
10 Lillo-Ródenas, M.A., Lozano-Castelló, D., Cazorla-Amorós, D., Linares-Solano, A., “Preparation of activated carbons from Spanish anthracite (II) Activation by NaOH”,, 39 (5), 751-759 (2001).
11 Lakatos, J., Brown, S.D., Snape, C.E., “Coals as sorbents for the removal and reduction of hexavalent chromium from aqueous waste streams”,, 81 (5), 691-698 (2002).
12 Burns, C.A., Boily, J.F., Crawford, R.J., Harding, I.H., “Cd(II) sorption onto chemically modified Australian coals”,, 84 (12-13), 1653-1660 (2005).
13 Hsu, L.Y, Teng, H., “Influence of different chemical reagents on the preparation of activated carbons from bituminous coal”,.., 64 (1-3), 155-166 (2000).
14 Alexeev, A.D., Vasilenko, T.A., Ulyanova, E.V., “Closed porosity in fossil coals”,, 78 (6), 635-638 (1999).
15 Jiang, X.M., Zheng, C.G, Yan, C., Liu, D.C., Qiu, J.R., Li, J.B., “Physical structure and combustion properties of super fine pulverized coal particle”, 81 (6), 793-797 (2002).
16 Liu, Z.N., Zhou, A.N., Jin, Q.T., “Mechanism of aniline adsorption on ultrafine coal powder”,, 34 (1), 20-24 (2006). (in Chinese)
17 Liu, Z.N., Zhou, A.N., Jin, Q.T., “Adsorption properties of Ni2+for different granularity coal powders”,, 30 (5), 627-631 (2005). (in Chinese)
18 Yu, Z.J., Zhou, A.N., Qu, J.L., Zhang, T.J., “Study on behavior and kinetics of sorption of Ag+by Shenfu coal”,..., 85 (1), 104-110 (2005).
19 Mittal, A., Malviya, A., Kaur, D., Mittal, J., Kurup, L., “Studies on the adsorption kinetics and isotherms for the removal and recovery of methyl orange from wastewaters using waste materials”,..., 148 (1-2), 229-240(2007).
20 Namasivayam, C., Yamuna, R.T., “Adsorption of direct red 12 B by biogas residual slurry: Equilibrium and rate processes”,.., 89 (1), 1-7 (1995).
21 Bulut, Y., Aydin, H., “A kinetics and thermodynamics study of methylene blue adsorption on wheat shells” ,, 194 (1-3), 259-267 (2006).
22 Gupta, V.K., Ali, I., “Utilisation of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater”,..., 18 (2), 131-140 (2000).
23 Annadurai, G., Ling, L.Y., Lee, J.F., “Biodegradation of phenol byon immobilized with chitin”,., 6 (3), 296-303 (2007).
24 Tan, I.A.W., Ahmad, A.L., Hameed, B.H., “Adsorption of basic dye using activated carbon prepared from oil palm shell: batch and fixed bed studies”,, 225 (1-3), 13-28 (2008).
25 Venkata, M.S., Chandrasekhar, R.N., Karthikeyan, J., “Adsorptive removal of direct azo dye from aqueous phase onto coal based sorbents: A kinetic and mechanistic study”,..., 90 (2), 189-204 (2002).
26 Matheswaran, M., Karunanithi, T., “Adsorption of Chrysoidine R by using fly ash in batch process”,..., 145 (1-2), 154-161 (2007).
2008-12-14,
2009-07-07.
the National Science Foundation for Post Doctoral Scientists of China (20070411124), Scientific and Technological Key Project of Shaanxi Province (2006k07-G19), and Industrialization Project of Shaanxi Provincial Department of Education (06JC11).
** To whom correspondence should be addressed. E-mail: zhuannianliu@163.com
 Chinese Journal of Chemical Engineering2009年6期
Chinese Journal of Chemical Engineering2009年6期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Working Temperature on the Resistance Characteristic of aPleated Stainless Steel Woven Filter*
- The Numerical Simulation of Collapse Pressure and Boundary of the Cavity Cloud in Venturi*
- Performance of Inner-core Supersonic Gas Separation Device with Droplet Enlargement Method*
- Void Fraction Distributions in Cold-gassed and Hot-sparged Three Phase Stirred Tanks with Multi-impeller*
- Kinetics of COD Removal in a Biological Aerated Filter in thePresence of 2,4,6-Trinitrophenol (Picric Acid)*
- Representation of Phase Behavior of Ionic Liquids Using the Equation of State for Square-well Chain Fluids with Variable Range*
