Separation of Sulfur/Gasoline Mixture with Polydimethylsiloxane/Polyetherimide Composite Membranes by Pervaporation*
ZHAO Changwei (赵长伟), LI Jiding (李继定), CHEN Jian (陈剑), QI Rongbin (亓荣彬) and LUAN Zhaokun (栾兆坤)
Separation of Sulfur/Gasoline Mixture with Polydimethylsiloxane/Polyetherimide Composite Membranes by Pervaporation*
ZHAO Changwei (赵长伟)1,**, LI Jiding (李继定)2, CHEN Jian (陈剑)2, QI Rongbin (亓荣彬)2and LUAN Zhaokun (栾兆坤)1
1State Key Laboratory of Environmental Aquatic Chemistry, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China2Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Worldwide environment has resulted in a limit on the sulfur content of gasoline. It is urgent to investigate the desulfurization of gasoline. The polydimethylsiloxane (PDMS)/polyetherimide (PEI) composite membranes were prepared by casting a PDMS solution onto porous PEI substrates and characterized by scanning electron microscope (SEM). The membranes were used for sulfur removal from gasoline by pervaporation. The effects of feed temperature, sulfur content in the feed and PDMS layer thickness on membrane performance were investigated, and an activation energy of permeation was obtained. Experimental results indicated that higher feed temperature yielded higher total flux and lower sulfur enrichment factor. The total flux varied little with the increase of sulfur content in the feed, but the sulfur enrichment factor first increased with the amount of thiophene added into the gasoline, and then the variation was little. The increase of PDMS layer thickness resulted in a smaller flux but a larger sulfur enrichment factor. The result indicates that the PDMS/PEI composite membranes are promising for desulfurization by pervaporation.
polydimethylsiloxane (PDMS)/polyetherimide (PEI) composite membranes, sulfur removal, gasoline, pervaporation
1 INTRODUCTION
Environmental concerns have resulted in the legislation of low sulfur content in gasoline. It is an urgent problem to develop a strategy for meeting clean fuel regulations economically and reliably [1-5]. In recent years, it has been proven that pervaporation (PV) is a feasible membrane technology with a number of potential advantages over conventional sulfur removal processes from gasoline [6-11].
Since fluid catalytic cracking (FCC) gasoline is an extraordinarily complex mixture composed of hundreds of compounds [12-14], in order to simplify and simulate the system for investigation, previous studies on the desulfurization were mainly focused on the modeled gasoline systems composed of hydrocarbon/ thiophene or-heptane/sulfur species [15-21]. Little has been reported on the separation performance of a real gasoline system for FCC gasoline desulfurization, which will have different effect on pervaporation separation performance. In this work, the aim is to investigate the separation performance by PV experiments for a real gasoline and the real gasoline with thiophene added. Polydimethylsiloxane (PDMS)/polyetherimide (PEI) composite membranes are prepared and employed in pervaporation separations. Different operating conditions are investigated in the desulfurization process for the gasoline.
2 EXPERIMENTAL
2.1 Materials
Hydroxy-terminated polydimethylsiloxane (PDMS) (viscosity 10 Pa×s,Beijing Chemical Plant, China), ethyl orthosilicate (analytical grade, Beijing Chemical Company, China), dibutyltin dilaurate (analytical grade, Beijing Chemical Company, China), and-heptane (analytical grade, Beijing Chemical Company, China) were used for the preparation of PDMS membrane. Polyetherimide (PEI) Ultem®1000 (General Electric Plastics, Shanghai Chemical Company, China) was dried at 423.15 K before used.,-dimethyl acetamide (DMAc) (analytical grade, Beijing Chemical Company, China) was used as solvent. The asymmetric microporous PEI membrane prepared in the laboratory was used as supports. Gasoline with 140 ng·μl-1sulfur content (Oil Station, China) was used as the real gasoline. Thiophene (99% pure, Acros) was chosen as the representative sulfur component to add into the real gasoline feed.
2.2 Membrane preparation
The asymmetric polyetherimide (PEI) membrane prepared in the laboratory was adopted as the microporous supporting layer in the flat-plate composite membranes. PEI was dissolved in DMAc solvent at 353.15K until a homogenous solution was obtained. After filtration, and degasification under vacuum, the solution was cast onto a polyester non-woven fabric, which was immediately immersed into a coagulation bath. Then, the membrane was washed and used as supporting layer. The PDMS prepolymer, crosslinking agent ethyl orthosilicate and initiator dibutyltin dilaurate were dissolved into-heptane at room temperature. After degassed under vacuum, the solution was cast onto the PEI membrane to form the skin layer. Then the composite membranes were vulcanized at room temperature to evaporate the solvent and complete the crosslinking. The carve knife thickness can be controlled to produce membranes with variable PDMS top layer thickness. The photomicrograph of the membrane was obtained by a scanning electron microscope (Hitachi S-450). The thickness of the top skin layer of the membrane was determined by SEM photograph.

Figure 1 Schematic diagram of pervaporation apparatus
2.3 Measurement for pervaporation performance
The schematic pervaporation apparatus is shown in Fig. 1. The membrane module was an annular chamber made of stainless steel and the membrane was supported on a porous stainless steel disc with an effective area of 28.3 cm2. The feed liquid in heated tank was pumped through the feed compartment of the membrane cell, where it came into direct contact with the PDMS top layer of the membrane. Steady state fluxes were determined by condensing and freezing the permeating vapor in two cold traps, which were set in parallel and used alternatively in order to collect permeate without breaking the vacuum.
The total flux is calculated by the following equation:
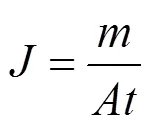
wheredenotes the total flux, andis the mass of permeate passing through the active membrane areaduring the time.
The sulfur concentrations of feed and permeate were analyzed by a gas chromatography (HP6890, USA). The sulfur enrichment factoris adopted to characterize the separation performance of the membrane:
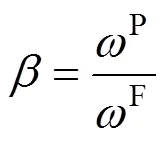
wherePandFrefer to the total sulfur content in the permeate liquid and in the feed liquid, respectively.
Figure 2 The cross-section morphology of the PDMS/PEI composite membranes
3 RESULTS AND DISCUSSION
3.1 SEM photographs of PDMS/PEI composite membranes
Figure 2 shows the cross-section morphology of the PDMS/PEI composite membrane. As demonstrated in the SEM photograph, the membrane consists of a thin skin layer and a porous substrate. The thickness of the PDMS layer was determined by the SEM photograph of cross-section of the membrane.
3.2 Effects of feed temperature on pervaporation properties
The effect of temperature in the range of 303.15 to 350.15 K on pervaporation performance with PDMS/ PEI composite membranes is illustrated in Fig. 3. The feed contained 500 ng·μl-1sulfur content with the gasoline and thiophene added. The operating pressure was at 300 Pa permeate pressure. As expected, as the feed temperature increases, the total flux increases but the sulfur enrichment factor decreases. This can be explained by that higher temperature enhances the mobility of polymer segments, which offers more free volume for permeating molecules to occupy and thus facilitates their movement both in the bulk feed solution and within the membrane.

Figure 3 Effect of feed temperature on pervaporation performance for gasoline mixtures
Figure 4 Flux of gasoline with thiophene added at different feed temperatures
The relationship between the permeation flux and feed temperature can normally be expressed by the Arrhenius-type formula as follows:

where0is the pre-exponential factor,is the activation energy of permeation,is the absolute temperature of the feed, andis the gas constant.
As shown in Fig. 4, the flux of pervaporation decreases exponentially with the reciprocal of temperature following Eq. (3). The activation energy of permeation,, was obtained as 23.89 kJ·mol-1.
3.3 Effects of sulfur content on pervaporation properties
Pervaporation for the real gasoline and the gasoline with thiophene added was conducted separately to investigate the effect of sulfur content on separation performance of the PDMS/PEI composite membranes. The corresponding sulfur contents by adding thiophene in the feed were in the range from 140 to 1200 ng·μl-1. It can be seen from Fig. 5 that variation of sulfur content in the feed has nearly negligible influence on the flux. Because the gasoline is an extraordinarily complex mixture with high content of alkanes, olefins, cycloparaffins, aromatics,., but low content of sulfur, a change in sulfur content in the feed has very limited impact on the total flux through the membrane. The sulfur enrichment factor is low for the real gasoline (140 ng·μl-1), and it first increases with the amount of thiophene added in the gasoline and then it varies a little. This shows that the PDMS/PEI composite membranes are more selective to thiophene.

Figure 5 Effects of sulfur content on pervaporation performance for gasoline mixtures
3.4 Effects of PDMS layer thickness on pervaporation properties
In our experiments, PDMS layer thickness was controlled by drawknife. The effect of varying PDMS layer thickness on pervaporation performance is shown in Fig. 6. The feed contained 500 ng·μl-1sulfur content with the gasoline and thiophene added. As expected, the flux decreases but sulfur enrichment factor increases with the increase in membrane dense layer thickness. When the membrane thickness increases the liquid molecules have to cross longer distance inside the membrane, which results in a decrease in flux. This is consistent with the solution-diffusion concept, which is generally applicable to membrane separation processes involving permeation through a dense layer.
In this study, the PDMS/PEI composite membranes were prepared and applied to the sulfur removal from gasoline by pervaporation. The effects of feed temperature, sulfur content in the feed and PDMS layer thickness on the desulfurization efficiency were investigated, and an activation energy of permeation was obtained. Experimental results indicated that higher feed temperature yielded higher total flux and lower sulfur enrichment factor. The total flux varied little as the sulfur content in the feed increased. The sulfur enrichment factor first increased with the amount of thiophene added in the gasoline, then the variation was little. The increase of PDMS layer thickness resulted in a smaller flux but a larger sulfur enrichment factor. The result demonstrated that the PDMS/PEI composite membranes could be effective for desulfurization by pervaporation.
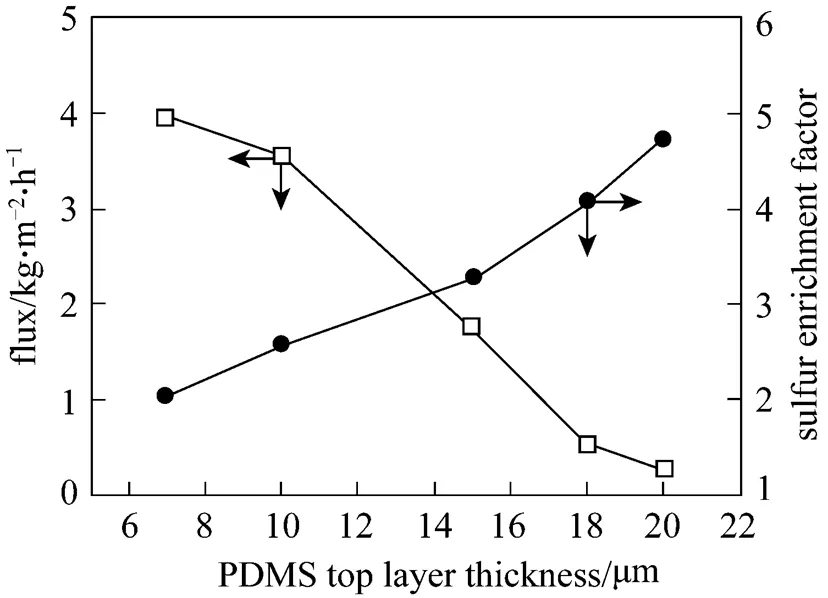
Figure 6 Effects of PDMS layer thickness on pervaporation performance for gasoline mixtures
1 Santos, N.D., Dulot, H., Marchal, N., Vrinat, M., “ New insight on competitive reactions during deep HDS of FCC gasoline”,..., 352 (1-2), 114-123 (2009).
2 Yao, Y.C., Tsai, J.H., Chang, A.L., Jeng, F.T., “Effects of sulfur and aromatic contents in gasoline on motorcycle emissions”,.., 42 (26), 6560-6564 (2008).
3 Arias, M., Laurenti, D., Geantet, C., Vrinat, M., “Gasoline desulfurization by catalytic alkylation over silica-supported heteropolyacids : from model reaction to real feed conversion”,.., 130 (1), 190-194 (2008).
4 Wu, Z.M., Ma, J.H., Yun, Z., Shi, M., “Desulfurization of gasoline using intermetallic SnSb”,.... (), 57 (8), 1974-1978 (2006). (in Chinese)
5 Chen, H.Z., Li, Y.D., Zhao, D.S., “Study on adsorptive desulfurization of FCC gasoline”,.. (), 34 (7), 1-5 (2006). (in Chinese)
6 Kong, Y., Lin, L.G., Zhang, Y.Z., “Studies on polyethylene glycol/polyethersulfone composite membranes for FCC gasoline desulphurization by pervaporation”,..., 44 (10), 3335-3343 (2008).
7 Minhas, B.S., Chuba, M.R., Saxton, R.J., “Membrane process for separating sulfur compounds from FCC light naphtha”, U.S. Pat., 6649061 (2003).
8 Saxton, R.J., Minhas, B.S., “Ionic membranes for organic sulfur separation from liquid hydrocarbon solutions”, U.S. Pat., 6702945 (2004).
9 Lin, L.G., Kong, Y., Wang, G., “Selection and crosslinking modification of membrane material for FCC gasoline desulfurization”,..., 285 (1/2), 144-151 (2006).
10 Chen, J., Li, J.D., Qi, R.B., “Pervaporation performance of crosslinked polydimethylsiloxane membranes for deep desulfurization of FCC gasoline I. Effect of different sulfur species”,..., 322 (1), 113-121 (2008).
11 Li, B., Xu, D., Jiang, Z.Y., Zhang, X.F., Liu, W.P., Dong, X., “Pervaporation performance of PDMS-Ni2+Y zeolite hybrid membranes in the desulfurization of gasoline”,..., 322 (2), 293-301 (2008).
12 Serafim, D.M., Stradiotto, N.R., “Determination of sulfur compounds in gasoline using mercury film electrode by square wave voltammetry”,, 87 (7), 1007-1013 (2008).
13 Zhang, J.C., Song, L.F., Hu, J.Y., Ong, S.L., “Investigation on gasoline deep desulfurization for fuel cell applications”,..., 46 (1), 1-9 (2005).
14 Shao, Z.N., “Current levels and development trend of world motor gasoline quality”,..., 38 (1), 1-6 (2008).
15 Zhao, C.W., Li, J.D., Qi, R.B., Qi, R.B., Luan, Z.K., “Pervaporation separation of-heptane/sulfur species mixtures with polydimethylsiloxane membranes”,..., 63 (1), 220-225 (2008).
16 Lin, L.G., Wang, G., Qu, H.M., Yang, J.R., Wang, Y.F., “Pervaporation performance of crosslinked polyethylene glycol membranes for deep desulfurization of FCC gasoline”,.., 280 (1/2), 651-658 (2006).
17 Kong, Y., Lin, L.G., Yang, J.R., Shi, D.Q., Qu, H.M., “FCC gasoline desulfurization by pervaporation: Effects of gasoline components”,..., 293 (1/2), 36-43 (2007).
18 Lin, L.G., Kong, Y., Yang, J.R., Shi, D.Q., Xie, K.K., “Scale-up of pervaporation for gasoline desulphurization (1) Simulation and design”,..., 298 (1/2), 1-13 (2007).
19 Qi, R.B., Zhao, C.W., Li, J.D., Wang, Y.J., Zhu, S.L., “Removal of thiophenes from-octane/thiophene mixtures by pervaporation”,..., 269 (1/2), 94-100 (2006).
20 Qi, R.B., Wang, Y.J., Li, J.D., Zhao, C.W., Zhu, S.L., “Pervaporation separation of alkane/thiophene mixtures with PDMS membrane”,..., 280 (1/2), 545-552 (2006).
21 Qi, R.B., Wang, Y.J., Li, J.D., Zhu, S.L., “Sulfur removal from gasoline by pervaporation: The effect of hydrocarbon species”,..., 51 (3), 258-264 (2006).
2008-12-02,
2009-03-23.
the National Basic Research Program of China (2009CB623404), the National Natural Science Foundation of China (50708109, 20736003), and the National High Technology Research and Development Program of China (2007AA06Z317).
** To whom correspondence should be addressed. E-mail: zhaocw@rcees.ac.cn
 Chinese Journal of Chemical Engineering2009年4期
Chinese Journal of Chemical Engineering2009年4期
- Chinese Journal of Chemical Engineering的其它文章
- Enhanced Methane Adsorption in Catenated Metal-organic Frameworks: A Molecular Simulation Study*
- Measurements of Hydrate Equilibrium Conditions for CH4, CO2, and CH4 + C2H6 + C3H8 in Various Systems by Step-heating Method*
- Effects of Temperature on the Preparation of Magnesium Carbonate Hydrates by Reaction of MgCl2 with Na2CO3*
- Adsorbents for Expanded Bed Adsorption: Preparation and Functionalization*
