Isobaric Vapor-Liquid Equilibrium of Binary System 2-Cyclohexen-1-one and 1,2-Epoxycyclohexane*
WANG Xuemeng (王学猛), JIANG Denggao (蒋登高)** and GENG Zaixin (耿再新)
Isobaric Vapor-Liquid Equilibrium of Binary System 2-Cyclohexen-1-one and 1,2-Epoxycyclohexane*
WANG Xuemeng (王学猛), JIANG Denggao (蒋登高)** and GENG Zaixin (耿再新)
School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, China
Green oxidation of cyclohexene using dioxygen as oxidizing agent is highly desirable because of its environmental compatibility and economic impact. Separation of its oxidation products depends on the reliable vapor- liquid equilibrium (VLE) data of relevant components, which are still lacking. The VLE data of binary system 1,2-epoxycyclohexane and 2-cyclohexen-1-one under ambient pressure were obtained using an improved VLE equipment EC-2 still in this work. The results showed that this binary system has no azeotropic point. Furthermore, the experimental VLE data were correlated with the Wilson thermodynamic model and the corresponding binary interaction parameters of the model were obtained. The results showed that the VLE data agreed well with the model and passed the thermodynamic consistency test of Herrington.
2-cyclohexen-1-one, 1,2-epoxycyclohexane, vapor-liquid equilibrium
1 INTRODUCTION
2 EXPERIMENTAL
2.1 Materials and instruments
1,2-epoxycyclohexane (≥99.3% purity, GC) and 2-cyclohexen-1-one (≥99.0% purity, GC) were commercially available from Shandong Silver Hawk Chemical Fiber Co., and Shanghai Haiqu Chemical Engineering, respectively. Gas Chromatogram (GC-900A) with FID detector was provided by Shanghai Kechuang Gas Chromatogram Co., Ltd. Mercury thermometers from 373.15 K to 423.15 K and from 423.15 K to 473.15 K were with accuracy of 0.02°C and the scale calibration,.., zero correction were done before used. The outside neck amendments of thermometers were considered when it was in use. The equilibrium still was improved EC-2 VLE. The basic properties of relevant substance were shown in Table 1.
2.2 Analysis
The compositions of vapor and liquid phases of the binary system were determined by gas chromatogram with FID detector. Toluene was used as internal standard to quantify molar fractions of 1,2-epocyclohexane and 2-cyclohexen-1-one.The specification of capillary column was SE-54 30 m×0.53 mm×1 μm. The injection temperature was 200°C and hydrogen microflare detector temperature was 190°C. The initial temperature of capillary oven was 50°C and was kept invariable for 4 min. Then, it increased to 140°C at the rate of 4°C·min-1and dropped suddenly. In proving the tests of benzene (1)—toluene (2), other items and conditions were the same as above besides cyclohexene, which was used as an internal standard.

Table 1 Basic properties of relevant substances

2.3 Verification of instruments
Ten groups of VLE data of binary system for benzene and toluene were determined in order to verify reliabilities of equipments and instruments at 101.33 kPa. Comparison between experimental data and previously reported data in Ref. [10] are shown in Fig. 1.
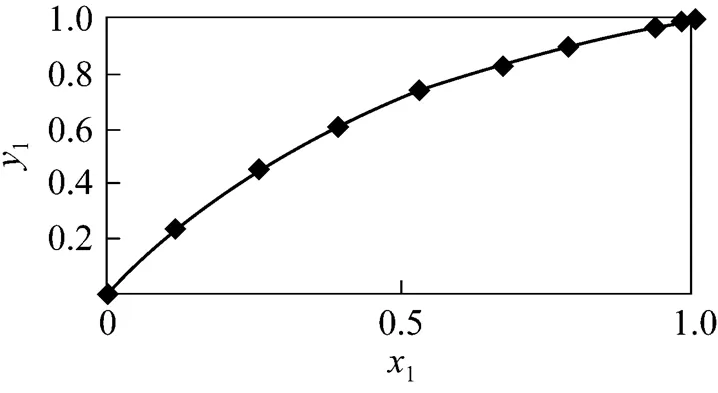
Figure 1 Vapor-liquid equilibrium phase diagram for the benzene (1)—toluene (2) system at 101.33 kPa
◆ experimental data; —— reference data
Figure 1 showed that the experimental data coincided with the data in Ref. [11], which indicated that it was reliable to determine VLE data of binary system at 101.33 kPa using such experiments and analysis equipments.
2.4 Experimental method
Improved EC-2 equilibrium still was used to determine the VLE data of binary systems. Equilibrium temperatures were measured by two-stage standard thermometers and the accuracy was 0.02°C. System pressure was controlled by two-stage self-action pressure-head switch, and accuracy was ±150 Pa. During the process of experiments, the external layers of still that were kept in vacuum, were covered with heat insulating material in order to intensify effectiveness. First, the binary system mixture was enclosed in EC-2 equilibrium still and system pressure was kept at 101.33 kPa. Then, it was slowly heated till the solution boils. After two hours, gas chromatogram was used to detect compositions of both phases at set intervals. It was believed that vapor and liquid phases achieved balance when their compositions did not further change. Corresponding temperature were noted down and samples for liquid and vapor were encased in refrigerator after being extracted. Three samples were taken in each equilibrium point, and the mean values of three samples were regarded as final VLE data. Series of VLE data were obtained when compositions of mixture of binary system were changed at 101.33 kPa.
3 RESULTS
3.1 Experimental results
The results of VLE data were displayed in Table 2 and the corresponding phase diagram was shown in Fig. 2. The boiling point of 1,2-epoxycyclohexane was 404.65 K at 101.33 kPa. This was in accordance with 404.85 K that was previously reported in Ref. [8].The boiling point of 2-cyclohexen-1-one was 445.35 K. It was very close to 445.00 K that was in Ref. [9]. From Fig. 2, it also can be seen that this system has no azeotropic point, which indicates that there is an ordinary deviation with ideal system in this imperfect compatible system.

Table 2 Vapor-liquid equilibrium data of 1,2-epoxycyclohexane (1)—2-cyclohexen-1-one (2) binary system at 101.33 kPa
 Chinese Journal of Chemical Engineering2009年1期
Chinese Journal of Chemical Engineering2009年1期
- Chinese Journal of Chemical Engineering的其它文章
- Modeling and Optimization for Scheduling of Chemical Batch Processes*
- Simulation of Droplet-gas Flow in the Effervescent Atomization Spray with an Impinging Plate*
- Numerical Investigation of Constructal Distributors with Different Configurations*
- The Kinetics of the Esterification of Free Fatty Acids in Waste Cooking Oil Using Fe2(SO4)3/C Catalyst
- Multiple Model Soft Sensor Based on Affinity Propagation, Gaussian Process and Bayesian Committee Machine*
- Measurement and Correlation of Solid-Liquid Equilibria of Phenyl Salicylate with C4 Alcohols
