Facile Preparation of Danazol Nanoparticles by High-Gravity Anti-solvent Precipitation (HGAP) Method*
ZHAO Hong (赵宏), WANG Jiexin (王洁欣), ZHANG Haixia (张海霞), SHEN Zhigang (沈志刚),, Jimmy Yun (甄崇礼)and CHEN Jianfeng (陈建峰),**
Facile Preparation of Danazol Nanoparticles by High-Gravity Anti-solvent Precipitation (HGAP) Method*
ZHAO Hong (赵宏)1, WANG Jiexin (王洁欣)1, ZHANG Haixia (张海霞)1, SHEN Zhigang (沈志刚)1,2, Jimmy Yun (甄崇礼)2and CHEN Jianfeng (陈建峰)1,**
1Sin-China Nano Technology Center, Key Lab for Nanomaterials, Ministry of Education, Beijing University of Chemical Technology, Beijing 10029, China2Nanomaterials Technology Pte. Ltd., Singapore
The nanoparticles of the hydrophobic drug of danazol with narrow size distribution are facilely prepared by controlled high-gravity anti-solvent precipitation (HGAP) process. Intensified micromixing and uniform nucleation environment are created by the high-gravity equipment (rotating packed bed) in carrying out the anti-solvent precipitation process to produce nanoparticles. The average particle size decreases from 55 µm of the raw danazol to 190 nm of the nanoparticles. The Brunauer-Emmett-Teller (BET) surface area sharply increases from 0.66 m2·g-1to 15.08 m2·g-1. Accordingly, the dissolution rate is greatly improved. The molecular state, chemical composition, and crystal form of the danazol nanoparticles remains unchanged after processing according to Fourier transform infrared (FTIR) and X-ray diffraction (XRD). The high recovery ratio and continuous production capacity are highly appreciated in industry. Therefore, the HGAP method might offer a general and facile platform for mass production of hydrophobic pharmaceutical danazol particles in nanometer range.
high-gravity antisolvent precipitation, rotating packed bed; danazol, nanoparticles, dissolution rate
1 INTRODUCTION
The fact that considerable proportion of drugs are poorly-water-soluble [1, 2] presents serious obstacles to the successful development and commercialization of the pharmaceutical industry. For many poorly-water- soluble drugs, the dissolution rate is an important limitation factor for the drug absorption rate, which is of great significance to its bioavailability. Dissolution kinetics is also the primary driving force behind the improved pharmacokinetic properties of poorly-water- soluble compounds [3]. Therefore, the dissolution rate is crucial for achieving appropriate blood-levels of these drugs. Many techniques have been developed to cope with the issue of slight aqueous solubility. One of the most facile and effective methods is to obtain particles with size in nanometer range to achieve enlarged surface area, thereby increasing the dissolution rate [4-6]. In principle, pharmaceutical nanoparticles are usually gained either by top-down method, which means reducing large particles to desired small size range, or by bottom-up method, which means growing particles from molecular state to a desired size range. Conventional pathways of the top-down method are mechanical techniques grounded on high shear or impaction including microfluidization, high-pressure homogenization, and milling [3, 7, 8]. However, these techniques are usually inefficient because of high-energy input or pharmaceuticals contamination and denaturation [9]. Bottom-up pathways are usually precipitation- based processes, such as spray drying [10, 11] and supercritical fluids techniques [12-14], which have received a particular emphasis on directly preparing particles with an optimal size but without pharmaceuticals denaturation. Among these technologies, the method of liquid anti-solvent precipitation (LASP) has promising properties, especially from an industrial viewpoint, such as its low cost, convenience in processing, as well as the ease for scale-up [15-17]. Preparation of ultrafine particles by this method relies on the supersaturation generated by the introducing of the liquid anti-solvent. However, the particle size of product by LASP process in conventional stirred tank is always of broad size distribution because of the un-uniform distribution of the spatial concentration caused by the poor micromixing within.
Various methods and equipments of process intensification are being developed to gain excellent micromixing in precipitation process in order to gain nanoparticles [18-23]. The high-gravity technology, which implemented by rotating packed bed (RPB) equipment, is prestigious for its massive production and easy scale-up for industrialization [22, 24, 25]. HGAP method has successfully developed to prepare nanoparticles of cefuroxime axetil and salbutamol sulfate [26]. Expanding the HGAP method into new pharmaceutical categories and ascertaining the optimal operation parameter is a significant work from the industrial viewpoint. In this study, high-gravity technology was used to create an environment of uniform spatial concentration distribution on the molecular scale of the solution by highly intensified micromixing to perfectly perform the LASP method to produce uniform nanoparticles. This facile strategy avoids the chemical reaction and the introduction of the additives.
The selected model drug, danazol (the molecular structure is shown in Fig. 1.), is a typical highly hydrophobic drug that belongs to the category of synthetic steroid for the treatment of endometriosis and hereditary angioedema,. The objective of this article is to use the controlled HGAP process to generate uniform hydrophobic pharmaceutical nanoparticles (.. danazol) without any chemical additives. The commercially attractive merits of controlled HGAP method lie on the facile industrial operating parameters, no scale-up effects, and high continuous production capacity [24].
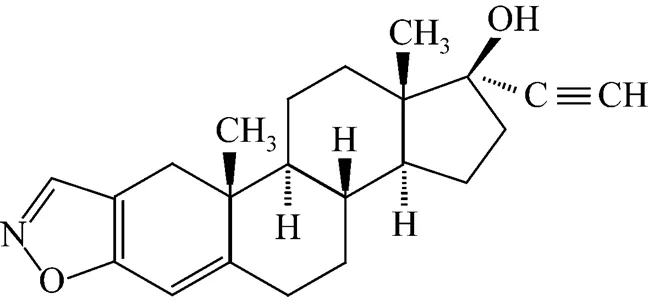
Figure 1 Molecular structure of danazol
2 MATERIALS AND METHODS
2.1 Materials and equipment
The raw danazol powder was purchased from Beijing Zizhu Tiangong Science and Technology Co. Ethanol (A.R. Grade) was supplied by Beijing Chemical Reagents Company. The experimental setup for the high-gravity anti-solvent precipitation is schematically displayed in Fig. 2. The key part of the RPB (high-gravity machine) is a packed rotator (Table 1), which could be referenced to our previous work for more details [22].
2.2 Preparation of danazol nanoparticles
Raw danazol was firstly dissolved in ethanol at 20°C and then filtrated through a 0.45 μm nylon filter to remove the solid impurities. Afterward, this solution (S) with a concentration of 25.8 mg·ml-1was placed in container 8 and kept at 20°C to avoid re-crystallization. Deionized water as the antisolvent was stored in container 9. The flow rates of the solution and antisolvent were 75 ml·min-1and 1500 ml·min-1, respectively. The two liquid streams from 8 and 9 containers were pumped through the flowmeters, and then sprayed onto the inside edge of the rotatorthe distributors, and mixed in packed bed zone. Nanoparticles were generated simultaneously when the two streams of solution and anti-solvent encountered. The rotating speed of the RPB was set at 2880 r·min-1, generating the centrifugal force with a high gravity environment of about 400(“” represents the gravitation acceleration, 9.81 m·s-2). The as-obtained danazol suspension was collected through the outlet and then centrifuged. Finally, the filtrate cake was dried at 60°C for 24 h to gain dry danazol nanoparticles.
2.3 The morphology and particle size studies
The particle size and the morphology of danazol were examined by a scanning electron microscope (SEM) of Cambridge S250MK3 (Cambridge Instruments Inc., U.K.). The danazol dry powder was fixed on a copper stubs using double-sided adhesive tapes. The sample on stub was sputter-coated with gold within a vacuum environment. The particle size distribution (PSD) was analyzed using the software of Image-Pro 5.1 (Media Cybernetics, Inc. USA.) according to the existing SEM images.
2.4 Chemical composition comparison
Fourier transform infrared (FT-IR) spectra of the raw danazol and danazol nanoparticles were recorded with a Nicolet 8700 (Thermo Electron, USA) spectrometer in the range 400-4000 cm-1using a resolution of 2 cm-1and 32 scans. Samples were diluted with KBr mixing powder at 1% and pressed to obtain self-supporting disks.
2.5 X-ray diffraction studies
X-ray diffraction (XRD) analysis was performed using XRD-6000 (SHIMADZU Inc., Japan) to detect the physical characteristics and crystallinity of the danazol particles. The measuring unit consists of a rotating anode in transmission technique and with the following specifications: Cu Kαradiation generated at 30 mA and 40 kV. The scanning speed is 10(°)·min-1from 5°-60° with a step size of 0.02°.
2.6 Specific surface area measurement
An ASAP 2010-M surface area analyzer (Micromeritics Instrument Corporation, American) was used to detect the specific surface area of raw danazol and the nanoparticles. Calculation was based on the Brunauer-Emmett-Teller (BET) equation. The samples were degassed for 4 h before measurement.

Figure 2 (a) Schematic illustration of the high gravity anti-solvent precipitation set-up; (b) Structure of the RPB; (c) Illustration of the fluid pattern of the LASP process within RPB
1—packed rotator; 2—casing; 3—motor; 4—pump; 5—outlet; 6—centrifuge; 7—flowmeters; 8—storage containers of antisolvent (AS); 9—solution (S); 10—liquid distributors

Table 1 RPB parameters in this study

Figure 3 SEM images of the raw danazol, danazol nanoparticles and the corresponding histogram of the particle size distribution
2.7 Concentration determination
An ultraviolet spectrophotometer (UV-3000, Shimadzu, Japan) was used here to determine the concentration of danazol solution. UV scanning of danazol aqueous solution yielded a stable absorption peak at 286 nm. All the subsequent UV analyses were set at 286 nm as the detection wavelength. Absorbance calibrations were conducted prior to any analysis. A series of danazol solutions were used and the corresponding empty solutions were used as references. An excellent correlation (2>0.999) indicated the Beer-Lambert law was obeyed in the danazol concentration ranges of interest.
2.8 Dissolution rate test
Dissolution rate tests were carried out following the CP (China Pharmacopoeia) Apparatus 2 (paddle) method (D-800LS, Tianjin). The paddle speed and bath temperature were set at 80 r·min-1and (37.0±0.5)ºC, respectively. The dissolution medium was the mixture of 2/5 volume of 0.1% (mol·L-1) HCl aqueous solution and 3/5 volume of isopropyl alcohol. The dissolution rate tests were conducted at sink conditions. 50 mg of raw danazol and as-prepared nanosized danazol particles were added into two vessels containing 1000 ml dissolution medium, respectively. 5 ml aliquot was taken each time at specific time intervals and filtered though a 0.45 µm filter. The as-obtained supernate was assayed by the UV to determine the dissolved drug in the medium.
3 RESULTS AND DISCUSSION
3.1 Preparation of danazol nanoparticles
The SEM images [Figs. 3 (a) and 3(b)] showed the morphologies of the raw danazol particles and the as-prepared nanoparticles. The width of the particles was defined as the specific particle size for assay and comparison. According to the particle size distribution histogram [Fig. 3 (c)], it can be seen that the average sizes of the raw danazol particles and the danazol nanoparticles were 55 μm and 190 nm, respectively. The particle sizes of more than 90% of the as-prepared danazol nanoparticles were tightly distributed within 100-300 nm. The average particle size was markedly reduced to only 1/280 of the original after the HGAP process.

3.2 Effects of AS/S ratio on the ultimate product

3.3 Chemical composition and physical characteristics studies
FT-IR and XRD (Figs. 5 and 6) were conducted to study the chemical composition and physical characteristics of the raw danazol and the nanoparticles by RPB. Comparison of FT-IR results using Omnic software showed that the peak position of the danazol nanoparticles matched that of the raw danazol very well, demonstrating that there were no changes in composition induced by the HGAP process. The same peak positions in XRD pattern (Fig. 6) with broader full width at half maximum (FWHM) values of the peaks of the nanoparticles indicated the smaller particle size and the same crystal form.
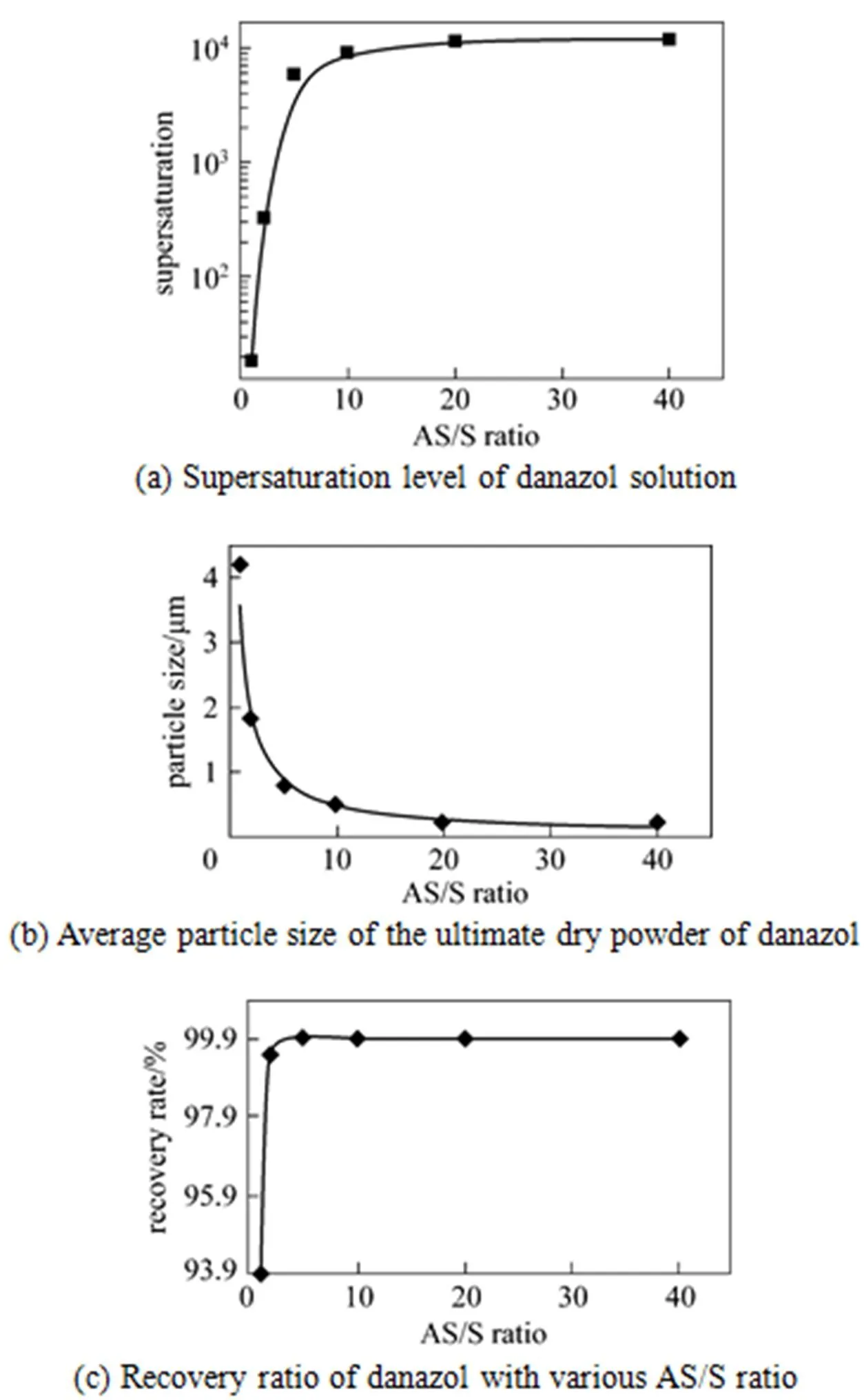
Figure 4 Profiles of the supersaturation level of danazol solution, the average particle size of the ultimate dry powder of danazol and the recovery ratio of danazol with various AS/S ratios
Figure 5 FT-IR spectra of the raw danazol and danazol nanoparticles

Figure 6 XRD pattern of the raw danazol and danazol nanoparticles
3.4 Dissolution rate test
Danazol is a class II drug, which is pooly water- soluble but highly permeable through the biomembrane, according to the Biopharmaceutics Classification System (BCS) [31]. The dissolution rate is the rate-limiting step of the total bioavailability of this drug. Therefore, the enhancement of the dissolution rate is of great significance for the bioavailability. The dissolution rate is in direct proportion to the surface area of the particle, whereas, it is in inverse proportion to the diffusion layer thickness around the particle. Reduction of the particle size will enlarge the surface area and decrease the diffusion layer thickness of the particle, which will be helpful to the enhancement of the dissolution rate. Fig. 7 exhibits the dissolution rate profiles of the raw danazol and danazol nanoparticles. After HGAP process, the average particle size decreased to 190 nm and the specific surface area increased to 15.08 m2·g-1. In this test, within 3 min, the dissolution rate of the as-prepared danazol nanoparticles reached 88%, whereas, that of the raw danazol is only 34% in the same period. The dissolution rate of the danazol nanoparticles was very close to 100% after 6 min. However, it cost about 60 min for the raw danazol to reach its 100% dissolution rate. Since there were no surfactants in raw or nanosized danazol, the nanonization process contributed to the rapid dissolution rate of danazol.

Figure 7 Dissolution rate profiles of the raw danazol and danazol nanoparticles
◆ particles by RPB;■ particles of raw danazol
4 CONCLUSIONS
In this study, danazol nanoparticles were successfully prepared by using the high-gravity anti-solvent precipitation (HGAP) process with no additives. The as-prepared danazol nanoparticles had an average size of approximately 190 nm with a narrow particle size distribution (PSD) from 100 to 300 nm. The specific surface area reached 15.08 m2·g-1, which is 22 times as much as that of raw drug. The size of danazol nanoparticles decreased with the increase of AS/S ratio from 1 to 40. The dissolution rate of danazol nanoparticles was significantly enhanced compared with raw drug, which was a result of the small particle size and large specific surface area. XRD and FT-IR results indicated that there was no change in composition and structure of drug nanoparticles after micronization. The drug recovery ratio achieved 99.9%, and the production capacity in lab scale rotating packed bed (RPB) achieved 116.1 g·h-1with continuous operation. Therefore, the HGAP process can be a feasible and effective pathway to prepare danazol nanoparticles for commercial mass production.
1 Lipinski, C., “Poor aqueous solubility—An industry wide problem in drug delivery”,..., 5, 82-85 (2002).
2 Radtke, M., “Pure drug nanoparticles for the formulation of poorly soluble drugs”,, 3, 62-68 (2001).
3 Liversidge, G.G., Cundy, K.C., “Particle size reduction for improvement of oral bioavailability of hydrophobic drygs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs”,..., 125, 91-97 (1995).
4 Rawlings, E.A., Tindall, B., Bentley’s Textbook of Pharmaceutics, Baillière Tindall London (1977).
5 Hu, J.H., Rogers, T.L., Brown, J., Young, T., Johnston, K.P., Williams, R.O., “Improvement of dissolution rates of poorly water soluble APIs using novel spray freezing into liquid technology”,.., 19, 1278-1284 (2002).
6 Rasenack, N., Müller, B.W., “Dissolution rate enhancement bymicronization of poorly water-soluble drugs”,.., 19, 1894-900 (2002).
7 Ripple, E.G., Powders: Remington’s Pharmaceutical Sciences, 17th Edition, Gennaro, A.R., eds., Mack Publishing Co., Easton, PA (1985).
8 Liversidge, G.G., Conzentino, P., “Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats”,..., 125, 309-313 (1995).
9 Vivek, K., Meenakshi, B., Harish, D., Deepak, K., “Nanoparticle technology for the delivery of poorly water-soluble drugs”,.., 30 (2), 82-92 (2006).
10 Esclisa-Díaz, M.T., Guimaraens-Méndez, M., Pérez-Marcos, M.B., Vila-Jato, J.L., Torres-Labandeira, J.J., “Characterization anddissolution behaviour of ketoconazole/β- and 2-hydroxypropyl-β- cyclodextrin inclusion compounds”,..., 143, 203-210 (1996).
11 Elversson, J., Millqvist-Fureby, A., Alderborn, G., Elofsson, U., “Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying”,..., 92, 900-910 (2003).
12 Domingo, C., Berends, E., van Rosmalen, G.M., “Precipitation of ultrafine organic crystals from the rapid expansion of supercritical solutions over a capillary and a frit nozzle”,.., 10, 39-55 (1997).
13 Reverchon, E., Porta, G.D., “Production of antibiotic micro- and nano-particles by supercritical anti-solvent precipitation”,., 106, 23-29 (1999).
14 Chattopadhyay, P., Gupta, R.B., “Production of griseofulvin nanoparticles using supercritical CO2antisolvent with enhanced mass transfer”,..., 228, 19-31 (2001).
15 Violanto, M.R., Fischer, H.W., “Method for making uniformly sized particles from water-insoluble organic compounds”, US Pat., 4826689 (1989).
16 Ruch, F., Matijević, E., “Preparation of micrometer size Budesonide particles by precipitation”,..., 229, 207-211 (2000).
17 Cushing, B.L., Kolesnichenko, V.L., O’Connor, C.J., “Recent advances in the liquid-phase syntheses of inorganic nanoparticles”,.., 104, 3893-3946 (2004).
18 Mersmann, A., “Crystallization and precipitation”,..., 38, 345-353 (1999).
19 Grenman, H., Murzina, E., Ronnholm, M., Eranen, K., Mikkola, J.P., Lahtinen, M., Salmi, T., Murzin, D.Y., “Enhancement of solid dissolution by ultrasound”,..., 46, 862-869 (2007).
20 Rao, D.P., Bhowal, A., Goswami, P.S., “Process intensification in rotating packed beds (higee): An appraisal”,...., 43, 1150-1162 (2004).
21 Akay, G., Tong, L., Addleman, R., “Process intensification in particle technology: intensive granulation of powders by thermomechanically induced melt fracture”,...., 41, 5436-5446 (2002).
22 Chen, J.F., Wang, Y.H., Guo, F., Wang, X.M., Zheng, C., “Synthesis of nanoparticles with novel technology: high gravity reactive precipitation”,...., 39, 948-954 (2000).
23 Hessel, V., Lowe, H., Schonfeld, F., “Micromixers—A review on passive and active mixing principles”,..., 60, 2479-2501 (2005).
24 Chen, J.F., Shao, L., “Mass production of nanoparticles by high gravity reactive precipitation technology with low cost”,, 1, 64-69 (2003).
25 Chen, J.F., Zhou, M.Y., Shao, L., Wang, Y.Y., Yun, J., Chew, N.Y., Chan, H.K., “Feasibility of preparing nanodrugs by high-gravity reactive precipitation”,..., 269, 267-274 (2004).
26 Hu, T.T., Wang, J.X., Shen, Z.G., Chen, J.F., “Engineering of drug nanoparticles by HGCP for pharmaceutical applications”,, 6, 239-251 (2008).
27 Yang, H.J., Chu, G.W., Zhang, J.W., Shen, Z.G., Chen, J.F., “Micromixing efficiency in a rotating packed bed: Experiments and simulation”,...., 44, 7730-7737 (2005).
28 Kucher, M., Babic, D., Kind, M., “Precipitation of barium sulfate: Experimental investigation about the influence of supersaturation and free lattice ion ratio on particle formation”,..., 45, 900-907 (2006).
29 Horn, D., Rieger, J., “Organic nanoparticles in the aqueous phase-theory, experiment, and use”,....., 40, 4330-4361 (2001).
30 Dirksen, J.A., Ring, T.A., “Fundamentals of crystallization: kinetic effects on particle size distributions and morphology”,..., 46, 2389-2427 (1991).
31 Lobenberg, R., Amidon, G.L., Modern bioavailability, “bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards”,...., 50, 3-12 (2000).
2008-09-03,
2008-11-11.
the National High Technology Research and Development Program of China (2006AA030202) and the Talent Training Program of Beijing (2007B022).
** To whom correspondence should be addressed. E-mail: chenjf@mail.buct.edu.cn
 Chinese Journal of Chemical Engineering2009年2期
Chinese Journal of Chemical Engineering2009年2期
- Chinese Journal of Chemical Engineering的其它文章
- Kinetics of Reactive Extraction of Nd from Nd2O3 with TBP-HNO3Complex in Supercritical Carbon Dioxide*
- Activity Coefficient Models to Describe Vapor-Liquid Equilibrium in Ternary Hydro-Alcoholic Solutions*
- Position Group Contribution Method for Predicting the Normal Boiling Point of Organic Compounds
- Experimental Study on the Initial Position Distribution of Taylor Bubbles in Cryogenic Upward Inclined Tubes*
- Enhancement of Proton Exchange Membrane Fuel Cell Performance Using a Novel Tapered Gas Channel*
- Tert-butylation of Toluene with Tert-butyl Alcohol over Realuminated H-mordenite Zeolite*
