腹部CT扫描定量腹腔积液对重症急性胰腺炎的预测
[摘要]目的评估定量腹腔积液预测急性胰腺炎(AP)严重程度的可靠性。
方法收集2018-2021年我院收治的273例AP病人,评估病人腹腔积液的发生情况并定量计算腹腔积液。收集病人的床旁急性胰腺炎严重程度指数(BISAP)评分、急性生理与慢性健康评估Ⅱ(APACHE Ⅱ)评分、CT严重程度指数(CTSI)。采用受试者工作特征曲线下面积(AUC)分析腹腔积液量及不同评分系统对AP严重程度的早期预测价值。
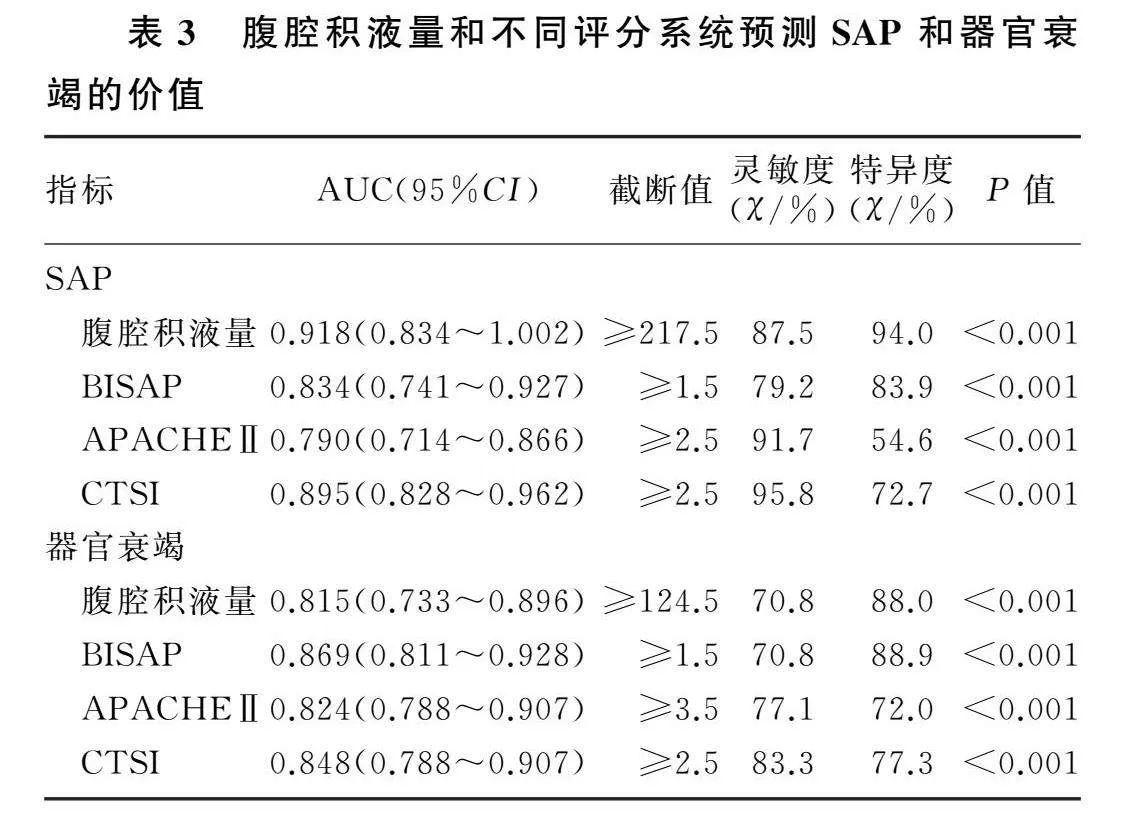
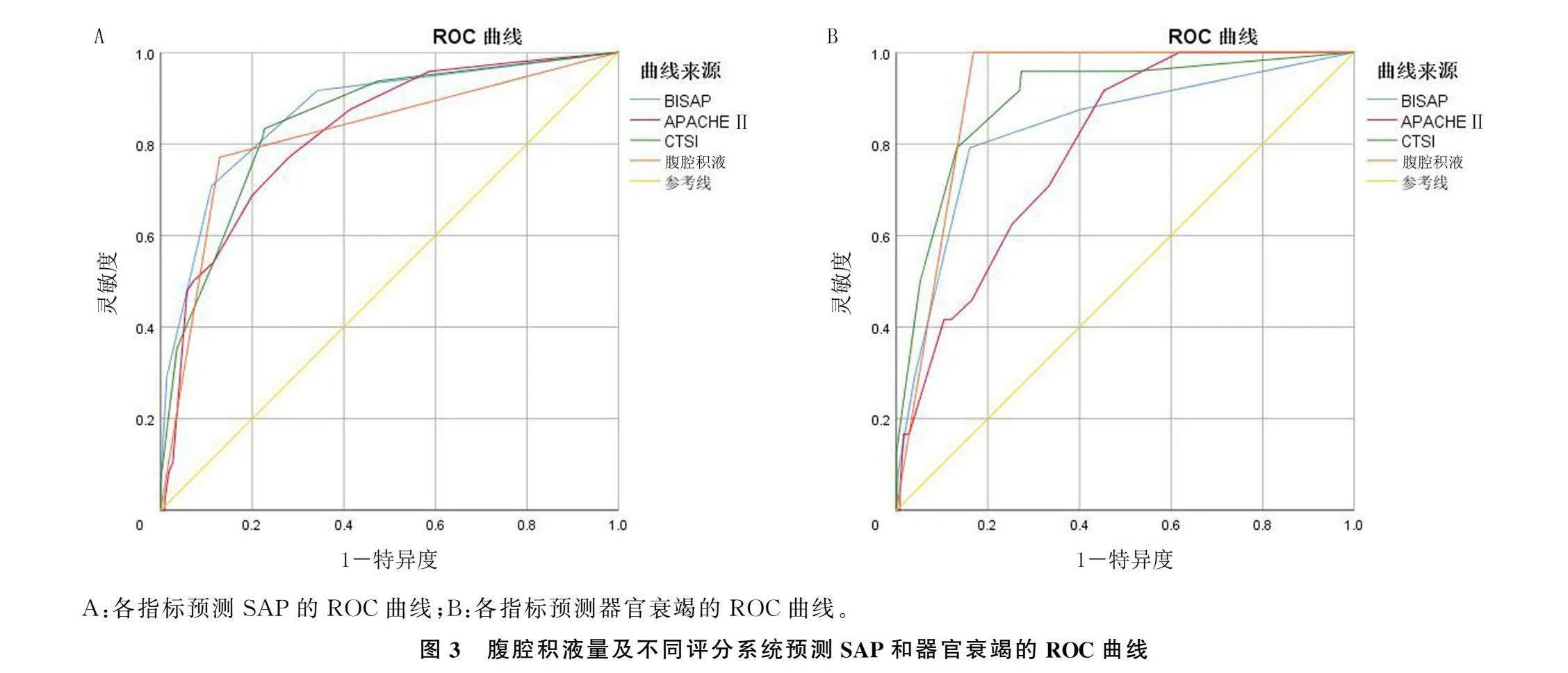
结果273例AP病人的腹腔积液发生率为23%。腹腔积液量与AP严重程度(χ2=35.88~156.21,P<0.001)及器官衰竭(t=5.00~12.14,P<0.001)有关,与各评分系统之间存在正相关(r=0.546~0.716,P<0.001)。腹腔积液量预测重症急性胰腺炎(SAP)的AUC、灵敏度、特异度分别为0.918、87.5%、94.0%;预测器官衰竭的AUC、灵敏度、特异度分别为0.815、70.8%、88.0%。腹腔积液量预测SAP的AUC高于BISAP评分、APACHE Ⅱ评分(Z=2.101、3.159,P<0.05)。
结论腹腔积液量与AP严重程度相关,与临床评分系统呈正相关,定量腹腔积液可用于早期预测SAP及器官衰竭的发生,且预测SAP的效能高于临床评分系统。
[关键词]胰腺炎;腹水;体层摄影术,X线计算机;器官衰竭;预测
[中图分类号]R576;R442.5
[文献标志码]A
[文章编号]2096-5532(2024)04-0518-05doi:10.11712/jms.2096-5532.2024.60.139
[开放科学(资源服务)标识码(OSID)]
[网络出版]https://link.cnki.net/urlid/37.1517.r.20240930.0936.002;2024-09-3015:22:38
Value of peritoneal effusion quantitatively measured by abdominal computed tomography in predicting severe acute pancreatitisSONG Zhimin, CUI Jiufa, ZHU Qingyun, HU Bin, PAN Xinting(Emergency Intensive Care Unit, The Affiliated Hospital of Qingdao University, Qingdao 266003, China); [Abstract]ObjectiveTo investigate the reliability of quantitative measurement of peritoneal effusion in predicting the severity of acute pancreatitis (AP).
MethodsA total of 273 patients with AP who were admitted to our hospital from 2018 to 2021 were enrolled to evaluate the onset of intra-abdominal fluid and quantitatively calculate the amount of peritoneal effusion. Related data were collected, including Bedside Index for Severity in Acute Pancreatitis (BISAP) score, Acute Physiology and Chronic Health Evaluation Ⅱ (APACHE Ⅱ) score, and Computed Tomography Severity Index (CTSI). The receiver operating characte-
ristic (ROC) curve and the area under the ROC curve (AUC) were used to investigate the value of peritoneal effusion volume and different scoring systems in the early prediction of the severity of AP.
ResultsThe incidence rate of peritoneal effusion was 23% among the 273 AP patients. Peritoneal effusion volume was associated with the severity of AP (χ2=35.88-156.21,P<0.001) and organ failure (t=5.00-12.14,P<0.001) and was positively correlated with various scoring systems (r=0.546-0.716,P<0.001). Peritoneal effusion volume had an AUC of 0.918, a sensitivity of 87.5%, and a specificity of 94.0% in predicting severe acute pancreatitis (SAP) and an AUC of 0.815, a sensitivity of 70.8%, and a specificity of 88.0% in predicting organ failure. Peritoneal effusion volume had a significantly larger AUC than BISAP score and APACHE Ⅱ score in predicting SAP (Z=2.101,3.159;P<0.05).
ConclusionPeritoneal effusion volume is associated with the severity of AP and is positively correlated with clinical scoring systems. Quantitative measurement of peritoneal effusion can be used to predict the onset of SAP and organ failure in the early stage, with a better efficacy than clinical scoring systems in predicting SAP.
[Key words]pancreatitis; ascites; tomography, X-ray computed; organ failure; forecasting
急性胰腺炎(AP)是常见的消化系统疾病,该病给病人带来多种局部或全身并发症[1-3]。AP的严重程度主要取决于全身炎症反应引起的器官衰竭及其持续时间[4-5]。早期重症急性胰腺炎(SAP)和器官衰竭对AP的危险分层尤为重要。临床上主要常用床旁急性胰腺炎严重程度指数(BISAP)评分[6-7]、急性生理和慢性健康检查Ⅱ(APACHEⅡ)评分[8]来评估和预测AP病人的严重程度,但APACHEⅡ评分最初用于重症监护病房病人病情的评估,而且它与BISAP评分系统需要大量的临床数据,在评估
SAP方面存在局限性[9-10],在临床中应用有限。腹部CT常用于诊断AP并确定AP的范围,具有较高的准确性和敏感性,CT严重程度指数(CTSI)可以评估AP的严重程度,特别是AP的局部并发症[9],然而造影剂可能会加重胰腺炎的病情[11]。腹腔积液作为AP的并发症之一,其中含有大量细胞因子、血管活性物质、胰酶等毒性物质,这些毒性物质引起的连锁反应可加重AP的病情[12-14]。本研究基于腹部CT图像评估AP病人腹腔积液的发生率,以及BISAP评分系统、APACHE Ⅱ评分系统和CTSI评分系统,分析腹腔积液量与AP严重程度及器官衰竭的关系。
1资料与方法
1.1研究对象
收集2018年1月—2021年12月我院连续收治的273例AP病人的临床资料。AP的诊断参照文献[15-16]。纳入标准:①急性发病;②在早期进行腹部CT检查;③临床资料完整;④年龄在18~80岁的住院病人。排除标准:①既往存在腹腔积液病史;②慢性胰腺炎。临床基础数据包括:住院时间、入院48 h BISAP评分、APACHEⅡ评分和Marshall评分等。APACHEⅡ评分分级为轻度(0~7分)和重度(≥8分)[9,16],BISAP评分分级为轻度(0~2分)和重度(≥3分)[17]。AP严重程度分级根据Atlanta分级(RAC分级)[18],其中器官功能障碍的诊断标准基于改进的Marshall评分系统[1]。本研究经医院伦理委员会批准,病人均签署知情同意书。
1.2CT检查
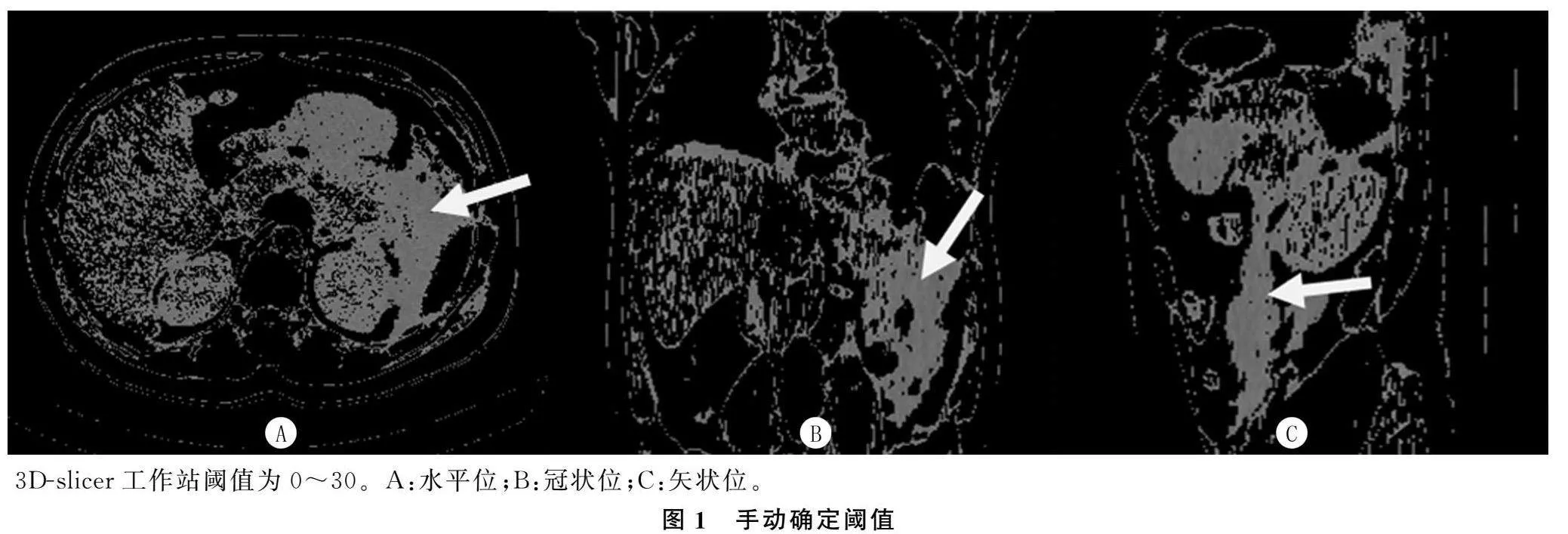
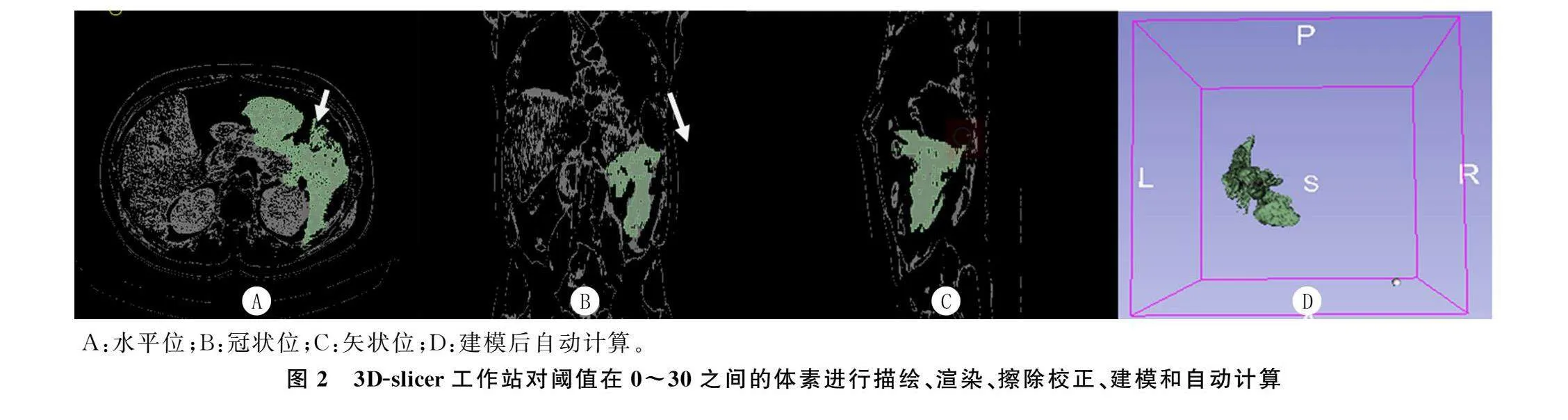
所有病人均使用CT扫描仪(东芝医疗系统Activion 16或西门子Somatom Definition Edge)进行CT检查。腹部CT检查的参数如下:120 kVp、200 mAs,探测器配置为64 mm×0.6 mm,矩阵 512×512,截面厚度5.0 mm,标准重建算法。扫描范围:膈肌圆顶至髂骨嵴。增强造影剂(碘普罗胺)的流量为3~5 mL/s。其中CTSI分级为轻度(0~3分)、中度(4~6分)和重度(7~10分)[19]。两名不知临床结果的放射科医生对图像进行分析,必要时在协商一致的情况下对图像进行校正。使用CT图像后处理站或3D-slicer(3D Slicer image computing platform|3D Slicer)[20-21]等开源软件,标记CT切片中所需测量区域的粗略轮廓,腹腔积液定义为该轮廓CT值在“0~30”之间的所有体素[22]。对所需体素进行渲染后,通过软件进行建模,自动计算体积。见图1、2。
1.3统计分析
采用SPSS 26.0统计学软件对数据进行统计分析。计数资料以例数(%)表示,组间比较采用χ2检验。计量资料以±s表示,组间比较采用两独立
样本t检验。指标间的相关性检验采用Spearman相关分析。采用受试者工作特征(ROC)曲线下面积(AUC)预测疾病的严重程度。以P<0.05为差异有显著性。
2结果
2.1一般情况
本研究共纳入273例病人,平均年龄(48.6±16.5)岁;其中男性174例,平均年龄(46.2±16.1)岁;女性99例,平均年龄(52.8±16.4)岁。AP原因:胆道113例(41.4%),高脂65例(23.8%),乙醇30例(11.0%),其他65例(23.8%)。AP程度:轻度180例(65.9%),中度69例(25.3%),重度24例(8.8%)。APACHEⅡ评分平均为(3.17±2.82)分(0~12分),其中轻度237例(86.8%),重度36例(13.2%)。BISAP评分平均为(0.73±0.97)分(0~4分),其中分级轻度256例(93.8%),重度17例(6.2%)。CTSI评分平均为(2.36±2.72)分(0~10分),其中轻度184例(67.4%),中度64例(23.4%),重度25例(9.2%)。平均住院时间(8.88±6.10)d(1~40 d)。其中器官衰竭48例(17.6%),死亡1例(0.4%)。
2.2腹腔积液量与各评分系统以及住院时间的相关性
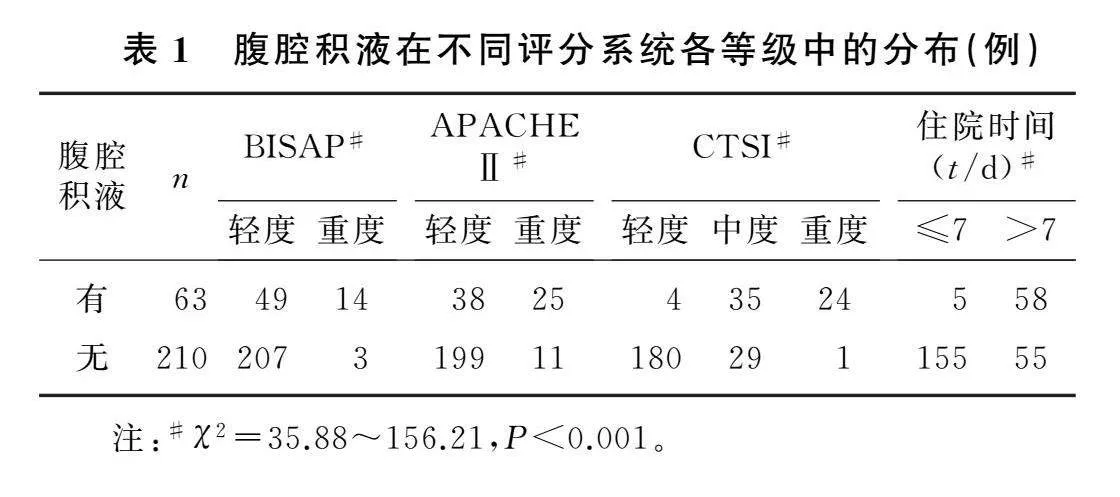
本文273例AP病人中,63例观察到腹腔积液,平均积液量为210 mL。腹腔积液量与CTSI评分(r=0.716,P<0.001)、BISAP评分(r=0.635,P<0.001)、APACHEⅡ评分(r=0.546,P<0.001)以及住院时间(r=0.657,P<0.001)呈正相关。
2.3不同评分系统各等级中腹腔积液的发生情况
不同评分系统各等级中腹腔积液的发生情况差异有统计学意义(χ2=35.88~156.21,P<0.001)。见表1。
2.4腹腔积液量与SAP和器官衰竭的关系
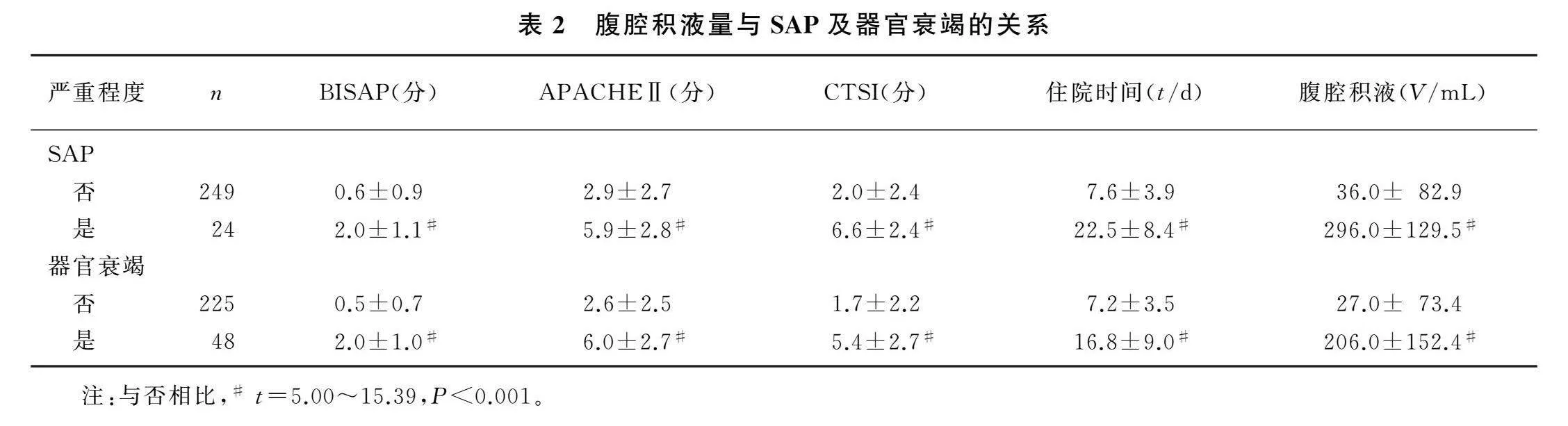
非SAP病人(即轻、中症AP病人)腹腔积液量低于SAP病人(t=9.65,P<0.01),BISAP评分、APACHEⅡ评分、CTSI评分和住院时间也低于SAP病人(t=5.00~15.39,P<0.001)。无器官衰竭病人腹腔积液量低于有器官衰竭病人(t=12.13,P<0.01),BISAP评分、APACHEⅡ评分、CTSI评分和住院时间也低于有器官衰竭病人(t=9.75~12.14,P<0.001)。见表2。
2.5腹腔积液量及不同评分系统对SAP和器官衰竭的预测价值
腹腔积液量预测SAP的AUC、灵敏度、特异度分别为0.918、87.5%和94.0%,预测器官衰竭的AUC、灵敏度、特异度分别为0.815、70.8%、88.0%。腹腔积液量预测SAP的AUC高于BISAP评分和APACHE Ⅱ评分(Z=2.101、3.159,P<0.05)。见图3、表3。
3讨论
AP作为世界上常见的疾病之一,其发病率在世界范围内呈上升趋势[23],其病死率在10%~15%之间[24],特别是SAP的病死率更可高达30%~50%[25]。早期、快速识别其病情严重程度及死亡风
险具有重要意义。腹腔积液作为AP的并发症之一,其中含有大量细胞因子、血管活性物质、胰酶等毒性物质,这些毒性物质引起的连锁反应是导致SAP早期多器官功能障碍综合征和死亡的重要原因之一[12-14,26],但通过定量检测腹腔积液来早期预测AP的严重程度的研究极少。
本研究结果表明,腹腔积液作为AP的并发症之一,随着AP的加重及器官衰竭的加重而加重,腹腔积液量也进一步增加,这与文献报道的腹腔积液可作为判断SAP的指标[26]、是死亡率的重要预测因子[14]的结果相符。
本研究结果表明,腹腔积液量与现存的AP评分系统具有相关性,且较现存评分系统具有更高的准确性,这与SAMANTA等[14]的研究相似。对于预测SAP和器官衰竭,腹腔积液量为217.5 mL或更高时,对SAP的预测特异度(94.0%)高于BISAP评分、APACHEⅡ评分和CTSI评分;腹腔积液量为124.5 mL或者更高时,对器官衰竭的预测的特异度(88.0%)高于APACHEⅡ评分和CTSI评分。本研究所采用的腹腔积液定量方法避免了造影剂在AP早期应用的危害[27],也避免了大量收集临床数据带来的偏倚[16]。腹腔积液的存在易于观察,积液量可用影像操作系统直接或间接检测,获取方便。腹腔积液的定量检测对于判断AP病情严重程度具有重要意义。
本文研究还存在一些局限性。本文分析中选择的是早期进行腹部CT检查的病人,可能存在选择偏倚。腹腔积液量与临床预后相关实验指标(如C反应蛋白水平、白细胞计数等)的关系在本研究未涉及,还需要进一步研究验证。
综上所述,AP病人腹部CT中常见的腹腔积液量与AP的严重程度相关,与器官衰竭的严重程度相关。定量测量腹腔积液量可作为SAP和器官衰竭的早期预测指标。
[参考文献]
[1]GARG P K, SINGH V P. Organ failure due to systemic injury in acute pancreatitis[J]. Gastroenterology, 2019,156(7):2008-2023.
[2]BICZO G, VEGH E T, SHALBUEVA N, et al. Mitochond-
rial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models[J]. Gastroenterology, 2018,154(3):689-703.
[3]SZATMARY P, GRAMMATIKOPOULOS T, CAI W H, et al. Acute pancreatitis: diagnosis and treatment[J]. Drugs, 2022,82(12):1251-1276.
[4]CHEN C Y, HUANG Z X, LI H, et al. Evaluation of extrapancreatic inflammation on abdominal computed tomography as an early predictor of organ failure in acute pancreatitis as defined by the revised Atlanta classification[J]. Medicine, 2017,96(15):e6517.
[5]COLVIN S D, SMITH E N, MORGAN D E, et al. Acute pancreatitis: an update on the revised Atlanta classification[J]. Abdominal Radiology, 2020,45(5):1222-1231.
[6]COLUOGLU I, COLUOGLU E, BINICIER H C, et al. The role of the BISAP score in predicting acute pancreatitis severity
according to the revised Atlanta classification: a single tertiary care unit experience from Turkey[J]. Acta Gastro-Enterologica Belgica, 2021,84(4):571-576.
[7]HAGJER S, KUMAR N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis-A prospective observational study[J]. International Journal of Surgery, 2018,54(Pt A):76-81.
[8]LARVIN M, MCMAHON M J. APACHE-Ⅱ score for assessment and monitoring of acute pancreatitis[J]. Lancet,1989,2(8656):201-205.
[9]HARSHIT KUMAR A, SINGH GRIWAN M. A comparison of APACHE Ⅱ, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification[J]. Gastroenterology Report, 2018,6(2):127-131.
[10]ZHOU H J, MEI X, HE X H, et al. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: a retrospective study[J]. Medicine, 2019,98(16):e15275.
[11]PENG R, ZHANG L, ZHANG Z M, et al. Chest computed tomography semi-quantitative pleural effusion and pulmonary consolidation are early predictors of acute pancreatitis severity[J]. Quantitative Imaging in Medicine and Surgery, 2020,10(2):451-463.
[12]MAYERLE J, SENDLER M, HEGYI E, et al. Genetics, cell biology, and pathophysiology of pancreatitis[J]. Gastroente-
rology, 2019,156(7):1951-1968.e1.
[13]GE P, LUO Y L, OKOYE C S, et al. Intestinal barrier da-
mage, systemic inflammatory response syndrome, and acute lung injury: a troublesome trio for acute pancreatitis[J]. Biomedecine & Pharmacotherapie, 2020,132:110770.
[14]SAMANTA J, RANA A, DHAKA N, et al. Ascites in acute pancreatitis: not a silent bystander[J]. Pancreatology: Official Journal of the International Association of Pancreatology, 2019,19(5):646-652.
[15]FOSTER B R, JENSEN K K, BAKIS G, et al. Revised Atlanta classification for acute pancreatitis: a pictorial essay-erratum[J]. Radiographics: a Review Publication of the Radiological Society of North America, Inc, 2019,39(3):912.
[16]MEDEROS M A, REBER H A, GIRGIS M D. Acute pancreatitis: a review[J]. JAMA, 2021,325(4):382-390.
[17]ARIF A, JALEEL F, RASHID K. Accuracy of BISAP score in prediction of severe acute pancreatitis[J]. Pakistan Journal of Medical Sciences, 2019,35(4):1008-1012.
[18]BANKS P A, BOLLEN T L, DERVENIS C, et al. Classification of acute pancreatitis: 2012: revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013,62(1):102-111.
[19]ALBERTI P, PANDO E, MATA R, et al. Evaluation of the modified computed tomography severity index (MCTSI) and computed tomography severity index (CTSI) in predicting severity and clinical outcomes in acute pancreatitis[J]. Journal of Digestive Diseases, 2021,22(1):41-48.
[20]FEDOROV A, BEICHEL R, KALPATHY-CRAMER J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network[J]. Magnetic Resonance Imaging, 2012,30(9):1323-1341.
[21]PINTER C, LASSO A, FICHTINGER G. Polymorph segmentation representation for medical image computing[J]. Computer Methods and Programs in Biomedicine, 2019,171:19-26.
[22]KERSCHBAUM M, SCHURR L A, RIEDL M, et al. Clinical value of CT for differentiation between ascites and hemorrhage: an experimental In-vitro study[J]. Journal of Clinical Medicine, 2020,10(1):76.
[23]BOXHOORN L, VOERMANS R, BOUWENSE S, et al. Acute pancreatitis[J]. The Lancet, 2020,396:726-734.
[24]LEE P J, PAPACHRISTOU G I. New insights into acute pancreatitis[J]. Nature Reviews Gastroenterology & Hepatology, 2019,16(8):479-496.
[25]SCHEPERS N J, BAKKER O J, BESSELINK M G, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis[J]. Gut, 2019,68(6):1044-1051.
[26]BUSH N, RANA S S. Ascites in acute pancreatitis: clinical implications and management[J]. Digestive Diseases and Sciences, 2022,67(6):1987-1993.
[27]XIAO B, XU H B, JIANG Z Q, et al. Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging[J]. Quantitative Imaging in Medicine and Surgery, 2019,9(12):1973-1985.
(本文编辑周晓彬)

