记忆辨别力受老化影响的认知神经机制及其应用
曾庆贺 崔晓宇 唐为 李娟
摘 要 记忆辨别力是对相似的记忆经验进行准确辨别的能力, 其依赖于名为模式分离的神经计算机制, 在人类被试中, 通常使用记忆相似性任务对其进行测量与研究。在老化过程中, 老年人的记忆辨别力会出现十分明显的衰退, 这种衰退与海马、内嗅皮层等内侧颞叶脑区的结构和功能衰退以及其他新皮层结构和功能老化关系密切。由于记忆辨别力高度依赖于内侧颞叶的结构和功能完整性, 因此, 它能够反映出认知障碍发展早期的异常脑结构和功能变化, 使得记忆相似性任务具备了应用于认知障碍早期识别的巨大潜力。未来研究需要采用更精细的成像技术单独考察海马齿状回和CA3亚区在记忆辨别中的作用及其老化的影响, 并更多关注前额叶等新皮层结构老化影响记忆辨别力的神经机制, 同时也需要建立大样本前瞻队列来验证记忆相似性任务在认知障碍早期识别中的有效性。
关键词 记忆辨别力, 模式分离, 老化, 认知神经机制, 认知障碍
分类号 B844
1 引言
情景记忆是个体对自己亲身经历的、发生在特定时间或地点的情景或事件的长时记忆, 是保证人们正常生活的重要认知功能(Tulving, 2002)。在日常生活中, 人们每天都会经历一些高度相似的事件, 比如老年人在吃药前常需要回忆今天是否已经吃过药了。可见, 准确区分相似的记忆经验是保证我们正常生活的前提之一。这种准确区分相似记忆经验的功能被称为记忆辨别功能(mnemonic discrimination), 记忆辨别功能高度依赖于名为模式分离(pattern separation)的神经计算机制(Marr, 1971; McClelland et al., 1995; Norman & O'Reilly, 2003; Yassa & Stark, 2011), 该机制是指将高度重叠的记忆信息输入表征为两个独立的、完全分离的神经信号输出。
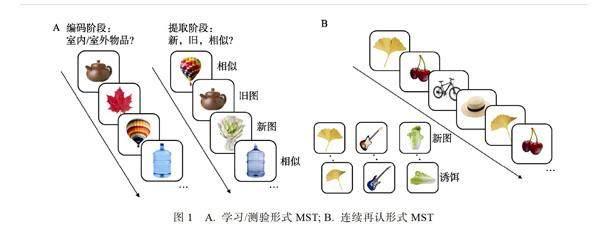
基于模式分离的基本原理, Stark等人开发出了记忆相似性任务(Mnemonic Similarity Task, MST)对记忆辨别功能进行测量与机制研究(Kirwan & Stark, 2007; Stark et al., 2013; Yassa et al., 2010a, 2011a), 其基本内容是要求被试对旧图、新图以及诱饵图片(与学习过的图片相似)进行识别(如图1所示), 其中, 对诱饵进行准确辨别(将诱饵判断为相似)的能力即代表了记忆辨别力(Sinha et al., 2018; Stark et al., 2013; Stark et al., 2019; Yassa et al., 2010a)。近些年, 众多研究者基于物体版MST的基本思路, 设计了空间版本(Granger et al., 2022; Reagh & Yassa, 2014)、场景版本(Berron et al., 2018; Güsten et al., 2021; Maass et al., 2019)、面孔版本(Chang et al., 2015; Stiernstr?mer et al., 2018)、情绪材料版本(Leal et al., 2019; Pagen et al., 2022; Szollosi et al., 2022)、单词版本(Ly et al., 2013)等多种变式任务对不同的问题进行研究, 但这些任务的核心都是对相似刺激进行准确辨别。
内侧颞叶(medial temporal lobe)是支持包括记忆辨别在内的情景记忆功能的关键结构。从解剖结构上看, 内侧颞叶包括海马(hippocampus)、内嗅皮层(entorhinal cortex, EC)、围嗅皮层(perirhinal cortex, PRC)和旁海马皮层(parahippocampal cortex,PHC)等结构, 海马也可以进一步划分为齿状回(dentate gyrus, DG)、海马角1区到4区(cornu ammonis 1-4, CA1-4)以及海马下托(subiculum)等亚区。自神经计算模型提出以来, 大量研究集中于探讨海马, 特别是海马DG对模式分离机制和记忆辨别功能的贡献, 但随着神经影像研究的不断深入, 研究者们逐渐意识到, 记忆辨别功能的实现也需要海马与其他内侧颞叶结构所组成的完整信息加工回路的支持, 以及额叶、顶叶、枕叶等新皮层结构对海马自上而下的调控(Amer & Davachi, 2023)。
随着年龄的增长, 许多认知功能都会出现不同程度的下降(Park & Bischof, 2013; Park et al., 2002), 记忆辨别功能也不例外。有研究者通过MST测量不同年龄被试的记忆辨别力, 结果发现记忆辨别力与年龄之间存在显著负相关, 即记忆辨别力随年龄增长而下降(Riphagen et al., 2020; Stark et al., 2013)。不仅如此, 当老年人出现神经退行性病变并发展为轻度认知障碍(mild cognitive impairment, MCI)或阿尔茨海默症(Alzheimers Disease, AD)时, 其记忆辨别力也会进一步降低(Bakker et al., 2015; Corona-Long et al., 2020; Lalani et al., 2022; Stark et al., 2013; Yassa et al., 2010a)。
目前, 众多研究者对老年人记忆辨别力衰退的认知神经机制进行了深入探讨, 在研究过程中, 研究者们还发现MST在识别认知障碍早期的轻微认知损伤方面具有应用价值。因此, 本文将重点对老年人记忆辨别力下降的认知神经机制研究进行梳理和归纳, 并介绍MST在老年认知障碍早期识别方面的应用, 最后针对当前研究中存在的问题进行讨论并对未来的研究进行展望。
2 记忆辨别的认知神经机制
2.1 记忆辨别的核心机制:以海马为中心的内侧颞叶模式分离
内侧颞叶是情景记忆的加工枢纽。在经过新皮层的处理后, 与物体或内容有关的信息会进入到PRC进行更复杂的加工, 然后被传递到外侧内嗅皮层(lateral entorhinal cortex, LEC), 与空间或位置有关的信息则会进入PHC进行加工, 然后被传递到内侧内嗅皮层(medial entorhinal cortex, MEC), 最终, 由海马对来自LEC和MEC的信息进行整合, 形成完整的情景记忆(Danieli et al., 2023)。作为情景记忆加工中的一个重要环节, 模式分离的实现也依赖于完整的内侧颞叶加工路径。
经典理论认为, 由于海马DG的颗粒细胞数目巨大且远超过向其进行投射的EC细胞数量, 故而DG颗粒细胞能够对来自EC的信息进行稀疏编码(Chawla et al., 2005; Deng et al., 2013), 即通过少数颗粒细胞对输入的信息进行编码。通过这种方式, 相似的事件将由不同的DG颗粒细胞进行表征, 从而实现信息分离表征。DG在模式分离中发挥关键作用已经得到了早期动物研究的证实, 比如, Leutgeb等人(2007)发现, DG颗粒细胞群对微小的环境变化高度敏感, 当环境发生十分微小的变化时, CA3锥体细胞群在两种环境下的放电模式十分相似, 但DG颗粒细胞群在两种环境下的放电模式显著改变(多个放电场不相干地变化), 说明相似的环境是由部分不同的DG颗粒细胞进行响应的, 这一结果在Neunuebel和Knierim (2014)的研究中也得到了验证。除了DG之外, 早期动物研究还发现, 海马CA3亚区也能执行模式分离(Lee et al., 2004; Leutgeb et al., 2004; Vazdarjanova & Guzowski, 2004), 只不过CA3亚区对微小的环境变化做出的响应远不及DG, 只有当环境变化较大时CA3亚区才会表现出模式分离的加工模式(Knierim & Neunuebel, 2016; Neunuebel & Knierim, 2014)。最近, 对猴子进行的研究也为DG/CA3混合区域神经元承担模式分离的观点提供了证据支持(Sakon & Suzuki, 2019)。由此可见, DG和CA3亚区对模式分离均有贡献, 但相比于CA3亚区, DG对微小特征变化更加敏感, 对相似刺激的分离表征主要由DG承担。
在人类研究中, 一方面, 有研究者应用MST对海马损伤患者的记忆辨别力进行测试, 结果发现这些患者的记忆辨别力严重受损(Baker et al., 2016; Hanert et al., 2019; Kirwan et al., 2012), 证明了海马在人类模式分离中的贡献; 另一方面, 研究者使用高分辨率功能磁共振成像(functional magnetic resonance imaging, fMRI)技术对模式分离进行研究, 揭示了DG/CA3亚区在模式分离中的关键性作用。比如, Bakker等人(2008)在MST研究中发现, 旧物体识别时, 海马DG/CA3亚区的活动水平显著低于新物体识别时的活动水平, 而当诱饵出现时, 海马DG/CA3亚区的活动水平则与新物体出现时的活动水平无显著差异, 其他海马亚区并没有出现类似的活动模式, 说明DG/CA3亚区能够按照处理新物体的方式对诱饵进行响应。还有研究者通过操作诱饵的相似性水平, 发现了海马DG/CA3亚区能够将差异较小的信息输入转变为差异较大的信号输出(Lacy et al., 2011; Reagh et al., 2018), 该结果与神经计算模型相吻合(Norman & O'Reilly, 2003), 进一步证实了DG/CA3亚区在模式分离中发挥着关键作用。
除了海马DG/CA3亚区之外, 其他内侧颞叶结构在模式分离中的作用也得到了研究者的关注。有研究显示, PHC能够根据场景特征对空间场景进行分类(Dilks et al., 2022)以及对距离等空间维度的信息进行表征(Baumann & Mattingley, 2021), MEC也能够区分物体的位置并推动海马的空间信息组织(Keene et al., 2016); PRC和LEC则对于感知具有较多重叠特征的物体十分重要, 特别是能够细粒度地表征主观感知到的物体相似程度(Ferko et al., 2022)。可见, PHC、PRC和EC在维持感知的准确性、解决相似信息干扰方面发挥着作用, 那么也可以认为, 高度重叠的记忆信息在进入内侧颞叶之后, 就已经开始进行模式分离了。这一观点已经得到了一定的证据支持, 比如, 有研究发现, 当成功辨别诱饵时, PRC-LEC在物体MST中会表现出更高的激活水平, 与DG/CA3亚区的活动模式相似(Reagh & Yassa, 2014; Stevenson et al., 2020), PHC-MEC则会在空间MST中表现出与DG/CA3亚区相似的活动模式(Reagh & Yassa, 2014)。不过, 根据相似度将诱饵划分为三个不同的水平后, 并没有发现PRC-LEC或PHC-MEC与DG/CA3的活动模式完全一致(Reagh et al., 2018)。对此结果, 研究者认为, 相比于海马DG/CA3亚区, EC与PRC、PHC在区分高度相似诱饵方面的能力较弱(Reagh et al., 2018), 也就是说, 这些脑区能够在一定程度上参与模式分离, 只不过这些脑区的表征更加模糊, 不能实现完全的分离表征(Reagh & Yassa, 2014)。
综上, 模式分离由内侧颞叶主导, 高度重叠的信息在进入海马之前, 首先经历信息的选择, 物体信息进入PRC-LEC通路, 并在此进行精细表征并将重叠的信息分离到一定程度, 空间或场景信息进入PHC-MEC通路并在此经历初步分离, 最终, 经过初步分离的信息进入海马DG/CA3亚区, 被加工成互不重叠的记忆表征(Amer & Davachi, 2023; Reagh & Yassa, 2014)。从模式分离的完整加工路径来看, 其他内侧颞叶结构对相似刺激的预先选择与初步分离加工, 是保证海马高效执行模式分离的基础之一。
2.2 记忆辨别的初始加工环节:知觉表征分离
记忆辨别是建立在知觉辨别的基础上的, 尽管内侧颞叶对于形成能够区分相似刺激的神经表征至关重要(Kent et al., 2016), 但枕叶感觉区在知觉表征分离中发挥的作用同样不容忽视。Bowman等人(2019)发现, 腹侧视觉区的视觉表征非常详细, 甚至可以依据腹侧视觉区的活动信号对目标以及与之高度相似的诱饵进行区分; 也有研究者在MST研究中发现, 诱饵正确辨别时与旧项目正确再认时的枕叶激活水平存在显著差异(Klippenstein et al., 2020), 枕叶感觉区甚至表现出了与海马模式分离相似的神经活动模式(Pidgeon & Morcom, 2016)。以上这些结果说明, 枕叶感觉区能够识别出先前呈现过的刺激的特征变化, 实现知觉分离表征。值得一提的是, 虽然现有研究发现枕叶感觉区的激活水平与记忆辨别力之间不存在显著相关(Klippenstein et al., 2020), 但枕叶却能够影响海马神经活动对行为成绩的预测, 比如Koolschijn等人(2019)在研究中发现, 当模式分离发生时对外侧枕叶进行经颅直流电刺激干扰后, 海马的神经活动不再能预测行为成绩, 这说明感觉区的知觉表征分离是内侧颞叶顺利进行模式分离的前提。
以上证据表明, 枕叶感觉区对相似刺激的特征差异也比较敏感, 其能够对相似的刺激进行知觉上的分离表征, 在一定程度上解决相似信息之间的干扰。作为记忆辨别的初始信息加工“车间”, 枕叶感觉区虽然不能单独实现记忆经验的分离表征, 但其进行的细颗粒度知觉表征对于由内侧颞叶主导的模式分离具有推动作用。
2.3记忆辨别中的监测与认知控制
记忆监测也是记忆功能的一个重要方面, 比如在记忆过程中, 需要对编码的质量或提取到的信息的准确性和相关性进行监测(Chua et al., 2009; Orth et al., 2023), 因此, 记忆监测对评估和判断记忆内容以及区分相似的记忆经验是必不可少的。已有研究证实, 记忆监测依赖于前额叶(Chua & Ahmed, 2016; Imperio & Chua, 2023; Shao et al., 2022), 那么, 在记忆辨别过程中, 刺激特征的微小变化也应该能够反映在前额叶的活动模式上。这一观点已经得到相关证据支持, 比如, 使用MST进行的研究发现, 前额叶表现出了和海马模式分离一致的活动模式(Nash et al., 2021), 更为重要的是, 通过操作诱饵相似性水平所得到的前额叶刺激信号输入?神经信号输出曲线与Lacy等人(2011)发现的海马DG/CA3亚区输入?输出函数曲线相吻合(Pidgeon & Morcom, 2016)。这些证据表明, 前额叶虽然不能对信息进行分离表征, 但能够监测到相似刺激间的差异, 并对相似的刺激进行差异化响应。
除了监测之外, 前额叶在情景记忆中的另一个重要功能是认知控制。已有研究表明, 在情景记忆的编码或提取阶段, 前额叶能够通过认知控制自上而下地调节海马的活动模式(Aly & Turk- Browne, 2016; Anderson & Hulbert, 2021; Malik et al., 2022; Zheng et al., 2021), 研究者在记忆辨别中也发现了类似的证据, 比如, Frank等人(2020)发现, 当由前额叶主导的预期被打破时, 海马DG/CA3亚区对高相似度物体的模式分离程度会进一步增加; Lohnas等人(2018)通过颅内电极记录被试的皮层脑电, 结果发现, 若要求被试将诱饵也判断为旧, 海马并不会表现出模式分离的电生理信号, 只有在要求被试对诱饵和旧物体进行区分时, 海马才会执行模式分离。这些证据说明, 前额叶能够根据任务要求向海马发送执行模式分离的指令并调节海马的模式分离活动。更为重要的是, Lohnas等人还发现, 无论是否要求区分诱饵和旧物体, 背外侧前额叶对诱饵和旧物体的响应始终存在显著差异, 也就是说, 前额叶始终能够监测到相似物体之间的差异, 在监测到差异后, 前额叶能够通过自上而下地调控来促进重叠信息分离, 进而实现记忆辨别。
综上所述, 记忆辨别依赖于大规模脑网络的协同活动:相似的刺激首先由枕叶感觉区对其特征进行感知与加工, 并对重叠的信号进行初步知觉表征分离, 前额叶在监测到输入信号的差异后, 根据任务要求启动并调控下游脑区的模式分离。在前额叶的监测和调控下, 来自枕叶感觉区的信息经由不同的信息加工路径进入内侧颞叶, 由PHC、PRC和EC进行初步的模式分离后进入海马, 再由海马DG/CA3亚区实现完全的分离表征, 最终将高度重叠的感知信号输入转变为互不重叠的神经信号输出。
3 记忆辨别力受老化影响的认知神经机制
3.1 海马老化影响记忆辨别力的认知神经机制
海马萎缩是老化中的常见现象(Kantarci et al., 2008; Raz et al., 2005), 尽管它被认为是导致老年人记忆能力下降的重要原因之一, 但现有研究发现, 海马总体积与健康老年人记忆辨别力之间的相关关系较弱。比如有研究者将老年人和青年人样本一起考察, 发现海马总体积与记忆辨别力之间存在显著正相关(Stark & Stark, 2017), 而Doxey和Kirwan (2015)在研究中并没有发现健康老年人的海马总体积与记忆辨别力之间存在关联, 还有研究者发现, 健康老年人只有在最相似物体的辨别成绩上才会表现出与海马总体积的显著正相关(Rizzolo et al., 2021)。当考察海马DG/CA3亚区体积与老年人记忆辨别力的关系时, 研究结果则趋向一致:Doxey和Kirwan (2015)在研究中发现, 健康老年人海马DG/CA3亚区的体积与记忆辨别力之间存在显著正相关, 即DG/CA3亚区的体积越小, 记忆辨别力越差, 还有研究者在对DG体积进行单独分析时也发现了一致的结果(Dillon et al., 2017; Riphagen et al., 2020)。这些证据说明海马DG/CA3亚区萎缩对老年人记忆辨别力的影响比海马总体萎缩造成的影响更为显著, 这可能是由于海马不同亚区在老化的过程中的萎缩速度不同(Bussy et al., 2021; Pereira et al., 2014), 且由于DG/CA3亚区仅占海马总体积的一小部分, 因此, DG/CA3亚区的体积萎缩很难反映在海马总体积的变化上。综上, DG/CA3亚区体积与记忆辨别力关系密切, 老化导致的DG/CA3亚区萎缩可能是导致老年人记忆辨别力下降的重要原因之一。
海马体积萎缩是一个连续的过程, 研究者认为在可测量的海马体积萎缩之前, 海马的微观结构破坏就已经发生了, 海马微观结构的完整性可能比海马体积更能有效预测健康老年人的记忆辨别力(Leal & Yassa, 2018)。最近一项研究发现, 通过超高分辨率扩散加权成像技术测量出的DG微观结构破坏(细胞密度降低)与更差的记忆辨别力显著相关, DG微观结构破坏对老年人记忆辨别力的预测作用甚至优于DG体积(Granger et al., 2022); Yassa等人(2011b)应用超高分辨率弥散张量成像技术也发现了DG/CA3亚区的树突结构完整性降低与健康老年人记忆辨别力下降有关。由此我们可以推测, 老年人记忆辨别力下降开始于海马微观结构破坏, 并随着海马结构完整性的降低而持续恶化, 也就是说, 模式分离加工的微神经环路破坏也是导致老年人记忆辨别力下降的原因之一。
除了海马结构完整性下降之外, 老年人的海马功能活动也会出现异常。大量研究表明, 兴奋性和抑制性活动平衡, 即E/I平衡, 是健康大脑的重要特征之一(Contreras & Wilent, 2005; Lopatina et al., 2019; Yizhar et al., 2011), 其中γ-氨基丁酸(γ-aminobutyric acid, GABA)是中枢神经系统中的主要抑制性神经递质之一(McCormick, 1989), 其在维持E/I平衡中发挥着重要作用(Bi et al., 2020)。在老化或认知障碍发展进程中, 海马内GABA能中间神经元和GABA受体数量减少(Levenga et al., 2013; Martín-Belmonte et al., 2020), 导致海马内GABA信号减弱, 抑制性神经活动不足。因此, 老年人的常见脑功能变化之一就是GABA能系统功能障碍导致的海马神经元兴奋性增加或过度激活(Jiménez-Balado & Eich, 2021; Tang et al., 2023)。在记忆辨别过程中, 研究者也发现了海马的过度激活:当正确辨别诱饵时, 健康老年人会表现出比年轻人更高的海马DG/CA3亚区激活水平(Reagh et al., 2018; Yassa et al., 2011a), 携带AD风险基因APOE ε4的健康老年人也会表现出比非携带者更高的DG/CA3激活水平(Sinha et al., 2018), 当老年人发展为MCI时, 其DG/CA3亚区的激活水平相比于健康老年人也会进一步提高(Corona-Long et al., 2020; Tran et al., 2017; Yassa et al., 2010a)。
目前, 尚未发展为AD的老年人在记忆辨别中表现出的海马过度激活已被证明是一种指示神经损伤和神经活动效率降低的标志。针对健康老年人的研究指出, 海马过度激活与记忆辨别力之间存在显著负相关, 老年人的海马激活水平越高, 记忆辨别力就越差(Berron et al., 2019; Reagh et al., 2018; Yassa et al., 2011a)。在轻度认知障碍群体中, 有研究者使用低剂量的抗癫痫药物对MCI患者进行治疗, 结果发现MCI患者的海马DG/CA3亚区的激活水平显著降低, 而记忆辨别力显著提升 (Bakker et al., 2012; Bakker et al., 2015)。除此之外, 来自AD病理机制的研究也发现, AD病理生物标记物tau蛋白和β-淀粉样蛋白(amyloid β-protein, Aβ)的含量不仅与老年人的记忆辨别力之间存在关联(Berron et al., 2019; Maass et al., 2019; Papp et al., 2021a), 而且与老年人在正确辨别诱饵时的海马激活水平也存在显著正相关(Berron et al., 2019), 即病理生物标记物的含量越高, 海马的激活水平越高。可见, 在发展为AD患者之前, 导致老年人记忆辨别力下降的另一个重要因素是海马功能障碍, 主要体现为海马因神经效率降低而出现过度激活。
已有研究指出, 海马与其他脑区间的功能连接对于记忆辨别也有重要作用, 比如动物研究发现, EC向海马DG的功能投射能够影响小鼠的位置辨别成绩(Yun et al., 2023), 针对人类被试的干预研究也发现, DG/CA3与PHC的功能连接增加能够提升记忆辨别力(Suwabe et al., 2018)。结合EC及其他内侧颞叶结构在记忆辨别中起到初步分离加工的作用, 可以推断内侧颞叶与海马的信息交流有助于加强海马与上游脑区的表征共享, 因此老化导致海马与其他脑区间的信息交流障碍也将影响进入海马的信息精度, 进而导致老年人记忆辨别力下降。这一观点已经得到相关证据支持:最近的一项研究发现, 在认知正常的老年人中, 物体记忆辨别力相对较差的老年人比物体记忆辨别力较好的老年人表现出了前侧LEC与海马DG/CA3亚区之间的静息态功能连接显著增加的情况, 且这一改变被证明是与Aβ病理发展和神经退行性病变有关(Adams et al., 2022); Stark等人(2021)则发现在记忆辨别中, 相比于年轻人, 老年人海马前部与PHC的功能连接显著降低, 这一较弱的功能连接与老年人较差的记忆辨别力有关。由此可见, 除海马自身的结构和功能外, 老化对海马与其他脑区间功能连接的影响, 也是导致老年人记忆辨别力下降的原因之一。
结合以上证据可以发现, 在记忆辨别中, 海马既作为模式分离的核心枢纽, 实现表征完全分离, 又作为一个收敛区, 对来自多个脑区的信息进行整合, 因此, 老化导致的海马DG/CA3亚区体积萎缩和微观结构破坏, 以及海马神经效率降低或与其他脑区的信息交流障碍都将导致老年人记忆辨别力受损。以海马为核心的脑老化是导致老年人记忆辨别力下降的关键原因。
3.2其他脑区老化影响记忆辨别力的认知神经机制
在除海马之外的其他内侧颞叶结构中, EC老化对记忆辨别力的影响是目前研究得最多的。尽管诸多研究报告了老年人的EC体积也会出现明显的萎缩(Devanand et al., 2008; Gellersen et al., 2023; Tran et al., 2017; Tran et al., 2022), 但是并没有发现记忆辨别力与EC的体积之间存在显著相关(Tran et al., 2022)。相比之下, EC功能与记忆辨别力的关系则更加明确。有研究指出, 在老化或认知障碍发展进程中, 诱饵正确辨别时的EC功能变化与海马的功能变化恰好相反, 在诱饵正确辨别时, 健康老年人的EC激活水平显著低于年轻人(Reagh et al., 2018), 当发展为MCI时, 患者的EC激活水平较健康老年人会进一步降低(Yassa et al., 2010a), 并且老年人在正确辨别诱饵时的EC的激活水平与记忆辨别力之间存在显著正相关, EC激活水平越低, 记忆辨别力越差(Reagh et al., 2018)。有临床试验采用极低剂量的抗癫痫药物左乙拉西坦对MCI患者进行治疗, 结果发现, 治疗后MCI患者的记忆辨别成绩显著提升, 此时海马激活水平并未出现显著变化, 但EC的激活水平提升到了与健康老年人相同的水平(Bakker et al., 2015)。从以上研究结果可知, EC的活动水平过低也会导致老年人记忆辨别力下降, EC活动不足反映了EC对相似信息进行初步分离加工的能力变差。
另外, 穿质通路(perforant path)老化的影响也不容忽视。穿质通路是EC向DG和CA3亚区进行信息传递的重要通道, 动物研究发现, 穿质通路纤维损失是导致记忆辨别力下降的一个独立因素(Burke et al., 2018)。 在人类研究中, Bennett和Stark (2016)采用超高分辨率弥散张量成像技术进行研究, 结果发现在控制老化对全脑白质的影响后, 穿质通路完整性能够显著预测老年人的记忆辨别力, 穿质通路越完整, 记忆辨别力越好, 该结果与前人研究结果相吻合(Yassa et al., 2010b)。值得一提的是, 在认知正常的老年人中, 穿质通路完整性并不能预测老年人的其他记忆成绩(Bennett & Stark, 2016), 也就是说, 尽管从EC到海马的信息传输通道正常运作是海马所有功能正常运作的前提, 但穿质通路的轻微损伤对记忆辨别以外的其他记忆功能影响并不显著, 这可能是由于穿质通路直接向海马DG进行投射, 因此穿质通路的轻微损伤也能对记忆辨别力产生直接影响, 这在一定程度上说明了穿质通路纤维损失也是导致老年人记忆辨别力损伤的一个重要原因。
近些年, 研究者也开始关注到老年人前额叶监测和认知控制功能衰退对记忆辨别力的影响, 比如行为研究发现, 由前额叶主导的老年人执行功能与通过MST测量的记忆辨别力之间存在显著正相关(Gellersen et al., 2021; Jensen et al., 2023; Pishdadian et al., 2020)。虽然目前仍然少有研究关注人类前额叶老化影响记忆辨别力的神经机制, 但神经影像学研究指出, 前额叶体积萎缩与功能障碍也会对老年人的情景记忆产生负面影响(Ankudowich et al., 2019; Brehmer et al., 2020; Maillet & Rajah, 2013; Shao et al., 2022), 老年人前额叶对海马的调控功能紊乱也与认知障碍的发展密切相关(Nyberg et al., 2019), 动物研究和人类研究还发现抑制前额叶的活动能够导致记忆辨别力显著下降(Johnson et al., 2021; Wais et al., 2018)。结合以上这些证据, 我们推测老年人的前额叶结构和功能完整性下降, 以及前额叶与内侧颞叶或其他脑区的信息传递与调控作用障碍, 也能够导致记忆辨别力严重受损, 但具体的认知神经机制还有待进一步探究。
另外, 还有研究团队发现, 记忆辨别也依赖于默认模式网络(Default mode network, DMN)。在DMN内, 老年人相较于年轻人表现出的前额叶与颞叶的静息态功能连接降低与老年人记忆辨别力下降有关(Wahlheim et al., 2022)。在最近的一项研究中, Cui等人(2023)也发现了老年人前部DMN和后部DMN之间的静息态功能连接的增加与老年人记忆辨别力的提升显著相关。尽管DMN在情景记忆中的作用通常被认为是海马或内侧颞叶与相邻脑区之间的功能连接主导的, 但不可否认的是, 其他脑区也会因老化的影响而产生或多或少的改变, 因此, 在未来还需要进一步探究具体新皮层结构在记忆辨别中的作用及其老化对记忆辨别力的影响程度。
总之, 现有证据表明, 记忆辨别是由大规模脑网络协同活动所支持的, 老化对各个脑区的影响都将或多或少导致记忆辨别力下降。除海马外的其他内侧颞叶结构老化主要影响了对信息的初步分离加工以及向海马的信息传输, 使得进入海马的信息完整性受损, 以至于无法形成准确的信息表征; 前额叶等控制网络内的脑区老化主要影响了对信息的监测以及对海马等内侧颞叶结构自上而下的调控。
4 MST在老化研究领域的应用
虽然目前尚无能够治愈认知障碍的治疗手段(Grabowska et al., 2023), 但已有大量研究证实, 早期干预能够延缓AD的进展(Gaugler et al., 2019; Rosenberg et al., 2018), 因此, 认知障碍的早期识别对患者进行疾病管理以及降低AD的发生和延缓AD的发展都具有重要意义。在认知障碍的评估中, 传统神经心理学测验扮演着重要角色:在临床和社区实践中, 研究者常依据多个认知领域的神经心理学测验结果对认知障碍风险群体进行风险等级划分(Edmonds et al., 2019; Langbaum et al., 2020)。然而, 老年人在完成成套神经心理学测验时, 需要花费大量时间, 且其施测和评分过程也依赖于临床医生或有经验的施测人员, 老年人难以独立进行, 因此, 简单易行且不依赖于专业施测人员的电子化认知评估范式成为认知障碍早期识别的重要发展方向。
MST在认知障碍的早期识别方面有着巨大的应用潜力。情景记忆损伤是AD的主要特征之一, 已有研究指出, 基线情景记忆成绩是认知能力衰退的重要预测因素(Johnson et al., 2009; Schaeverbeke et al., 2021), 且情景记忆成绩与AD病理发展程度存在显著负相关(Albert, 2011; Bennett et al., 2006; Moscoso et al., 2019), 故而大多数研究者使用情景记忆范式对认知障碍进行早期识别, 其中, 再认测验是情景记忆范式中最常见的测验形式。不过, 已有研究证实, 简单再认能力无法被用于识别存在轻微记忆损伤或携带AD风险基因APOE ε4的认知障碍高风险个体(Sinha et al., 2018; Stark et al., 2013)。虽然MST采用了再认的形式进行测验, 但相比之下, 其效果显著优于简单再认任务, 通过其测量的记忆辨别力能够有效地反映出由于认知障碍早期发展所导致的轻微记忆损伤。比如, 有研究指出, 在健康老年人中, 回忆功能(听觉词语学习测验成绩)受损的被试相比回忆功能正常的被试表现出了显著降低的记忆辨别力(Stark et al., 2013), 存在主观记忆下降老年人的记忆辨别力显著低于健康老年人(De Simone et al., 2022), AD风险基因APOE ε4的携带者也比非携带者在MST上表现得更差(Sinha et al., 2018)。还有研究发现, 通过MST测量的记忆辨别力在区分正常老年人和MCI患者以及区分主观认知下降老年人和MCI患者方面具有较高的准确度(Belliart- Guérin & Planche, 2023; Kim et al., 2023)。可见, MST是一项对认知功能衰退非常敏感的范式, 能够有效地揭示AD等神经退行性疾病发展早期就开始出现的轻微记忆损伤, 十分有潜力成为社区认知障碍风险预警和临床认知障碍早筛的有效工具。
尽管研究者们基于模式分离的基本原理开发出了多种版本的MST对不同的问题进行研究, 但在老化研究领域中, 应用比较广泛的仍然是物体版本的MST和空间/场景版本的MST, 相比之下, 物体版本的MST更加适用于认知障碍的早期识别。已有研究发现, 老年人对物体进行记忆辨别的能力比对空间/场景进行记忆辨别的能力下降得更快、下降得更早(Güsten et al., 2021; Reagh et al., 2016), 跨物种研究也发现了相似的结果(Johnson et al., 2017)。这种差异产生的原因在于物体与空间/场景模式分离加工依赖于不同的加工通路。如前文所述, 空间/场景的模式分离则更多依赖于PHC-MEC通路, 物体的模式分离则更多依赖于PRC-LEC通路, 而PRC-LEC通路更加容易受到老化的影响而出现功能衰退(Burke et al., 2014), 在AD临床前阶段, 也发现了LEC的功能障碍更为显著(Khan et al., 2014), 这也许是物体版本的MST能够比另外两种任务反映出更早期的AD病理生理变化, 以及在认知障碍的早期识别方面更具优势的原因。基于此, 目前的研究团队更多地尝试将物体版本的MST应用于智能手机、平板电脑等便携式电子设备, 进行无监督式认知评估(Papp et al., 2021a, 2021b), 同时也有研究人员不断对MST进行优化(Stark et al., 2023; Villarreal et al., 2022), 如通过自适应设计等方式缩短评估时间, 提升使用体验, 以推动其在社区和临床的广泛应用。
5 问题与展望
近些年来, 研究者们应用MST对记忆辨别的认知神经机制进行了深入的探索, 也揭示了老年人记忆辨别力下降的规律及原因, 基于这些发现, 研究者们也逐渐将MST推广到社区和临床研究中, 推动实现认知障碍风险预警和早期识别。虽然当前已有的研究取得很多重要成果, 但也仍然存在一些亟待解决的问题。
首先, 在应用fMRI技术考察海马不同亚区在执行模式分离时的功能活动时, 由于分辨率限制, 大多数研究无法将DG和CA3亚区完全分开, 只是笼统地考察DG/CA3亚区的活动对模式分离的贡献。然而动物研究早已指出, DG和CA3亚区在模式分离中的贡献程度是不同的(Knierim & Neunuebel, 2016; Neunuebel & Knierim, 2014), 因此, 在人类研究中精确地考察DG和CA3在模式分离中的功能活动以及二者与其他脑结构之间的功能连接是十分有必要的。7T超高场强fMRI技术的发展为解决这一问题提供了契机, 在此精细的成像技术之下, 不仅有研究者发现了DG是海马中唯一能够对相似场景形成完全不同的神经表征的结构(Berron et al., 2016), 还有研究者发现不同APOE基因型的青年被试在相似空间信息的模式分离中表现出了DG、CA3的功能活动差异以及DG和CA3之间的功能连接差异(Lee et al., 2020)。可见, 在人类被试中将DG和CA3亚区分开考察, 能够为模式分离的机制研究提供更多信息, 也能够为认知障碍的发生与发展机制提供重要补充。因此, 在未来的研究中, 应该更多地尝试在老年人群体中单独考察DG和CA3在执行模式分离时的功能活动以及其与不同脑区之间的相互作用对记忆辨别力的影响。
其次, 尽管现有研究强调了内侧颞叶, 特别是海马在记忆辨别中的作用, 但前额叶老化对记忆辨别的影响可能不亚于内侧颞叶老化。有研究指出, 相比于诱饵虚报为旧的条件, 诱饵正确辨别时, 双侧额下回与内侧颞叶的功能连接显著增加(Wais et al., 2017), 这种差异在一定程度上说明了, 在记忆辨别时需要对内侧颞叶进行更强的调控。因此, 前额叶老化导致调控功能减弱, 可能对海马的过度激活也有贡献, 进而间接影响了老年人的记忆辨别力。这一观点不乏证据支持, 比如, 最近一项动物研究指出, 前额叶能够通过长程GABA能投射(long-range GABAergic projection)抑制海马的活动(Malik et al., 2022), 在青年人的工作记忆研究中也发现了前额叶对海马的调控减弱与海马的过度激活有关联(Xiong et al., 2021), 还有研究发现, 在情景记忆编码阶段, 老年人前额叶激活水平改变对海马过度激活也有贡献(Nyberg et al., 2019)。因此, 未来的研究应该更加注重前额叶与海马的相互作用对记忆辨别力的影响, 多角度、整体性地探究老年人记忆辨别力下降的认知神经机制, 以便在老化早期采取行之有效的干预方案来维持老年人的认知健康。
最后, 现阶段针对老年人记忆辨别功能进行的研究仍然以小样本横断研究为主, 缺乏大样本研究和前瞻性队列研究。虽然MST在认知障碍风险预警与早期识别方面有着良好的应用潜力, 但想要使MST成为辅助临床诊断和进行认知障碍风险评估与预测的有效工具, 还需要通过前瞻队列确定不同记忆辨别力的老年人向MCI或AD转归的情况, 以此划分认知障碍风险等级, 同时也需要通过大样本研究建立老年人记忆辨别力常模。因此, 未来的研究应侧重于应用MST进行大样本研究, 并尝试建立前瞻性队列。
6 总结
大量研究对记忆辨别功能进行了深入探讨, 并从结构和功能两方面揭示了老年人记忆辨别力下降的认知神经机制。当前研究已经证实了海马等内侧颞叶结构完整性降低和功能异常, 以及海马与其他内侧颞叶脑区之间的结构和功能连接改变是记忆辨别力下降的关键原因, 此外, 广泛分布的新皮层区域老化对记忆辨别力也存在显著影响。未来的研究应结合更加先进的神经影像技术手段, 单独考察海马DG和CA3亚区在记忆辨别中的作用及其老化的影响, 同时也应该更加关注前额叶等新皮层结构老化影响记忆辨别力的神经机制。另一方面, 通过MST测得的记忆辨别力, 能够有效地反映出早期老化及异常老化对大脑结构和功能的影响, 因此, MST在老年人的认知障碍早筛与风险预警方面有着巨大的应用潜力, 但目前仍然需要通过大样本研究和前瞻队列研究来进一步验证其在认知障碍早期识别中的有效性, 同时也要对MST进行改进, 使其成为能够满足老年人主动监测认知健康需求的电子化评估工具。
参考文献
Adams, J. N., Kim, S., Rizvi, B., Sathishkumar, M., Taylor, L., Harris, A. L., ... Yassa, M. A. (2022). Entorhinal- hippocampal circuit integrity is related to mnemonic discrimination and amyloid-β pathology in older adults. The Journal of Neuroscience, 42(46), 8742?8753.
Albert, M. S. (2011). Changes in cognition. Neurobiology of Aging, 32(Suppl. 1), S58?S63.
Aly, M., & Turk-Browne, N. B. (2016). Attention promotes episodic encoding by stabilizing hippocampal representations. Proceedings of the National Academy of Sciences of the United States of America, 113(4), E420?E429.
Amer, T., & Davachi, L. (2023). Extra-hippocampal contributions to pattern separation. eLife, 12, e82250.
Anderson, M. C., & Hulbert, J. C. (2021). Active forgetting: Adaptation of memory by prefrontal control. Annual Review of Psychology, 72, 1?36.
Ankudowich, E., Pasvanis, S., & Rajah, M. N. (2019). Age- related differences in prefrontal-hippocampal connectivity are associated with reduced spatial context memory. Psychology and Aging, 34(2), 251?261.
Baker, S., Vieweg, P., Gao, F., Gilboa, A., Wolbers, T., Black, Sandr E., & Rosenbaum, R. S. (2016). The human dentate gyrus plays a necessary role in discriminating new memories. Current Biology, 26(19), 2629?2634.
Bakker, A., Albert, M. S., Krauss, G., Speck, C. L., & Gallagher, M. (2015). Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clinical, 7, 688?698.
Bakker, A., Kirwan, C. B., Miller, M., & Stark, C. E. L. (2008). Pattern separation in the human hippocampal CA3 and dentate gyrus. Science, 319(5870), 1640?1642.
Bakker, A., Krauss, G. L., Albert, M. S., Speck, C. L., Jones, L. R., Stark, C. E., … Gallagher, M. (2012). Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron, 74(3), 467?474.
Baumann, O., & Mattingley, J. B. (2021). Extrahippocampal contributions to spatial navigation in humans: A review of the neuroimaging evidence. Hippocampus, 31(7), 640?657.
Belliart-Guérin, G., & Planche, V. (2023). Mnemonic discrimination performance in a memory clinic: A pilot study. Journal of Alzheimer's Disease, 94(4), 1527?1534.
Bennett, D. A., Schneider, J. A., Arvanitakis, Z., Kelly, J. F., Aggarwal, N. T., Shah, R. C., & Wilson, R. S. (2006). Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology, 66(12), 1837?1844.
Bennett, I. J., & Stark, C. E. L. (2016). Mnemonic discrimination relates to perforant path integrity: An ultra-high resolution diffusion tensor imaging study. Neurobiology of Learning and Memory, 129, 107?112.
Berron, D., Cardenas-Blanco, A., Bittner, D., Metzger, C. D., Spottke, A., Heneka, M. T., ... Düzel, E. (2019). Higher CSF tau levels are related to hippocampal hyperactivity and object mnemonic discrimination in older adults. The Journal of Neuroscience, 39(44), 8788?8797.
Berron, D., Neumann, K., Maass, A., Schütze, H., Fliessbach, K., Kiven, V., ... Düzel, E. (2018). Age-related functional changes in domain-specific medial temporal lobe pathways. Neurobiology of Aging, 65, 86?97.
Berron, D., Schütze, H., Maass, A., Cardenas-Blanco, A., Kuijf, H. J., Kumaran, D., & Düzel, E. (2016). Strong evidence for pattern separation in human dentate gyrus. The Journal of Neuroscience, 36(29), 7569?7579.
Bi, D., Wen, L., Wu, Z., & Shen, Y. (2020). GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer's disease. Alzheimers & Dementia, 16(9), 1312?1329.
Bowman, C. R., Chamberlain, J. D., & Dennis, N. A. (2019). Sensory representations supporting memory specificity: Age effects on behavioral and neural discriminability. The Journal of Neuroscience, 39(12), 2265?2275.
Brehmer, Y., Nilsson, J., Berggren, R., Schmiedek, F., & L?vdén, M. (2020). The importance of the ventromedial prefrontal cortex for associative memory in older adults: A latent structural equation analysis. NeuroImage, 209, 116475.
Burke, S. N., Maurer, A. P., Nematollahi, S., Uprety, A., Wallace, J. L., & Barnes, C. A. (2014). Advanced age dissociates dual functions of the perirhinal cortex. The Journal of Neuroscience, 34(2), 467?480.
Burke, S. N., Turner, S. M., Desrosiers, C. L., Johnson, S. A., & Maurer, A. P. (2018). Perforant path fiber loss results in mnemonic discrimination task deficits in young rats. Frontiers in Systems Neuroscience, 12, 61.
Bussy, A., Plitman, E., Patel, R., Tullo, S., Salaciak, A., Bedford, S. A., ... Alzheimers Dis, N. (2021). Hippocampal subfield volumes across the healthy lifespan and the effects of MR sequence on estimates. NeuroImage, 233, 117931.
Chang, A., Murray, E., & Yassa, M. A. (2015). Mnemonic discrimination of similar face stimuli and a potential mechanism for the "other race" effect. Behavioral Neuroscience, 129(5), 666?672.
Chawla, M. K., Guzowski, J. F., Ramirez-Amaya, V., Lipa, P., Hoffman, K. L., Marriott, L. K., ... Barnes, C. A. (2005). Sparse, environmentally selective expression of arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus, 15(5), 579?586.
Chua, E. F., & Ahmed, R. (2016). Electrical stimulation of the dorsolateral prefrontal cortex improves memory monitoring. Neuropsychologia, 85, 74?79.
Chua, E. F., Schacter, D. L., & Sperling, R. A. (2009). Neural correlates of metamemory: A comparison of feeling- of-knowing and retrospective confidence judgments. Journal of Cognitive Neuroscience, 21(9), 1751?1765.
Contreras, D., & Wilent, W. B. (2005). Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nature Neuroscience, 8(10), 1364?1370.
Corona-Long, C. A., Tran, T. T., Chang, E., Speck, C. L., Gallagher, M., & Bakker, A. (2020). Comparison of male and female patients with amnestic mild cognitive impairment: Hippocampal hyperactivity and pattern separation memory performance. Alzheimer's & Dementia: Diagnosis, Assessment & Disease monitoring, 12(1), e12043.
Cui, X. Y., Gui, W. J., Miao, J. W., Liu, X. M., Zhu, X. Y., Zheng, Z. W., ... Li, J. (2023). A combined intervention of aerobic exercise and video game in older adults: The efficacy and neural basis on improving mnemonic discrimination. Journals of Gerontology Series A-Biological Sciences and Medical Sciences, 78(8), 1436?1444.
Danieli, K., Guyon, A., & Bethus, I. (2023). Episodic memory formation: A review of complex hippocampus input pathways. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 126, 110757.
De Simone, M. S., Rodini, M., De Tollis, M., Fadda, L., Caltagirone, C., & Carlesimo, G. A. (2022). The diagnostic usefulness of experimental memory tasks for detecting subjective cognitive decline: Preliminary results in an Italian sample. Neuropsychology, 37(6), 636?649.
Deng, W., Mayford, M., & Gage, F. H. (2013). Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife, 2, e00312.
Devanand, D. P., Liu, X., Tabert, M. H., Pradhaban, G., Cuasay, K., Bell, K., ... Pelton, G. H. (2008). Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer's disease. Biological Psychiatry, 64(10), 871?879.
Dilks, D. D., Kamps, F. S., & Persichetti, A. S. (2022). Three cortical scene systems and their development. Trends in Cognitive Sciences, 26(2), 117?127.
Dillon, S. E., Tsivos, D., Knight, M., McCann, B., Pennington, C., Shiel, A. I., ... Coulthard, E. J. (2017). The impact of ageing reveals distinct roles for human dentate gyrus and CA3 in pattern separation and object recognition memory. Scientific Reports, 7, 14069.
Doxey, C. R., & Kirwan, C. B. (2015). Structural and functional correlates of behavioral pattern separation in the hippocampus and medial temporal lobe. Hippocampus, 25(4), 524?533.
Edmonds, E. C., McDonald, C. R., Marshall, A., Thomas, K. R., Eppig, J., Weigand, A. J., ... Alzheimers Dis Neuroimaging, I. (2019). Early versus late MCI: Improved MCI staging using a neuropsychological approach. Alzheimer's & Dementia, 15(5), 699?708.
Ferko, K. M., Blumenthal, A., Martin, C. B., Proklova, D., Minos, A. N., Saksida, L. M., ... K?hler, S. (2022). Activity in perirhinal and entorhinal cortex predicts perceived visual similarities among category exemplars with highest precision. eLife, 11, e66884.
Frank, D., Montemurro, M. A., & Montaldi, D. (2020). Pattern separation underpins expectation-modulated memory. Journal of Neuroscience, 40(17), 3455?3464.
Gaugler, J., James, B., Johnson, T., Marin, A., Weuve, J., & Alzheimer's, A. (2019). 2019 Alzheimer's disease facts and figures. Alzheimer's & Dementia, 15(3), 321?387.
Gellersen, H. M., Trelle, A. N., Farrar, B. G., Coughlan, G., Korkki, S. M., Henson, R. N., & Simons, J. S. (2023). Medial temporal lobe structure, mnemonic and perceptual discrimination in healthy older adults and those at risk for mild cognitive impairment. Neurobiology of Aging, 122, 88?106.
Gellersen, H. M., Trelle, A. N., Henson, R. N., & Simons, J. S. (2021). Executive function and high ambiguity perceptual discrimination contribute to individual differences in mnemonic discrimination in older adults. Cognition, 209, 104556.
Grabowska, M. E., Huang, A., Wen, Z. X., Li, B. S., & Wei, W. Q. (2023). Drug repurposing for Alzheimer's disease from 2012-2022?A 10-year literature review. Frontiers in Pharmacology, 14, 1257700.
Granger, S. J., Colon-Perez, L., Larson, M. S., Phelan, M., Keator, D. B., Janecek, J. T., ... Yassa, M. A. (2022). Hippocampal dentate gyrus integrity revealed with ultrahigh resolution diffusion imaging predicts memory performance in older adults. Hippocampus, 32(9), 627? 638.
Güsten, J., Ziegler, G., Duzel, E., & Berron, D. (2021). Age impairs mnemonic discrimination of objects more than scenes: A web-based, large-scale approach across the lifespan. Cortex, 137, 138?148.
Hanert, A., Pedersen, A., & Bartsch, T. (2019). Transient hippocampal CA1 lesions in humans impair pattern separation performance. Hippocampus, 29(8), 736?747.
Imperio, C. M., & Chua, E. F. (2023). HD-tDCS over the left DLPFC increases cued recall and subjective question familiarity rather than other aspects of memory and metamemory. Brain Research, 1819, 148538.
Jensen, A., Karpov, G., Collin, C. A., & Davidson, P. S. R. (2023). Executive function predicts older adults' lure discrimination difficulties on the mnemonic similarity task. Journals of Gerontology Series B-Psychological Sciences and Social Sciences, 78(10), 1642?1650.
Jiménez-Balado, J., & Eich, T. S. (2021). GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer's disease. Seminars in Cell & Developmental Biology, 116, 146?159.
Johnson, D. K., Storandt, M., Morris, J. C., & Galvin, J. E. (2009). Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of Neurology, 66(10), 1254?1259.
Johnson, S. A., Turner, S. M., Santacroce, L. A., Carty, K. N., Shafiq, L., Bizon, J. L., Maurer, A. P., & Burke, S. N. (2017). Rodent age-related impairments in discriminating perceptually similar objects parallel those observed in humans. Hippocampus, 27(7), 759?776.
Johnson, S. A., Zequeira, S., Turner, S. M., Maurer, A. P., Bizon, J. L., & Burke, S. N. (2021). Rodent mnemonic similarity task performance requires the prefrontal cortex. Hippocampus, 31(7), 701?716.
Kantarci, K., Petersen, R. C., Przybelski, S. A., Weigand, S. D., Shiung, M. M., Whitwell, J. L., ... Jack, C. R. (2008). Hippocampal volumes, proton magnetic resonance spectroscopy metabolites, and cerebrovascular disease in mild cognitive impairment subtypes. Archives of Neurology, 65(12), 1621?1628.
Keene, C. S., Bladon, J., McKenzie, S., Liu, C. D., O'Keefe, J., & Eichenbaum, H. (2016). Complementary functional organization of neuronal activity patterns in the perirhinal, lateral entorhinal, and medial entorhinal cortices. Journal of Neuroscience, 36(13), 3660?3675.
Kent, B. A., Hvoslef-Eide, M., Saksida, L. M., & Bussey, T. J. (2016). The representational-hierarchical view of pattern separation: Not just hippocampus, not just space, not just memory? Neurobiology of Learning and Memory, 129, 99?106.
Khan, U. A., Liu, L., Provenzano, F. A., Berman, D. E., Profaci, C. P., Sloan, R., ... Small, S. A. (2014). Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nature Neuroscience, 17(2), 304?311.
Kim, S., Adams, J. N., Chappel-Farley, M. G., Keator, D., Janecek, J., Taylor, L., ... Yassa, M. A. (2023). Examining the diagnostic value of the mnemonic discrimination task for classification of cognitive status and amyloid-beta burden. Neuropsychologia, 191, 108727.
Kirwan, C. B., Hartshorn, A., Stark, S. M., Goodrich- Hunsaker, N. J., Hopkins, R. O., & Stark, C. E. L. (2012). Pattern separation deficits following damage to the hippocampus. Neuropsychologia, 50(10), 2408?2414.
Kirwan, C. B., & Stark, C. E. L. (2007). Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning & Memory, 14(9), 625?633.
Klippenstein, J. L., Stark, S. M., Stark, C. E. L., & Bennett, I. J. (2020). Neural substrates of mnemonic discrimination: A whole-brain fMRI investigation. Brain and Behavior, 10(3), e01560.
Knierim, J. J., & Neunuebel, J. P. (2016). Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiology of Learning and Memory, 129, 38?49.
Koolschijn, R. S., Emir, U. E., Pantelides, A. C., Nili, H., Behrens, T. E. J., & Barron, H. C. (2019). The hippocampus and neocortical inhibitory engrams protect against memory interference. Neuron, 101(3), 528?541.
Lacy, J. W., Yassa, M. A., Stark, S. M., Muftuler, L. T., & Stark, C. E. L. (2011). Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning & Memory, 18(1), 15?18.
Lalani, S. J., Reyes, A., Kaestner, E., Stark, S. M., Stark, C. E. L., Lee, D., ... McDonald, C. R. (2022). Impaired behavioral pattern separation in refractory temporal lobe epilepsy and mild cognitive impairment. Journal of the International Neuropsychological Society, 28(6), 550?562.
Langbaum, J. B., Ellison, N. N., Caputo, A., Thomas, R. G., Langlois, C., Riviere, M. -E., ... Hendrix, S. B. (2020). The Alzheimer's prevention initiative composite cognitive test: A practical measure for tracking cognitive decline in preclinical Alzheimer's disease. Alzheimer's Research & Therapy, 12(1), 66.
Leal, S. L., Ferguson, L. A., Harrison, T. M., & Jagust, W. J. (2019). Development of a mnemonic discrimination task using naturalistic stimuli with applications to aging and preclinical Alzheimer's disease. Learning & Memory, 26(7), 219?228.
Leal, S. L., & Yassa, M. A. (2018). Integrating new findings and examining clinical applications of pattern separation. Nature Neuroscience, 21(2), 163?173.
Lee, H., Stirnberg, R., Wu, S., Wang, X., St?cker, T., Jung, S., Montag, C., & Axmacher, N. (2020). Genetic Alzheimer's disease risk affects the neural mechanisms of pattern separation in hippocampal subfields. Current Biology, 30(21), 4201?4212.
Lee, I., Knierim, J. J., Yoganarasimha, D., & Rao, G. (2004). Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature, 430(6998), 456?459.
Leutgeb, J. K., Leutgeb, S., Moser, M. B., & Moser, E. I. (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315(5814), 961?966.
Leutgeb, S., Leutgeb, J. K., Treves, A., Moser, M. -B., & Moser, E. I. (2004). Distinct ensemble codes in hippocampal areas CA3 and CA1. Science, 305(5688), 1295?1298.
Levenga, J., Krishnamurthy, P., Rajamohamedsait, H., Wong, H., Franke, T. F., Cain, P., Sigurdsson, E. M., & Hoeffer, C. A. (2013). Tau pathology induces loss of GABAergic interneurons leading to altered synaptic plasticity and behavioral impairments. Acta Neuropathologica Communications, 1, 34.
Lohnas, L. J., Duncan, K., Doyle, W. K., Thesen, T., Devinsky, O., & Davachi, L. (2018). Time-resolved neural reinstatement and pattern separation during memory decisions in human hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 115(31), E7418?E7427.
Lopatina, O. L., Malinovskaya, N. A., Komleva, Y. K., Gorina, Y. V., Shuvaev, A. N., Olovyannikova, R. Y., ... Salmina, A. B. (2019). Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Reviews in the Neurosciences, 30(8), 807?820.
Ly, M., Murray, E., & Yassa, M. A. (2013). Perceptual versus conceptual interference and pattern separation of verbal stimuli in young and older adults. Hippocampus, 23(6), 425?430.
Maass, A., Berron, D., Harrison, T. M., Adams, J. N., La Joie, R., Baker, S., ... Jagust, W. J. (2019). Alzheimer's pathology targets distinct memory networks in the ageing brain. Brain, 142(8), 2492?2509.
Maillet, D., & Rajah, M. N. (2013). Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: A review. Ageing Research Reviews, 12(2), 479?489.
Malik, R., Li, Y., Schamiloglu, S., & Sohal, V. S. (2022). Top-down control of hippocampal signal-to-noise by prefrontal long-range inhibition. Cell, 185(9), 1602?1617.
Marr, D. (1971). Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 262(841), 23?81.
Martín-Belmonte, A., Aguado, C., Alfaro-Ruíz, R., Moreno- Martínez, A. E., de la Ossa, L., Martínez-Hernández, J., ... Luján, R. (2020). Density of GABAB receptors is reduced in granule cells of the hippocampus in a mouse model of Alzheimer's disease. International Journal of Molecular Sciences, 21(7), 2459.
McClelland, J. L., McNaughton, B. L., & O'Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419?457.
McCormick, D. A. (1989). GABA as an inhibitory neurotransmitter in human cerebral cortex. Journal of Neurophysiology, 62(5), 1018?1027.
Moscoso, A., Silva-Rodríguez, J., Aldrey, J. M., Cortés, J., Fernández-Ferreiro, A., Gómez-Lado, N., ... Alzheimer's Dis Neuroimaging, I. (2019). Staging the cognitive continuum in prodromal Alzheimer's disease with episodic memory. Neurobiology of Aging, 84, 1?8.
Nash, M. I., Hodges, C. B., Muncy, N. M., & Kirwan, C. B. (2021). Pattern separation beyond the hippocampus: A high-resolution whole-brain investigation of mnemonic discrimination in healthy adults. Hippocampus, 31(4), 408?421.
Neunuebel, J. P., & Knierim, J. J. (2014). CA3 retrieves coherent representations from degraded input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron, 81(2), 416?427.
Norman, K. A., & O'Reilly, R. C. (2003). Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychological Review, 110(4), 611?646.
Nyberg, L., Andersson, M., Lundquist, A., Salami, A., & W?hlin, A. (2019). Frontal contribution to hippocampal hyperactivity during memory encoding in aging. Frontiers in Molecular Neuroscience, 12, 229.
Orth, M., Wagnon, C., Neumann-Dunayevska, E., Kaller, C. P., Kl?ppel, S., Meier, B., Henke, K., & Peter, J. (2023). The left prefrontal cortex determines relevance at encoding and governs episodic memory formation. Cerebral Cortex, 33(3), 612?621.
Pagen, L. H. G., Poser, B. A., van Boxtel, M. P. J., Priovoulos, N., van Hooren, R. W. E., Verhey, F. R. J., & Jacobs, H. I. L. (2022). Worry modifies the relationship between locus coeruleus activity and emotional mnemonic discrimination. Brain Sciences, 12(3), 381.
Papp, K. V., Rentz, D. M., Maruff, P., Sun, C. K., Raman, R., Donohue, M. C., ... Team, A. S. (2021a). The computerized cognitive composite (C3) in A4, an Alzheimer's disease secondary prevention trial. The Journal of Prevention of Alzheimer's Disease, 8(1), 59?67.
Papp, K. V., Samaroo, A., Chou, H. C., Buckley, R., Schneider, O. R., Hsieh, S., ... Amariglio, R. E. (2021b). Unsupervised mobile cognitive testing for use in preclinical Alzheimers disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring, 13(1), e12243.
Park, D. C., & Bischof, G. N. (2013). The aging mind: Neuroplasticity in response to cognitive training. Dialogues in Clinical Neuroscience, 15(1), 109?119.
Park, D. C., Lautenschlager, G., Hedden, T., Davidson, N. S., Smith, A. D., & Smith, P. K. (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17(2), 299?320.
Pereira, J. B., Valls-Pedret, C., Ros, E., Palacios, E., Falcon, C., Bargallo, N., ... Junque, C. (2014). Regional vulnerability of hippocampal subfields to aging measured by structural and diffusion MRI. Hippocampus, 24(4), 403?414.
Pidgeon, L. M., & Morcom, A. M. (2016). Cortical pattern separation and item-specific memory encoding. Neuropsychologia, 85, 256?271.
Pishdadian, S., Hoang, N. V., Baker, S., Moscovitch, M., & Rosenbaum, R. S. (2020). Not only memory: Investigating the sensitivity and specificity of the mnemonic similarity task in older adults. Neuropsychologia, 149, 107670.
Raz, N., Lindenberger, U., Rodrigue, K. M., Kennedy, K. M., Head, D., Williamson, A., ... Acker, J. D. (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15(11), 1676?1689.
Reagh, Z. M., Ho, H. D., Leal, S. L., Noche, J. A., Chun, A., Murray, E. A., & Yassa, M. A. (2016). Greater loss of object than spatial mnemonic discrimination in aged adults. Hippocampus, 26(4), 417?422.
Reagh, Z. M., Noche, J. A., Tustison, N. J., Delisle, D., Murray, E. A., & Yassa, M. A. (2018). Functional imbalance of anterolateral entorhinal cortex and hippocampal dentate/CA3 underlies age-related object pattern separation deficits. Neuron, 97(5), 1187?1198.
Reagh, Z. M., & Yassa, M. A. (2014). Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proceedings of the National Academy of Sciences of the United States of America, 111(40), E4264?E4273.
Riphagen, J. M., Schmiedek, L., Gronenschild, E. H. B. M., Yassa, M. A., Priovoulos, N., Sack, A. T., Verhey, F. R. J., & Jacobs, H. I. L. (2020). Associations between pattern separation and hippocampal subfield structure and function vary along the lifespan: A 7 T imaging study. Scientific Reports, 10, 7572.
Rizzolo, L., Narbutas, J., Van Egroo, M., Chylinski, D., Besson, G., Baillet, M., ... Collette, F. (2021). Relationship between brain AD biomarkers and episodic memory performance in healthy aging. Brain and Cognition, 148, 105680.
Rosenberg, A., Ngandu, T., Rusanen, M., Antikainen, R., Backman, L., Havulinna, S., ... Kivipelto, M. (2018). Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimer's & Dementia, 14(3), 263?270.
Sakon, J. J., & Suzuki, W. A. (2019). A neural signature of pattern separation in the monkey hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 116(19), 9634?9643.
Schaeverbeke, J. M., Gabel, S., Meersmans, K., Luckett, E. S., De Meyer, S., Adamczuk, K., ... Vandenberghe, R. (2021). Baseline cognition is the best predictor of 4-year cognitive change in cognitively intact older adults. Alzheimer's Research & Therapy, 13(1), 75.
Shao, X. H., Liu, W. Z., Guo, Y., & Zhu, B. (2022). Age effects on neural discriminability and monitoring process during memory retrieval for auditory words. Frontiers in Aging Neuroscience, 14, 884993.
Sinha, N., Berg, C. N., Tustison, N. J., Shaw, A., Hill, D., Yassa, M. A., & Gluck, M. A. (2018). APOE ε4 status in healthy older African Americans is associated with deficits in pattern separation and hippocampal hyperactivation. Neurobiology of Aging, 69, 221?229.
Stark, C. E. L., Noche, J. A., Ebersberger, J. R., Mayer, L., & Stark, S. M. (2023). Optimizing the mnemonic similarity task for efficient, widespread use. Frontiers in Behavioral Neuroscience, 17, 1080366.
Stark, S. M., Frithsen, A., & Stark, C. E. L. (2021). Age-related alterations in functional connectivity along the longitudinal axis of the hippocampus and its subfields. Hippocampus, 31(1), 11?27.
Stark, S. M., Kirwan, C. B., & Stark, C. E. L. (2019). Mnemonic similarity task: A tool for assessing hippocampal integrity. Trends in Cognitive Sciences, 23(11), 938?951.
Stark, S. M., & Stark, C. E. L. (2017). Age-related deficits in the mnemonic similarity task for objects and scenes. Behavioural Brain Research, 333, 109?117.
Stark, S. M., Yassa, M. A., Lacy, J. W., & Stark, C. E. L. (2013). A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia, 51(12), 2442?2449.
Stevenson, R. F., Reagh, Z. M., Chun, A. P., Murray, E. A., & Yassa, M. A. (2020). Pattern separation and source memory engage distinct hippocampal and neocortical regions during retrieval. The Journal of Neuroscience, 40(4), 843?851.
Stiernstr?mer, E. S., Wolgast, M., Johansson, M., Innes-Ker, ?., & Carde?a, E. (2018). The effect of variations of emotional expressions on mnemonic discrimination and traditional recognition memory. Journal of Cognitive Psychology, 30(5?6), 547?557.
Suwabe, K., Byun, K., Hyodo, K., Reagh, Z. M., Roberts, J. M., Matsushita, A., ... Soya, H. (2018). Rapid stimulation of human dentate gyrus function with acute mild exercise. Proceedings of the National Academy of Sciences of the United States of America, 115(41), 10487?10492.
Szollosi, A., Keri, S., & Racsmany, M. (2022). The key to superior memory encoding under stress: The relationship between cortisol response and mnemonic discrimination. Learning & Memory, 29(1), 7?15.
Tang, Y., Yan, Y., Mao, J., Ni, J., & Qing, H. (2023). The hippocampus associated GABAergic neural network impairment in early-stage of Alzheimers disease. Ageing Research Reviews, 86, 101865.
Tran, T. T., Speck, C. L., Gallagher, M., & Bakker, A. (2022). Lateral entorhinal cortex dysfunction in amnestic mild cognitive impairment. Neurobiology of Aging, 112, 151?160.
Tran, T. T., Speck, C. L., Pisupati, A., Gallagher, M., & Bakker, A. (2017). Increased hippocampal activation in ApoE-4 carriers and non-carriers with amnestic mild cognitive impairment. NeuroImage Clinical, 13(C), 237?245.
Tulving, E. (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53(1), 1?25.
Vazdarjanova, A., & Guzowski, J. F. (2004). Differences in hippocampal neuronal population responses to modifications of an environmental context: Evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. The Journal of Neuroscience, 24(29), 6489?6496.
Villarreal, M., Stark, C. E. L., & Lee, M. D. (2022). Adaptive design optimization for a mnemonic similarity task. Journal of Mathematical Psychology, 108, 102665.
Wahlheim, C. N., Christensen, A. P., Reagh, Z. M., & Cassidy, B. S. (2022). Intrinsic functional connectivity in the default mode network predicts mnemonic discrimination: A connectome-based modeling approach. Hippocampus, 32(1), 21?37.
Wais, P. E., Jahanikia, S., Steiner, D., Stark, C. E. L., & Gazzaley, A. (2017). Retrieval of high-fidelity memory arises from distributed cortical networks. NeuroImage, 149, 178?189.
Wais, P. E., Montgomery, O., Stark, C. E. L., & Gazzaley, A. (2018). Evidence of a causal role for mid-ventrolateral prefrontal cortex based functional networks in retrieving high-fidelity memory. Scientific Reports, 8, 14877.
Xiong, B., Chen, C., Tian, Y., Zhang, S., Liu, C., Evans, T. M., … Qin, S. (2021). Brain preparedness: The proactive role of the cortisol awakening response in hippocampal- prefrontal functional interactions. Progress in Neurobiology, 205, 102127.
Yassa, M. A., Lacy, J. W., Stark, S. M., Albert, M. S., Gallagher, M., & Stark, C. E. L. (2011a). Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus, 21(9), 968?979.
Yassa, M. A., Mattfeld, A. T., Stark, S. M., & Stark, C. E. L. (2011b). Age-related memory deficits linked to circuit- specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 108(21), 8873?8878.
Yassa, M. A., Muftuler, L. T., Stark, C. E. L., & Squire, L. R. (2010b). Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences of the United States of America, 107(28), 12687?12691.
Yassa, M. A., & Stark, C. E. L. (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34(10), 515?525.
Yassa, M. A., Stark, S. M., Bakker, A., Albert, M. S., Gallagher, M., & Stark, C. E. L. (2010a). High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage, 51(3), 1242?1252.
Yizhar, O., Fenno, L. E., Prigge, M., Schneider, F., Davidson, T. J., Ogshea, D. J., ... Deisseroth, K. (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature, 477(7363), 171?178.
Yun, S. H., Soler, I., Tran, F. H., Haas, H. A., Shi, R., Bancroft, G. L., ... Eisch, A. J. (2023). Behavioral pattern separation and cognitive flexibility are enhanced in a mouse model of increased lateral entorhinal cortex-dentate gyrus circuit activity. Frontiers in Behavioral Neuroscience, 17, 1151877.
Zheng, L., Gao, Z. Y., McAvan, A. S., Isham, E. A., & Ekstrom, A. D. (2021). Partially overlapping spatial environments trigger reinstatement in hippocampus and schema representations in prefrontal cortex. Nature Communications, 12(1), 6231.
The cognitive neural mechanisms of age-related decline in mnemonic discrimination and its application
ZENG Qinghe, CUI Xiaoyu, TANG Wei, LI Juan
(CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing 100101, China)(Department of Psychology, University of Chinese Academy of Sciences, Beijing 100049, China)
Abstract: Mnemonic discrimination (MD) refers to the ability to accurately distinguish similar memory experiences, which relies on a neural computing mechanism known as pattern separation. Currently, mnemonic similarity task (MST) is commonly employed to measure and study MD. The elderly tend to exhibit a noticeable decline in MD. This decline is proved to be associated with damage to the structural and functional integrity of the medial temporal lobe, which occurs during the aging process. Some researchers have also suggested that the aging of the neocortex can influence MD. Given its reliance on the medial temporal lobe, MD can reflect abnormal brain structural damage and functional decline in the early stages of cognitive impairment. Thus, MST has significant potential in early identification of cognitive impairment. To further explore the causes of the decline in MD, future studies should employ more advanced imaging techniques to separately investigate the impact of aging in the dentate gyrus and CA3 subregions on MD. It is also critical to research more about the cognitive neural mechanisms underlying the impact of neocortical dysfunction on MD, with a particular focus on age-related changes in cortical-hippocampal interaction mechanisms. Large-scale prospective cohorts should also be established to validate the effectiveness of MST in early identification of cognitive impairment.
Keywords: mnemonic discrimination, pattern separation, aging, cognitive neural mechanism, cognitive impairment

