氢吗啡酮对脂多糖诱导小鼠肺泡巨噬细胞高尔基体应激影响
[摘要]目的探讨氢吗啡酮(HM)对脂多糖(LPS)诱导的小鼠肺泡巨噬细胞高尔基体应激的影响及其机制。
方法以10 mg/L LPS处理小鼠肺泡巨噬细胞系MH-S,构建肺泡巨噬细胞高尔基体应激模型。将细胞随机分为Control组(A组,正常培养)、LPS组(B组,给予10 mg/L的LPS)、LPS+HM组(C组,给予1 μmol/L HM和10 mg/L LPS)、Nrf2 siRNA+LPS+HM组(D组,Nrf2 siRNA转染后给予1 μmol/L HM和10 mg/L LPS)和NC siRNA+LPS+HM组(E组,NC siRNA转染后给予1 μmol/L HM和10 mg/L LPS)。采用ELISA法检测细胞上清液白细胞介素1β(IL-1β)和白细胞介素6(IL-6)含量;检测丙二醛(MDA)含量与超氧化物歧化酶(SOD)活性;采用Western blot法检测核因子E2相关因子2(Nrf2)、血红素加氧酶-1(HO-1)、高尔基体基质蛋白130(GM130)、高尔基体蛋白97(Golgin-97)、甘露糖苷酶Ⅱ(Mannosidase Ⅱ)、钙转运ATP酶2C型成员1(ATP2C1)蛋白表达水平;透射电镜下观察细胞高尔基体形态学改变。
结果与A组比较,B组IL-1β、IL-6、MDA含量显著升高及Nrf2、HO-1蛋白表达上调,SOD活性显著降低及GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1蛋白的表达下调(F=2.33~47.61,P<0.05);与B组比较,C组IL-1β、IL-6、MDA含量显著降低,SOD活性显著升高及Nrf2、HO-1、GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1蛋白表达上调(P<0.05);与C组比较,D组IL-1β、IL-6、MDA含量显著升高,SOD活性显著降低及Nrf2、HO-1、GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1蛋白表达下调(P<0.05)。透射电镜显示,C组细胞高尔基体损伤、空泡化与碎片化较B组减轻;D组细胞高尔基体损伤、空泡化与碎片化较C组加重。
结论HM通过调控Nrf2/HO-1信号通路抑制LPS诱导的肺泡巨噬细胞高尔基体应激,减轻细胞炎症与氧化应激反应。
[关键词]氢吗啡酮;脂多糖类;巨噬细胞,肺泡;高尔基体;氧化性应激;血红素加氧酶-1;小鼠
[中图分类号]R965
[文献标志码]A
[文章编号]2096-5532(2024)04-0523-05doi:10.11712/jms.2096-5532.2024.60.135
[开放科学(资源服务)标识码(OSID)]
[网络出版]https://link.cnki.net/urlid/37.1517.R.20240927.1331.002;2024-09-2908:36:49
Effect of hydromorphone on Golgi stress in lipopolysaccharide-attacked mouse alveolar macrophages
LI Shaona, JIA Changxin, WU Xiuyun, ZHU Youzhuang, ZHAO Qin, XU Yexiang
(Department of Anesthesiology, The Affiliated Hospital of Qingdao University, Qingdao 266000, China)
; [Abstract]ObjectiveTo investigate the effect of hydromorphone (HM) on Golgi stress in lipopolysaccharide (LPS)-attacked mouse alveolar macrophages and its mechanism.
MethodsThe mouse alveolar macrophage cell line MH-S was attacked with 10 mg/L LPS to establish a model of Golgi stress, and then the cells were randomly divided into control group (group A, normal culture), LPS group (group B treated with 10 mg/L LPS), LPS+HM group (group C treated with 1 μmol/L HM and 10 mg/L LPS), Nrf2 siRNA+LPS+HM group (group D treated with 1 μmol/L HM and 10 mg/L LPS after Nrf2 siRNA transfection), and NC siRNA+LPS+HM group (group E treated with 1 μmol/L HM and 10 mg/L LPS after NC siRNA transfection). ELISA was used to measure the content of interleukin 1β (IL-1β) and interleukin 6 (IL-6) in cell supernatant; the content of malondialdehyde (MDA) and the activity of superoxide dismutase (SOD) were measured; Western blot was used to measure the protein expression levels of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), Golgi Matrix Protein of 130 (GM130), Golgin-97, Mannosidase Ⅱ, and ATP2C1; transmission electron microscopy was used to observe the morphological changes of Golgi apparatus.
ResultsCompared with group A, group B had significant increases in the content of IL-1β, IL-6, and MDA and the protein expression levels of Nrf2 and HO-1 and significant reductions in the activity of SOD and the protein expression levels of GM130, Golgin-97, Mannosidase Ⅱ, and ATP2C1 (F=2.33-47.61,P<0.05). Compared with group B, group C had significant reductions in the content of IL-1β, IL-6, and MDA and significant increases in the activity of SOD and the protein
expression levels of Nrf2, HO-1, GM130, Golgin-97, Mannosidase
Ⅱ, and ATP2C1 (P<0.05). Compared with group C, group D had significant increases in the content of IL-1β, IL-6, and MDA and significant reductions in the activity of SOD and the protein expression levels of Nrf2, HO-1, GM130, Golgin-97, Mannosidase Ⅱ, and ATP2C1 (P<0.05). Transmission electron microscopy showed that compared with group B, group C had alleviation of 76S6jeEz77S5xcTvvFmbFA==the damage, vacuolization, and fragmentation of Golgi apparatus, and compared with group C, group D had aggravation of the da-
mage, vacuolization, and fragmentation of Golgi apparatus.
ConclusionHM inhibits Golgi stress in LPS-attacked alveolar macrophages by regulating the Nrf2/HO-1 signaling pathway, thereby alleviating cellular inflammation and oxidative stress response.
[Key words]hydromorphone; lipopolysaccharides; macrophages, alveolar; Golgi apparatus; oxidative stress; heme oxyge-
nase-1; mice
内毒素急性肺损伤(ALI)是一种由脓毒症诱发的严重且难以控制的肺部炎症反应,目前尚无特效的临床治疗方法,是重症监护室病人死亡的主要原因之一[1]。肺泡巨噬细胞作为肺组织抵御致病微生物和外源性颗粒的第一道防线,在维持机体免疫系统稳态和调控炎症反应方面发挥着至关重要的作用[2]。高尔基体是肺泡巨噬细胞内的一个细胞器,主要参与脂质和蛋白质等物质的细胞内运输。高尔基体应激反应可影响肺组织细胞的功能状态,从而对ALI的发病和病程产生重要影响[3]。作为机体重要的内源性保护机制之一,核因子E2相关因子2(Nrf2)/血红素加氧酶-1(HO-1)信号通路的激活可抑制过度的高尔基体应激反应,从而为ALI提供保护[4]。氢吗啡酮(HM)是吗啡的衍生物,由于其作用强于吗啡且起效快,近年来被广泛用于各种急慢性疼痛的综合治疗。研究证实,HM对ALI有保护作用[5],但其作用机制尚不清楚。本研究旨在探讨HM是否能通过调控Nrf2/HO-1信号通路减轻肺泡巨噬细胞的高尔基体应激反应,从而为ALI的临床治疗提供新的依据。
1材料与方法
1.1实验材料
1.1.1细胞株本实验所用小鼠肺泡巨噬细胞系MH-S,购自通派(上海)生物科技有限公司。
1.1.2药品与试剂盐酸HM注射液(宜昌人福药业有限公司);脂多糖(LPS,Sigma公司);白细胞介素1β(IL-1β)和白细胞介素6(IL-6)试剂盒(北京索莱宝科技有限公司);丙二醛(MDA)试剂盒与超氧化物歧化酶(SOD)试剂盒(南京建成生物工程研究所);Nrf2抗体和高尔基体基质蛋白130(GM130)抗体(上海优宁维生物科技股份有限公司);HO-1抗体和高尔基体蛋白97(Golgin-97)抗体(北京友谊中联生物科技有限公司);钙转运ATP酶2C型成员1(ATP2C1)和甘露糖苷酶Ⅱ(Mannosidase Ⅱ)抗体(上海拓然生物科技有限公司);Nrf2 siRNA、Negative Control siRNA(NC siRNA,上海吉玛制药技术有限公司);Lipofectamine 3000转染试剂盒(美国Invitrogen公司)。
1.1.3仪器电泳仪和转膜槽(北京六一生物科技有限公司);全自动化学/荧光/凝胶成像分析系统(中国雪科电器有限公司);H-7500型透射电镜(日本HITACHI公司)。
1.2实验方法
1.2.1细胞培养和建模根据文献方法[6],用MH-S细胞培养液在37 ℃、体积分数0.05 CO2条件下培养MH-S细胞。选择生长状态良好的MH-S细胞,用10 mg/L LPS处理细胞,构建LPS诱导小鼠肺泡巨噬细胞模型。
1.2.2细胞的分组与处理将培养的MH-S肺泡巨噬细胞分为Control组(A组)、LPS组(B组)、LPS+HM组(C组)、Nrf2 siRNA+LPS+HM组(D组)和NC siRNA+LPS+HM组(E组)。A组细胞正常培养,C组在实验前2 h加入1 μmol/L HM,D组和E组肺胞巨噬细胞在实验前48 h分别转染Nrf2 siRNA和NC siRNA,并在实验前2 h加入1 μmol/L HM。B、C、D、E组分别在实验0 h加入10 mg/L LPS建立LPS诱导MH-S细胞模型。经LPS作用24 h后,收集各组细胞及其上清液进行相关实验检测。
1.2.3ELISA法检测细胞培养上清液IL-1β、IL-6含量根据文献方法[7],收集各组细胞培养上清液,1 000 r/min离心15 min。采用ELISA法检测各组细胞培养上清液中IL-1β和IL-6的含量,严格按照ELISA试剂盒说明书进行操作,使用酶标仪在波长450 nm处测定吸光度(A)值,绘制标准曲线,计算IL-1β和IL-6的含量。
1.2.4细胞MDA含量与SOD活性检测收集各组细胞,用PBS冲洗,1 000 r/min离心10 min,倒掉上清液,留取细胞沉淀,加入等渗PBS制备细胞悬液,用二奎琳甲酸(BCA)法测定蛋白浓度,应用MDA试剂盒和SOD试剂盒测定细胞中MDA含量和SOD活性,严格按照试剂盒说明书操作。
1.2.5Western blot法测定蛋白表达将RIPA裂解液加入含有细胞的六孔板中,用刮刀轻轻刮掉细胞并吸入离心管内,置于冰上裂解15 min。将离心管上下混匀,置于离心机内低温(4 ℃)12 000 r/min离心20 min,去上清液用BCA法测定蛋白浓度,计算上样量。每份样品取40 μg,95 ℃变性10 min,加入凝胶孔中,将电压调至60 V进行电泳,然后转移至PVDF膜上。将反应膜置于封闭液中,37 ℃封闭1 h,然后将反应膜分别放入稀释后的一抗中,4 ℃反应过夜。用TBST洗涤3次以清除游离的一抗,每次洗涤10 min,再放入稀释的二抗中缓慢摇动60 min,用TBST洗涤3次以清除游离的二抗。将反应膜浸于ECL发光液中,室温下反应3 min。使用全自动化学/荧光/凝胶成像分析系统成像并用Image J软件对条带进行灰度值分析,计算目的蛋白灰度值与内参蛋白β-actin灰度值的比值,对各组目的蛋白的表达情况进行统计分析。
1.2.6透射电镜观察细胞高尔基体的形态学变化
根据文献方法[8],将各组细胞用刮刀刮下,置于离心机中1 000 r/min离心10 min,弃上清液,留取细胞沉淀,加入25 g/L戊二醛固定,将细胞制片,将切片浸入醋酸铀中染色20 min,随后浸入枸橼酸铅中继续染色5 min,将染色后的切片置于单口铜网中,在透射电镜下观察细胞高尔基体形态学变化。
1.3统计学分析
使用GraphPad Prism 9.0软件进行统计学分析。所得计量资料数据以±s表示,多组比较采用单因素方差分析,组间两两比较采用LSD-t检验。P<0.05表示差异具有统计学意义。
2结果
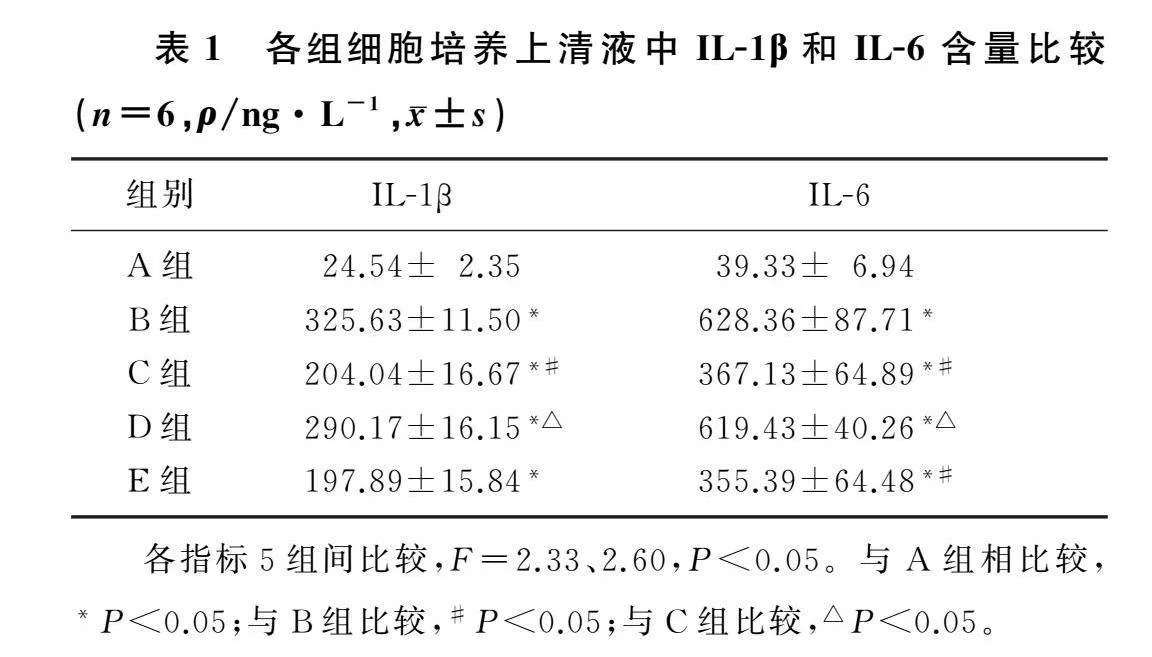
2.1各组MH-S细胞培养上清液中IL-1β和IL-6含量比较
本文5组IL-1β和IL-6含量比较,差异均有显著性(F=2.33、2.60,P<0.05)。与A组比较,B组IL-1β和IL-6含量显著增加(P<0.05);与B组比较,C组IL-1β和IL-6含量显著降低(P<0.05);与C组比较,D组IL-1β和IL-6含量显著增加(P<0.05);C组与E组比较,IL-1β和IL-6含量差异无统计学意义(P>0.05)。见表1。
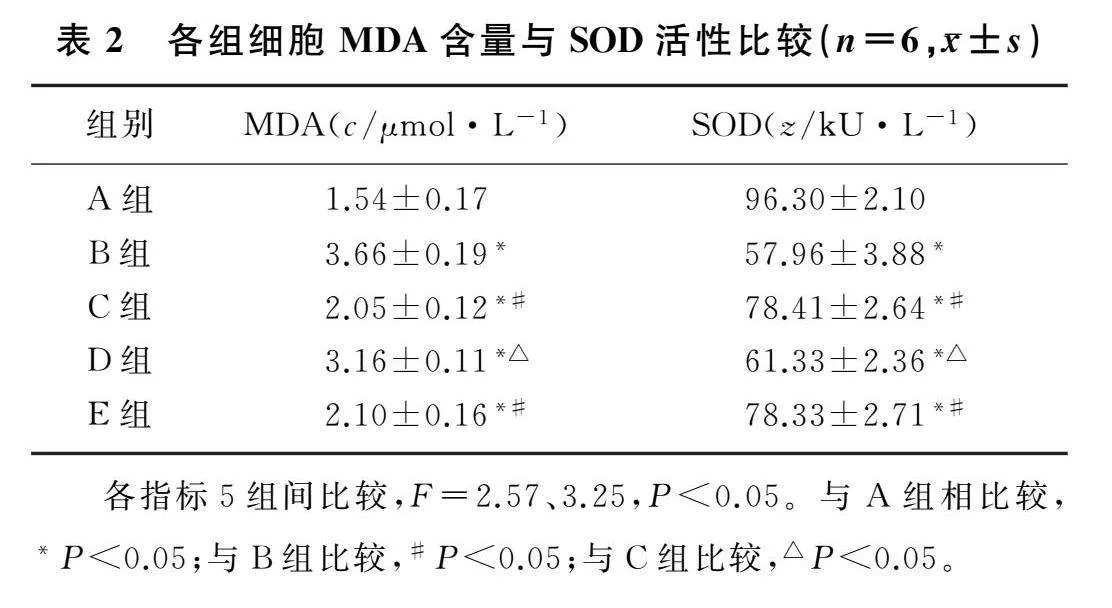
2.2各组MH-S细胞MDA含量和SOD活性比较
本文5组MDA含量和SOD活性比较,差异均有统计学意义(F=2.57、3.25,P<0.05)。与A组比较,B组细胞MDA含量显著增加,SOD活性显著下降(P<0.05);与B组比较,C组细胞MDA含量显著降低,SOD活性显著上升(P<0.05);与C组比较,D组细胞MDA含量显著增加,SOD活性显著下降(P<0.05);C组与E组比较,MDA含量与SOD活性差异无统计学意义(P>0.05)。见表2。
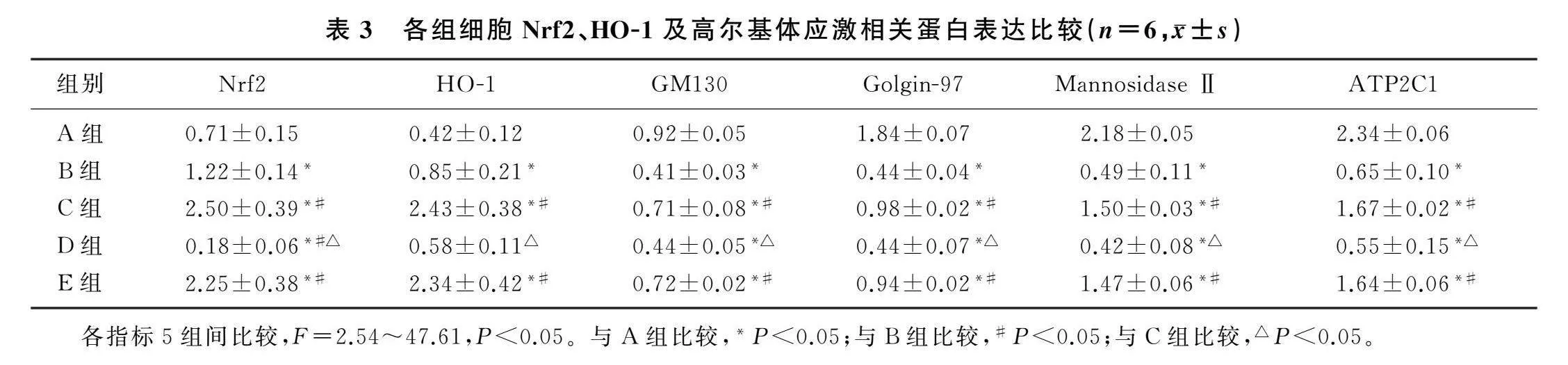
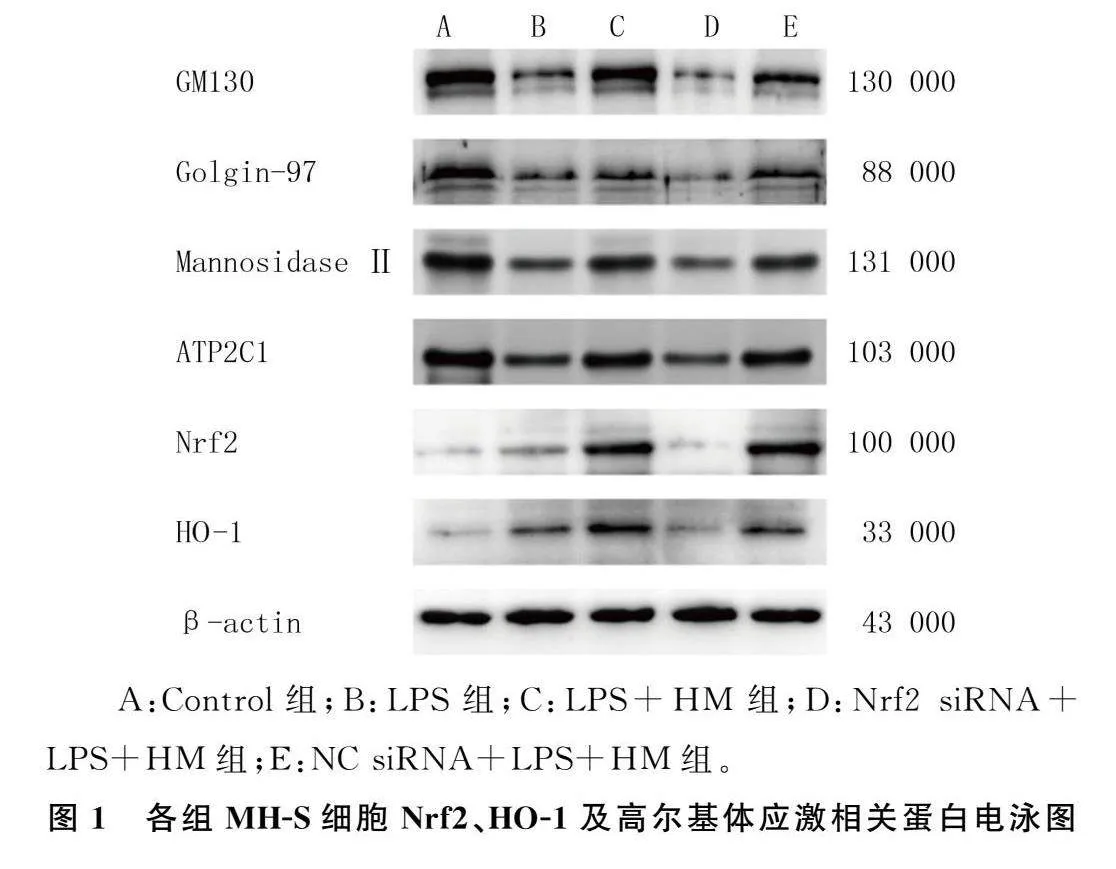
2.3各组MH-S细胞Nrf2、HO-1及高尔基体应激相关蛋白表达比较
本文5组细胞Nrf2、HO-1、GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1蛋白表达比较,差异有统计学意义(F=2.54~47.61,P<0.05)。与A组比较,B组细胞Nrf2、HO-1蛋白的表达显著上调,GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1蛋白表达显著下调(P<0.05);与B组比较,C组细胞Nrf2、HO-1、GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1蛋白表达显著上调(P<0.05);与C组相比较,D组细胞Nrf2、HO-1、GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1蛋白表达水平显著下调(P<0.05);C组与E组比较,Nrf2、HO-1、GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1蛋白表达差异无统计学意义(P>0.05)。见图1、表3。
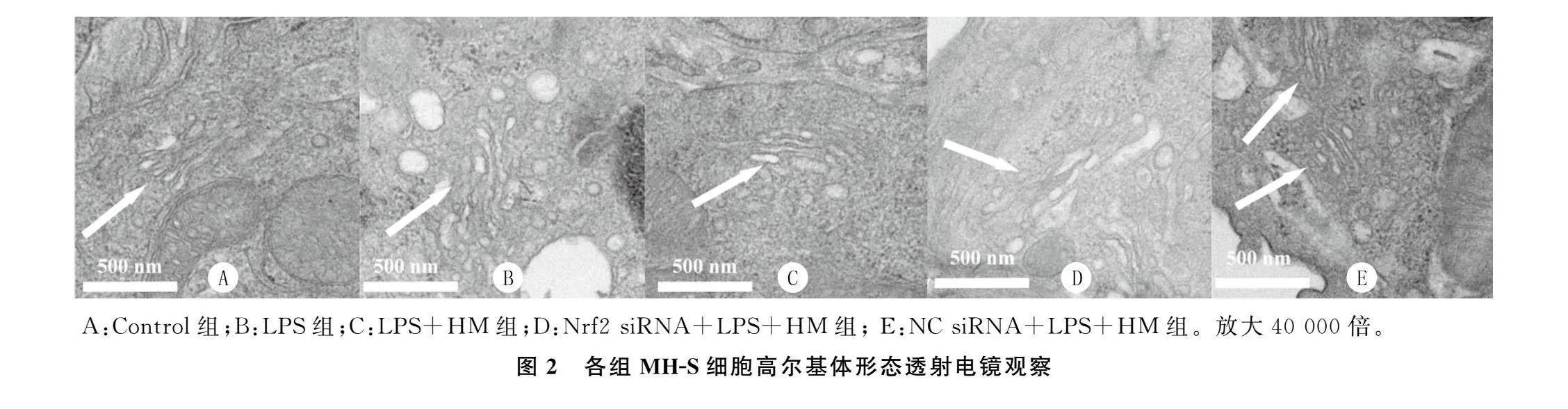
2.4各组MH-S细胞高尔基体形态学变化比较
A组细胞高尔基体形态学正常,排列致密;其余
4组均可见不同程度的高尔基体损伤、空泡化与碎片化。与B组比较,C组细胞高尔基体损伤减轻,空泡化与碎片化减轻;与C组比较,D组细胞高尔基体损伤程度加重,空泡化与碎片化均加重;C组与E组细胞高尔基体损伤程度类似。见图2。
3讨论
肺泡巨噬细胞是肺内主要的抗原反应细胞,在受到内毒素等炎症介质刺激后,会迅速激活一系列信号通路,包括Nrf2/HO-1信号通路、核因子κB和丝裂原激活的蛋白激酶信号通路,进而引发一系列生化反应[9]。高尔基体应激是指当细胞在受到外界刺激时,高尔基体功能紊乱,产生大量未折叠蛋白而引发的细胞应激反应[10]。其主要特点包括刺激源多样、反应迅速、涉及多种信号通路等[11]。适当的高尔基体应激可通过增加抗氧化酶活性、增加细胞壁厚度、激活细胞自噬与凋亡等机制保护细胞免受内毒素损伤,但是过度的高尔基体应激会导致细胞蛋白质合成和降解失衡、物质运输障碍,甚至细胞死亡,因此对高尔基体应激的调控至关重要[12]。
GM130是位于高尔基体顺面的一种基质蛋白[13];Golgin-97是高尔基体特异性连接蛋白,主要位于高尔基体反面[14];Mannosidase Ⅱ位于高尔基体中间体,参与糖蛋白的合成[15];ATP2C1是一种高尔基体特异性Ca2+/Mn2+ATP酶,对维持高尔基体功能至关重要[16]。因此,本研究选择GM130、Golgin-97、Mannosidase Ⅱ、ATP2C1作为高尔基体应激的标志物。本文研究结果显示,给予MH-S肺泡巨噬细胞10 mg/L LPS刺激24 h后,LPS组细胞培养上清液中炎症因子IL-1β和IL-6的表达水平显著升高,细胞MDA含量增加,SOD活性降低,高尔基体应激相关蛋白GM130、Golgin-97、Mannosidase Ⅱ和ATP2C1表达下调。
Nrf2/HO-1信号通路激活是生物体内重要的内源性防御机制,有助于细胞抵抗氧化应激损伤。Nrf2是一种转录因子,当细胞受到氧化应激等刺激时,Nrf2从细胞质转移至细胞核,并与DNA上的启动子区域结合,促进HO-1等抗氧化基因的表达,进而生成一氧化碳、胆绿素和亚铁离子,发挥清除氧自由基等抗氧化作用[17]。HM是一种阿片类镇痛药,通过作用于阿片受体和抑制疼痛信号传递发挥镇痛作用[18]。此外,HM还具有抗炎作用,其机制
可能与抑制炎症因子和前列腺素合成、调节免疫细胞活化和增殖有关,但具体作用机制尚未研究清楚[19]。本文研究结果显示,HM抑制了LPS诱导的MH-S细胞的高尔基体应激反应,上调了Nrf2和HO-1的蛋白表达,减轻了细胞的炎症与氧化应激反应。当应用siRNA技术敲减Nrf2基因后,HM对肺泡巨噬细胞的保护作用被部分逆转,并伴随着细胞Nrf2和HO-1蛋白表达的下调。
综上所述,HM通过调控Nrf2/HO-1信号通路减轻高尔基体应激反应,从而减轻LPS诱导的MH-S肺DoZsDd57xKN584Sbug+J8zoeR/uPfaEtzOqF1Xx4akk=泡巨噬细胞的炎症与氧化应激反应。本实验的局限性在于HM对肺泡巨噬细胞的作用机制仅在离体细胞水平得到验证,尚需进一步的动物实验和临床试验研究,从而为HM的临床应用提供理论依据。
[参考文献]
[1]LAI K, SONG C K, GAO M L, et al. Uridine alleviates sepsis-induced acute lung injury by inhibiting ferroptosis of macrophage[J]. International Journal of Molecular Sciences, 2023,24(6):5093.
[2]MA Y, WANG Z X, WU X Y, et al. 5-Methoxytryptophan ameliorates endotoxin-induced acute lung injury in vivo and in vitro by inhibiting NLRP3 inflammasome-mediated pyroptosis through the Nrf2/HO-1 signaling pathway[J]. Inflammation Research: Official Journal of the European Histamine Research Society, 2023,72(8):1633-1647.
[3]KIM W K, CHOI W, DESHAR B, et al. Golgi stress response: new insights into the pathogenesis and therapeutic targets of human diseases[J]. Molecules and Cells, 2023,46(4):191-199.
[4]LI S N, XU Y X, HE S M, et al. Tetramethylpyrazine ame-
liorates endotoxin-induced acute lung injury by relieving Golgi stress via the Nrf2/HO-1 signaling pathway[J]. BMC Pulmonary Medicine, 2023,23(1):286.
[5]SHI J, DU S H, YU J B, et al. Hydromorphone protects against CO2 pneumoperitoneum-induced lung injury via heme oxygenase-1-regulated mitochondrial dynamics[J]. Oxidative Medicine and Cellular Longevity, 2021,2021:9034376.
[6]WU X Y, WU L L, WU Y, et al. Heme oxygenase-1 ameliorates endotoxin-induced acute lung injury by modulating macrophage polarization via inhibiting TXNIP/NLRP3 inflammasome activation[J]. Free Radical Biology & Medicine, 2023,194: 12-22.
[7]ZHANG J, LI J, AN Z Z, et al. Hydromorphone mitigates cardiopulmonary bypass-induced acute lung injury by repres-
sing pyroptosis of alveolar macrophages[J]. Shock, 2023,60(1):92-99.
[8]SHI J, YU T X, SONG K, et al. Dexmedetomidine ameliorates endotoxin-induced acute lung injury in vivo and in vitro by preserving mitochondrial dynamic equilibrium through the HIF-1a/HO-1 signaling pathway[J]. Redox Biology, 2021,41: 101954.
[9]BETTER J, ESTIRI M, MATT U. Cultured mouse alveolar macrophages: a new step toward targeted cell therapy?[J]. American Journal of Respiratory Cell and Molecular Biology, 2022,66(1):3-4.
[10]VIETTRI M, ZAMBRANO J L, ROSALES R, et al. Flavivirus infections induce a Golgi stress response in vertebrate and mosquito cells[J]. Scientific Reports, 2021,11(1):23489.
[11]OH-HASHI K, HASEGAWA T, MIZUTANI Y, et al. Elucidation of brefeldin A-induced ER and Golgi stress responses in Neuro2a cells[J]. Molecular and Cellular Biochemistry, 2021,476(10):3869-3877.
[12]MENG S Q, LIU J F, WANG Z W, et al. Inhibition of Golgi stress alleviates sepsis-induced cardiomyopathy by reducing inflammation and apoptosis[J]. International Immunopharmacology, 2024,133: 112103.
[13]MEI M, BAO S L. Generation of GM130 conditional knockout mouse[J]. Methods in Molecular Biology (Clifton, N J), 2023,2557:61-81.
[14]GRIMALDI G, FILOGRANA A, SCHEMBRI L, et al. PKD-dependent PARP12-catalyzed mono-ADP-ribosylation of Golgin-97 is required for E-cadherin transport from Golgi to plasma membrane[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022,119(1):e2026494119.
[15]ARMSTRONG Z, KUO C L, LAHAV D, et al. Manno-epi-cyclophellitols enable activity-based protein profiling of human α-mannosidases and discovery of new Golgi mannosidase Ⅱ inhibitors[J]. Journal of the American Chemical Society, 2020,142(30):13021-13029.
[16]ZONFRILLI A, TRUGLIO F, SIMEONE A, et al. Loss of ATP2C1 function promotes trafficking and degradation of NOTCH1: Implications for Hailey-Hailey disease[J]. Experimental Dermatology, 2023,32(6):787-798.
[17]LIU X J, LV Y F, CUI W Z, et al. Icariin inhibits hypoxia/reoxygenation-induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1 signaling pathway[J]. FEBS Open Bio, 2021,11(11):2966-2976.
[18]WANG Y H, LIU Y, LIU J T, et al. Coadministration of curcumin and hydromorphone hydrochloride alleviates postoperative pain in rats[J]. Biological & Pharmaceutical Bulletin, 2022,45(1):27-33.
[19]Hydromorphone. In: LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2020.
(本文编辑牛兆山)

