杉木无性系圃地测定性状遗传变异分析及超早期选择



摘要:【目的】利用立地条件相对均一的圃地建立短期测定林,通过分析67个参试杉木无性系苗期生长性状的遗传变异情况及遗传-环境互作效应对各性状选择的影响程度,探讨无性系苗期超早期选择策略,进一步对大量候选无性系开展快速初筛和超早期选择,以降低长期测定成本,提高无性系选育效率。【方法】利用杉木第3代种子园子代实生群体选择优良单株,扦插繁育成无性系,于圃地做性状短期测定。参试无性系67个,重复10次,12株小区,完全随机区组设计。造林1 a后,测定苗高、地径、侧枝数和最长侧枝长度共4个生长性状指标,通过构建表型方差分析模型,估算遗传方差分量,以及遗传与环境互作效应方差分量的值,并利用ASReml软件分别估算遗传力和重复力。【结果】在圃地栽植1 a后,参试无性系的苗高、地径、侧枝数和最长侧枝长度均值分别为0.640 m、1.010 cm、10.30条和0.28 m,4个观察性状的表型变异系数分别为12.86%、14.88%、21.34%和14.89%;参试67个无性系在苗高、地径、侧枝数和最长侧枝长度性状上存在显著遗传差异,测定性状的重复力均达0.74左右,遗传力估值均稳定在0.48左右;所测4个性状的遗传与环境互作方差分量占总遗传方差的35%左右;地径与苗高、侧枝数和最长侧枝长度存在显著相关,遗传相关系数均达0.9以上;以地径性状为指标进行选择,苗高、地径、侧枝数和最长侧枝长度的遗传增益估值随着入选率的降低逐渐增高,但苗高、侧枝数和最长侧枝长度重复力、遗传力以及遗传-环境互作方差比例均保持在较为稳定的范围内,呈现出一定程度的波状变化;而地径则随着入选率的降低,重复力和遗传力估值下降,遗传-环境互作方差比例增大。当入选率降低到40%以下时,杉木无性系的苗高、侧枝数和最长侧枝长度3个性状的遗传-环境互作方差比例分别达到41.18%~48.61%、37.82%~40.13%和39.61%~54.37%,但地径的遗传-环境互作方差比例由45.91%快速上升至94.33%,当入选无性系数量由19个降至16个时,地径遗传力和遗传-环境互作方差比例发生显著变化,地径遗传力由0.226 3降至0.091 4,遗传-环境互作方差比例由63.09%迅速增加到83.26%;取30%左右入选率,筛选出19个无性系用于山地造林长期测定,初选材料的苗高、地径、侧枝数和最长侧枝长度的均值分别为0.73 m、1.20 cm、12.4条和0.33 m,遗传增益均值分别为10.81%、15.45%、16.66%和13.88%,分别比群体均值高出14.06%、18.81%、20.39%和17.86%。【结论】遗传-环境互作效应对杉木无性系表型性状的影响不可忽视,其互作方差在总遗传方差中具有较大的占比;杉木无性系苗高生长和侧枝生长受遗传与环境互作作用影响相对较小,地径可能对圃地微环境变化等因素更为敏感,因而将苗高和地径性状综合起来进行杉木无性系超早期选择能够取得较为理想的结果;降低入选率并不能剔除遗传-环境互作效应对地径和最长侧枝长度的影响,高强度的选择反而会增加遗传-环境互作的影响,但适当的入选强度既能保留无性系间目标性状遗传变异的丰富度,又能固定大部分的遗传-环境互作效应;短期圃地测定,能对大量待测杉木无性系进行快速初筛,缩小长期测定林面积,降低测定成本;对参试无性系性状遗传与环境互作效应特征进行早期解析,可为充分利用遗传与环境的有益互作效应提供重要依据。
关键词:杉木;无性系;短期测定;遗传和环境互作分量;重复力;早期选择
中图分类号:S791.27 文献标志码:A开放科学(资源服务)标识码(OSID):
文章编号:1000-2006(2024)03-0063-08
Genetic variation analysis and selection of clones based on short-term nursery testing on Cunninghamia lanceolata
XIAO Hui1, LIN Zezhong1, SU Shunde1, JIANG Xiaoli2, CHEN Haiqiang3,WU Wei2, LUO Shuijin2, PAN Longying4, ZHENG Renhua1*
(1. Fujian Academy of Forestry, Key Laboratory of National Forestry and Grassland Administration on Timber Forest Breeding and Cultivation for Mountainous Areas in Southern China, Fujian Key Laboratory of Forest Cultivation and Forest Products Processing, Fuzhou 350012, China; 2. National Forest Farm of Jiangle, Jiangle 353300,China;3. Fujian Jinshuo Biotechnology Co., Ltd., Jiangle 353300, China; 4. Fujian Jinsen Forestry Co., Ltd., Jiangle 353300, China)
Abstract:【Objective】 The efficiency of selection and long-term testing costs for clonal propagation candidates of Cunninghamia lanceolata were improved by implementing a short-term nursery test with 67 clonal propagation candidates. By analyzing the genetic variation of growth traits and the impact of genetic environmental interactions on the selection of various traits of clones during the seedling stage, this study explores strategies for ultra early selection of clone seedlings. 【Method】 A selection procedure was conducted from a population of two million seedlings, with 275 well performing individuals selected for further prorogation. The seeds were collected from a local third generation C. lanceolata seed orchard.The selected plants were propagated into clones by hedged cutting. Of the propagated clones, 67 individuals with a fine rooting ability were selected for further testing under a completely random block design with 12 plants per plot and 10 replications. Four traits (seedling height, diameter above ground, number of branches and the length of the longest branch) were measured after one year’s growth. Furthermore, a phenotypic analysis of variance model was constructed to estimate the values of genetic variance component and genetic environmental interaction effect variance component, and ASReml software was used to estimate in heritance and repeatability, respectively.
【Result】 After planting in the nursery for one year, the average seedling height, ground diamete, number of lateral branches and longest lateral branch length of the tested clones were 0.640 m, 1.010 cm, 10.30 and 0.28 m, respectively. The phenotypic variation coefficients of the four observed traits were 12.86%, 14.88%, 21.34% and 14.89%, respectively. There were notable genetic differences found in the traits of seedling height, diameter above ground, number of lateral branches, and length of the longest lateral branches among the tested clones, and the repeatability of the measured traits exceeded 0.74, and the estimated heritability remained stable at around 0.48. The variance component of the genetic and environmental interaction accounted for about 35% of the total genetic variance. There is a significant correlation between ground diameter and seedling height, number of lateral branches, and length of the longest lateral branch, with genetic correlation coefficients above 0.9. The genetic gain estimates of seedling height, number of lateral branches, and longest lateral branch length gradually increase with the decrease of selection rate based on the ground diameter trait. However, the variance ratios of repeatability, heritability, and genetic environmental interaction of seedling height, number of lateral branches, and longest lateral branch length remain within a relatively stable range, exhibiting varying degrees of wavy fluctuations. As the selection rate decreases, the value of repeatability and heritability of ground diameter decrease, while the variance ratio of genetic environmental interaction increases. When the selection rate decreased to below 40%, the genetic environmental interaction variance ratios of the three traits of seedling height, number of lateral branches, and longest lateral branch length of C. lanceolata clones reached 41.18%-48.61%, 37.82%-40.13% and 39.61%-54.37%, respectively. However, the genetic environmental interaction variance ratio of diameter rapidly increased from 45.91% to 94.33%.When the number of selected clones decreased from 19 to 16, the genetic environmental interaction variance ratios of ground diameter heritability and genetic environmental interaction variance ratios changed significantly, with diameter heritability decreasing from 0.226 3 to 0.091 4 and genetic environmental interaction variance ratios rapidly increasing from 63.09% to 83.26%. Based on a selection rate of approximately 30%, 19 clones were selected for further evaluation in multiple sites in a long-term afforestation project in a mountain area. The average seedling height, ground diameter, number of lateral branches, and longest lateral branch length of the selected clones were 0.73 m, 1.20 cm, 12.4 branches and 0.33 m, respectively. The estimated average genetic gains of the four observed traits were 10.81%, 15.45%, 16.66% and 13.88%, which were 14.06%, 18.81%, 20.39% and 17.86% higher than the population average, respectively. 【Conclusion】 The effect of genetic environmental interaction on the phenotypic traits of C. lanceolata clones cannot be ignored, and its interaction variance accounts for a large proportion of the total genetic variance. The growth of height and lateral branches of C. lanceolata clones are relatively less affected by the genetic environmental interaction effect, while the growth of ground diameter are more sensitive to changes in the microenvironment of the nursery or from unknown factors. Therefore, combining the growth performance of tree height and ground diameter of C. lanceolata clones for short-term testing can achieve ideal of selection. Reducing the selection rate does not eliminate the influence of genetic environmental interaction on ground diameter and longest lateral branch length. High intensity selection can actually increase the influence of genetic environmental interaction. Appropriate selection intensity can not only retain the richness of genetic variation in target traits between clones, but also fix most of the genetic environmental interaction effects. Short-term nursery testing can serve as a rapid preliminary screening technique, especially when there is a large amount of clonal candidates to be tested. Several benefits were apparent, including forest-land use and the long-term cost efficiency of testing. The clonal traits, genetic components, and interaction between genetics and environment could be evaluated in the super-early stage of clonal evaluation.
Keywords:Cunninghamia lanceolata; clones; short-term testing; genetic and environmental interaction components; repeatability; early selection
杉木(Cunninghamia lanceolata),是我国南方主要的针叶造林用材树种。近半世纪以来,应用无性系造林在世界人工林培育中发挥了重要作用[1-2]。20世纪80年代开始,我国对杉木无性系选育和造林应用潜力开展了较多的研究[2-3],特别是在无性系性状遗传差异和无性系的规模繁育方面开展了许多有益的探索,为杉木无性系选育和推广应用奠定了良好基础[4-14]。杉木的遗传改良是一项系统工程[15-18],而无性系选择和利用是杉木长期遗传改良程序中,固定杂种优势和正向的遗传和环境互作效应,实现遗传增益最大化的重要途径。有关杉木无性系的生长[8,10-11]、树形和材性[9]等性状的重复力,遗传与环境交互作用,早晚相关与早期选择林龄等[12,15]方面报道较多。但是有关超早期的遗传和环境互作效应对杉木无性系表现评价的影响研究报道较少。本研究利用立地条件相对一致的苗圃地,进行杉木无性系测定和初筛,旨在通过控制环境异质性,解析遗传和环境互作效应对无性系生长的影响,并探索无性系的超早期选择可行性,以期改善杉木无性系及选育的时空利用效率。
1 材料与方法
1.1 试验材料
2016年春,从杉木第3代良种播种培育的苗木中挑选275株超级苗,在福建省将乐国有林场营建采穗圃。2020年春,以采穗圃穗条扦插育苗,培育供试无性系苗木,其中有67个无性系苗高超过30 cm,株型发育完好,且扦插成苗率达到90%以上的无性系,用于圃地超短期测定试验。
1.2 样地概况及试验方法
试验圃地位于福建省将乐县万安镇坊头村(117.48°E,26.73°N),海拔225 m,年均气温17.7 ℃,平均日照时间1 730 h,无霜期288 d,年均降水量1 726.3 mm。圃地前作为水稻,2020年秋水稻收割后排水晒田(通常稻田土质和肥力分布较均匀)。2021年2月全垦深挖整地,施基肥钙镁磷750 kg/hm2,经2~3次翻耕和土块破碎后整畦作床,畦面宽1.2 m,高30 cm,步道宽30 cm,四周开设排水沟。
试验采用完全随机区组设计(RCB设计),参试无性系67个,10次重复,12株小区,株行距30 cm×30 cm。2021年5月栽植。2022年5月,按小区调查每个无性系的苗高、地径、侧枝数量、最长侧枝长度。
1.3 统计分析方法
1.3.1 方差分析和遗传-环境互作方差分解模型
按完全随机区组设计进行方差分析,并估算遗传方差分量以及遗传与环境互作效应方差分量的大小,来衡量两者对无性系表型方差的贡献大小和相对重要性。表型方差可以分解为基因型方差与环境方差两部分[17],表达式为σ2P=σ2G+σ2E。式中:σ2P为表型方差;σ2G为遗传方差;σ2E为环境随机误差方差。在完全随机区组试验设计条件下,无性系的总遗传方差又可以拆分为基因型遗传方差和遗传与环境交互作用方差(简称遗传-环境互作)分量[19]:σ2G=σ2cG+σ2ge。因此,无性系的表型方差可表达为σ2=σ2cG+σ2ge+σ2E。式中:σ2cG为遗传方差;σ2ge为遗传-环境互作方差。无性系重复力和遗传力定义分别为H2=σ2Gσ2P=(σ2cG+σ2ge)(σ2cG+σ2ge+σ2E),h2=σ2cG(σ2cG+σ2ge+σ2E)。式中:H2为重复力;h2为遗传力。遗传-环境互作方差比例(NR)定义为遗传力与重复力的比值h2H2,即(σ2cGσ2cG+σ2ge),可度量无性系(基因型)的遗传稳定性,其比值越趋近于1,表明遗传与环境互作方差愈小,无性系表型性状表现愈稳定;反之,则说明无性系对环境的变化反应敏感性强。如设h2H2=a,则有σ2ge=[(1-a)/a]σ2cG,即总的遗传方差中,遗传-环境互作方差占比为(1-a)×100%。
1.3.2 遗传参数估算
利用ASReml分别估算遗传力和重复力[20]。以个体模型进行分析,yijk=μ+Bi+Tijk+EBPik+eijk。式中:yijk为个体观测值,μ为总平均值,Bi为区组效应,EBPik为区组内小区效应,Tijk为加性遗传效应,eijk为随机误差;μ、Bi为固定效应,其余为随机效应。在ASReml中,将个体作为随机因子,其值代表遗传-环境互作效应。无性系的σ2cG值则代表加性遗传效应,无性系值(Gi)与遗传增益(ΔG)计算公式为Gi=H2(x-);ΔG=ι/×100%。式中:ι为入选无性系的无性系值均值[19,21]。
2 结果与分析
2.1 无性系遗传变异分析
在圃地栽植1 a后,参试无性系的苗高、地径、侧枝数和最长侧枝长度均值分别为0.640 m、1.010 cm、10.30条和0.28 m,4个观察性状的表型变异系数分别为12.86%、14.88%、21.34%和14.89%(表1)。由表1还可见,参试无性系间苗高、地径、侧枝数及长度性状上出现了明显的变异,其中分生侧枝数的变异系数大于其他3个性状。
无性系超早期测定的4个性状的方差分析、重复力和遗传力估算结果见表1。由表1可见,参试无性系的苗高、地径、侧枝数和最长侧枝长度4个性状的遗传方差分量,均达到了极显著差异水平。4个性状的无性系重复力估值均稳定在0.74左右,遗传力估值均稳定在0.48左右。说明待测无性系间存在真实的遗传变异,利用立地条件均一的圃地,进行杉木无性系生长性状的超早期测定,获取无性系的遗传差异信息是可行的。试验结果还说明,超早期测定同时能很好地估计杉木无性系(基因型)与环境的交互作用效应。本试验中,参试无性系苗高、地径、侧枝数和最长侧枝长度性状上分离到的遗传和环境交互作用方差分量均占总遗传方差的35%左右,显见遗传-环境交互作用对杉木无性系总遗传方差分量的贡献,值得深入分析。
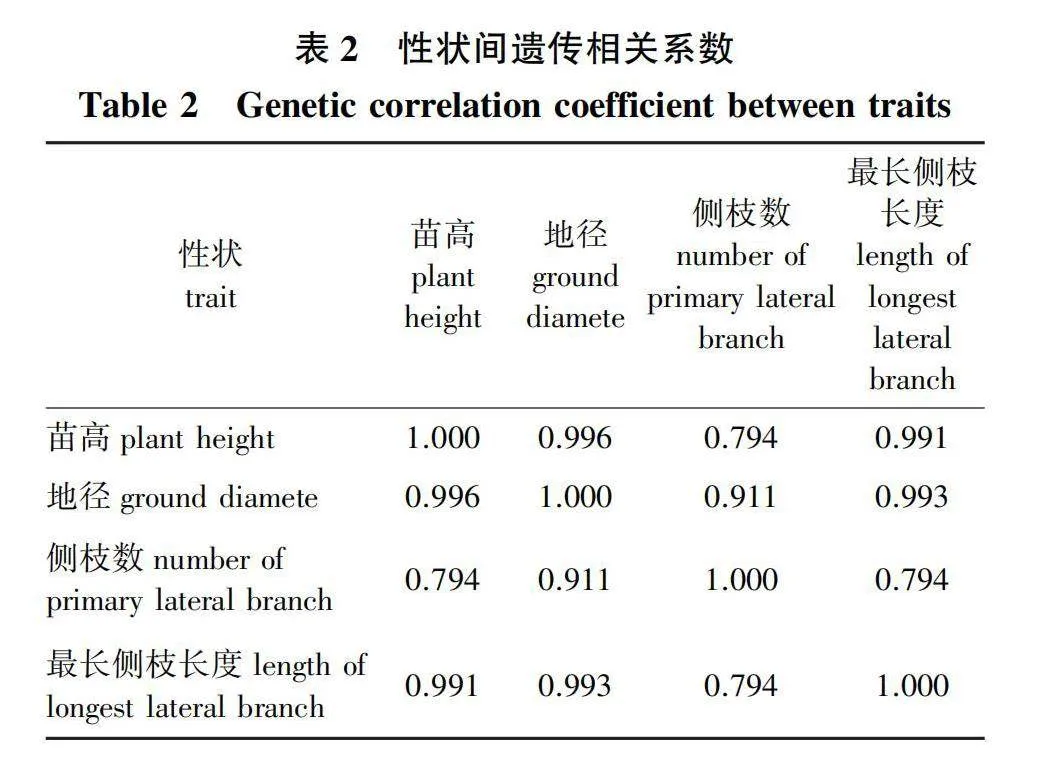
2.2 遗传-环境互作效应对苗期性状选择的影响
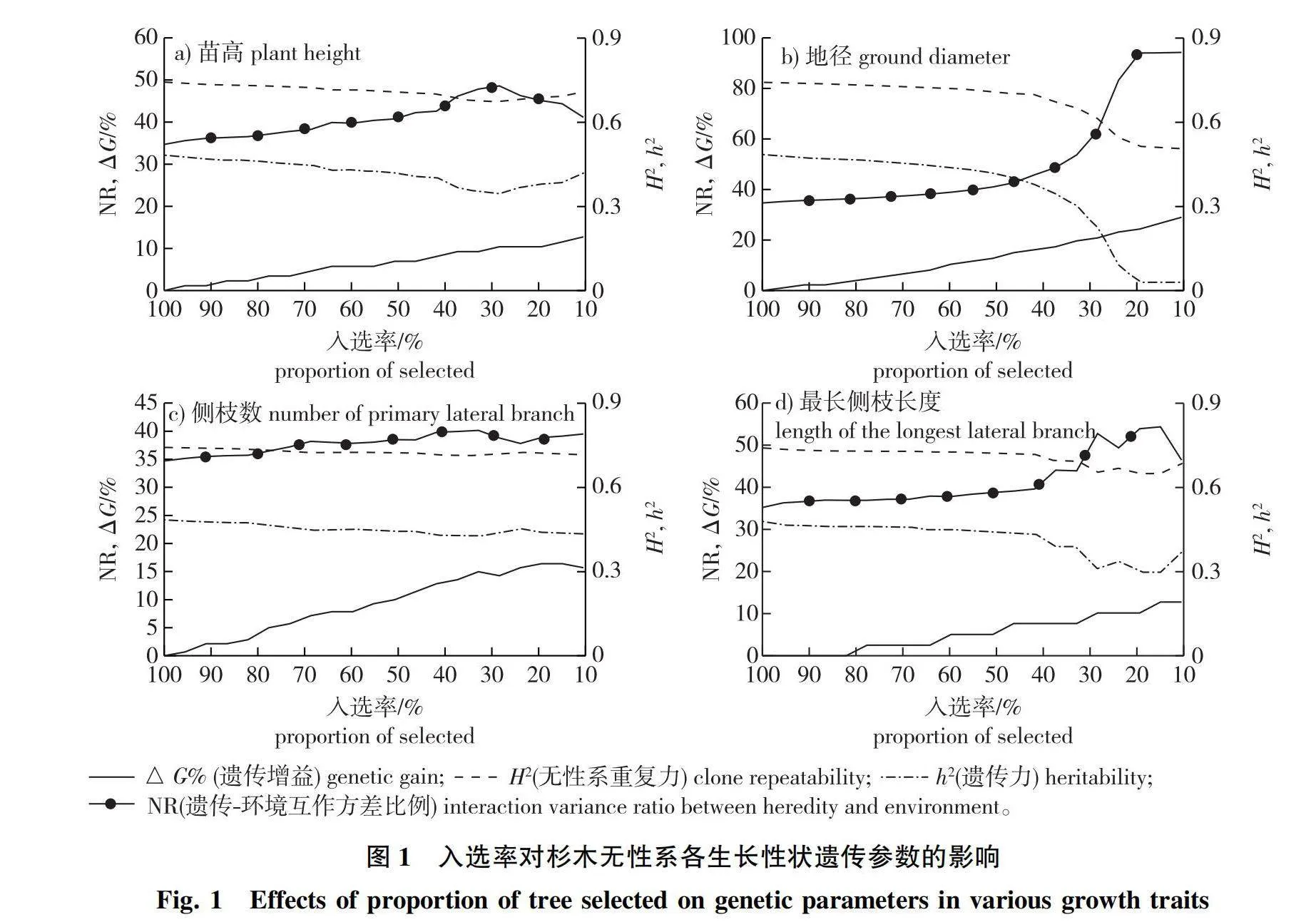
苗高、地径、侧枝数量和最长侧枝长度间遗传相关性如表2所示。地径与苗高、侧枝数量和最长侧枝长度的遗传相关系数均达0.9以上,说明地径与其他几个性状的遗传相关性最高。据此,将参试无性系按地径均值大小排序,每次剔除地径生长排序倒数三位的3个无性系,并估算每次剩余无性系后的遗传增益,同时再依据剩余无性系的观测值重新估算各性状的遗传参数。结果表明,随着剔除末位无性系数量的增多,入选率降低,苗高、地径、侧枝数和最长侧枝长度的遗传增益估值逐渐增高(图1)。由图1可见,苗高、侧枝数的重复力和遗传力,以及遗传-环境互作方差比例,并未随着入选率的降低而出现明显的规律性变化,均保持在较为稳定的范围内(图1a、1c)。而地径、最长侧枝长度,当入选率降低到40%以下时,重复力和遗传力估值下降,遗传-环境互作方差比例增大(图1b、1d)。以上分析表明,对于杉木扦插繁殖的无性系苗木,选择强度提高并不能剔除遗传-环境互作效应对地径和最长侧枝长度的影响,高强度的选择反而会增加遗传-环境互作的影响。这是否意味着提高苗期测定选择强度,有利于固定地径等性状上的正向遗传-环境互作效应,并通过扦插在无性世代间传递,有待深入探讨。
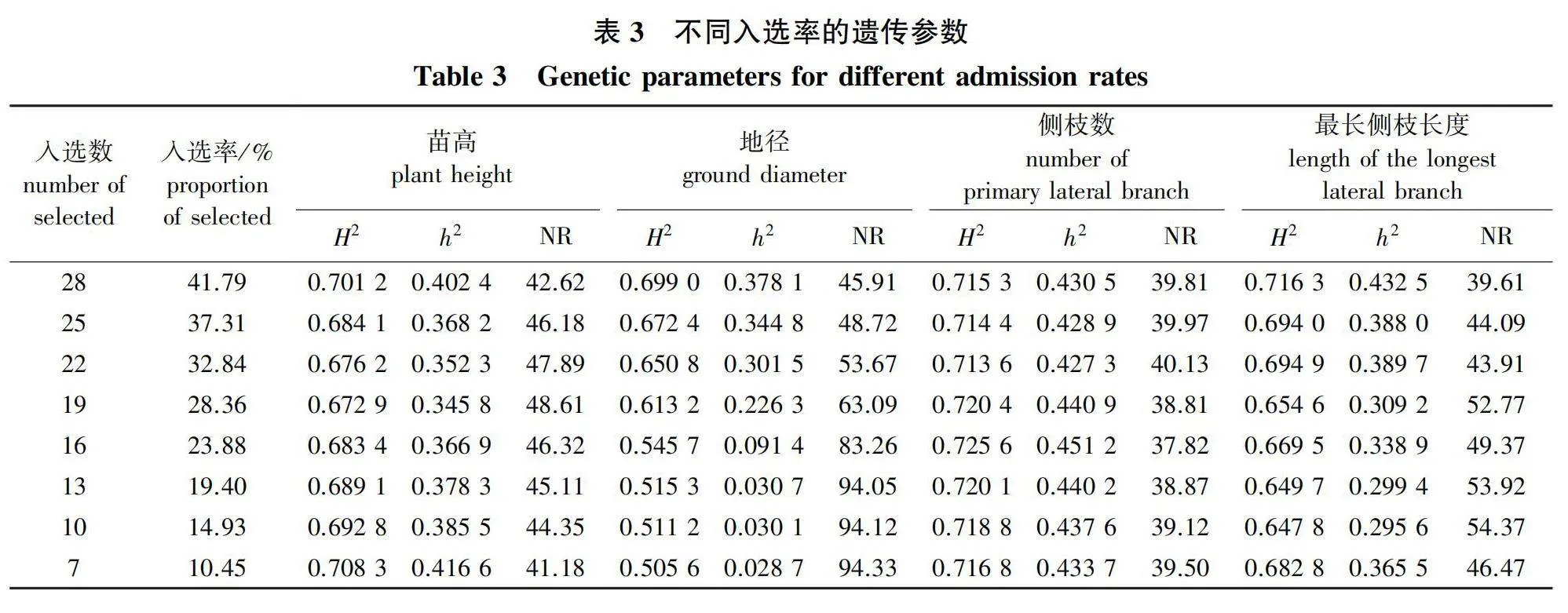
2.3 超短期测定遗传参数变化与杉木无性系初筛策略
不同入选率下遗传参数见列表3。由表3可见,当入选率低于42%时,重复力、遗传力以及遗传-环境互作方差比例在苗高、侧枝数和最长侧枝长度中均呈现出波状浮动,并且这种波动现象在三者中存在明显区别。当67个测定材料中入选数量由28个降低至7个无性系,即入选率由41.79%降低至10.45%时,苗高各遗传参数值浮动呈现明显的先降后升或者先升后降趋势,其中重复力在0.672 9~0.708 3间波动,遗传力在0.345 8~0.416 6间波动,遗传-环境互作方差比例在41.18%~48.61%间波动;侧枝数各遗传参数在不同入选率下的值较为稳定,其中重复力在0.713 6~0.725 6间波动,遗传力在0.427 3~0.451 2间波动,遗传-环境互作方差比例在37.82%~40.13%间波动;最长侧枝长度各遗传参数值的浮动幅度较大,其中重复力在0.647 8~0.716 3间波动,遗传力在0.295 6~0.432 5间波动,遗传-环境互作方差比例在39.61%~54.37%间波动。因此,可通过无性繁殖固定遗传-环境互作效应,使遗传-环境互作方差比例控制在一定范围内(表3)。
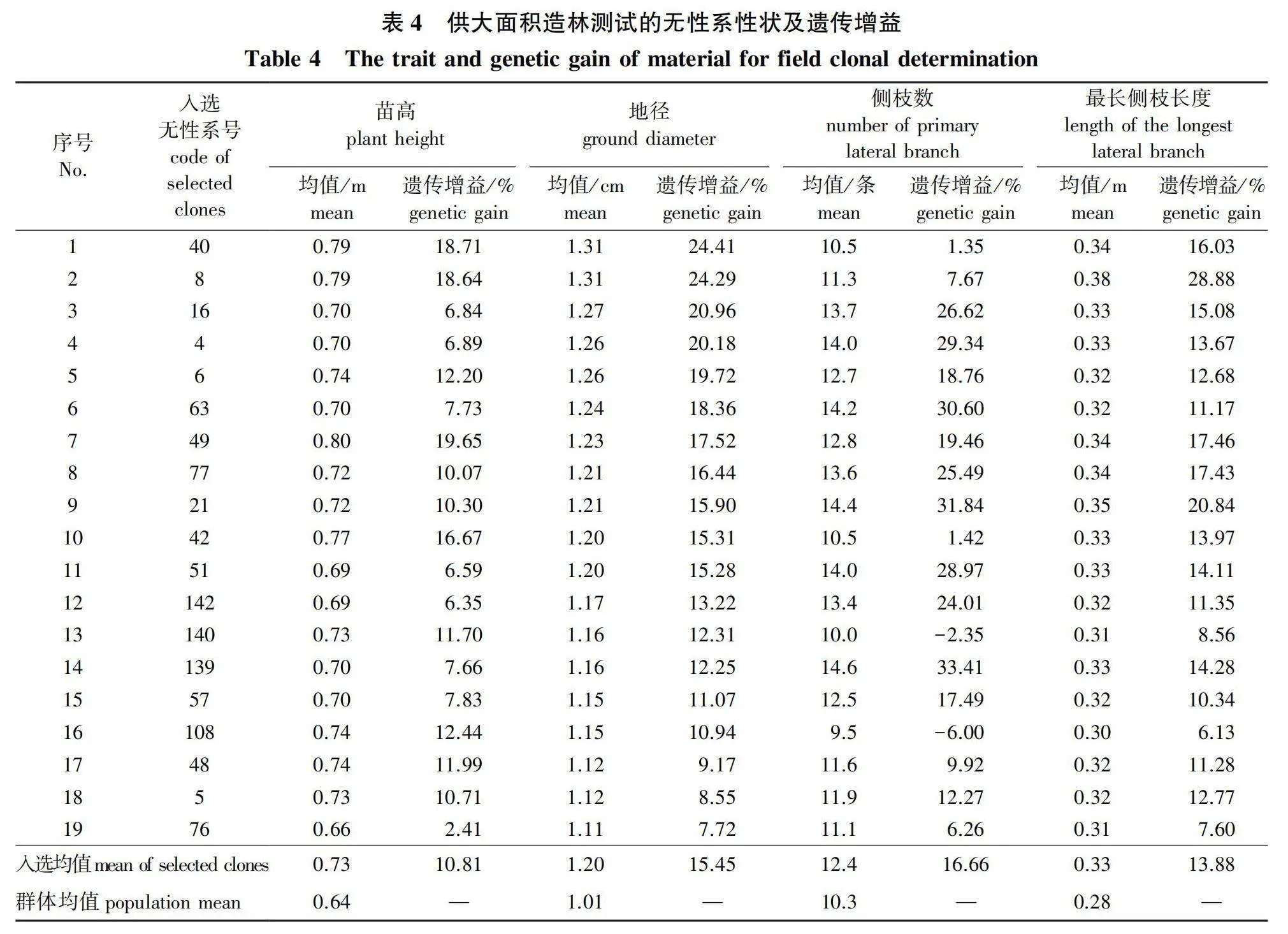
但随着入选率降低(即淘汰强度提高),地径的重复力降低趋势较慢,遗传力降低趋势较快,遗传-环境互作效应急剧增大。当入选无性系数量为19个时,地径遗传力为0.226 3,遗传-环境互作方差比例达63.09%;当入选无性系数量为16个时,地径遗传力降低到0.091 4,遗传-环境互作方差比例迅速增加到83.26%。足见遗传-环境互作效应在杉木无性系苗期测定中的重要性。对于杉木无性系超短期苗期测定和初筛,应充分重视遗传-环境互作效应。筛选排序前19名的无性系作为进入大面积的多点造林测试的材料,既能保留无性系间目标性状遗传变异的丰富度,又能固定60%左右的遗传-环境互作效应,达到圃地超短期测定筛选无性系测定材料的目的。本试验初步筛选的19个供大面积造林测试的无性系性状及遗传增益见表4。
由表4可见,初选材料的苗高、地径、侧枝数量和最长侧枝长度的均值分别为0.73 m、1.20 cm、12.4条和0.33 m,遗传增益均值分别为10.81%、15.45%、16.66%和13.88%,分别比群体均值高出14.06%、18.81%、20.39%和17.86%。其中,苗高和地径生长是圃地超早期测定的主要观察指标,而侧枝数量和最长侧枝长度则反映了植株初期树冠发育的重要评价指标。
3 讨 论
在现代林木遗传育种策略中,充分利用种间或种内杂交获得杂种优势群体,再通过无性系选择和无性繁殖技术大量克隆优选无性系,是发展无性系营林产业链中不可或缺的关键技术环节。而加速大量候选材料的遗传测定和优选无性系种苗的产业化、标准化生产,在不降低无性系选择精度的情况下,缩短无性系的测定周期,加速优质无性系苗木的繁殖,节省无性系选择和良种繁育的时间、人力、物力成本,是目前无性系营林产业链上要解决的“卡脖子”问题。
本研究通过对67个无性系1 a圃地测定,检测到无性系间在苗高、地径、侧枝数量和最长侧枝长度性状上,存在着统计学水准的极显著差异,估计的重复力和遗传力分别为0.74和0.48左右。依据参试地径生长量排序结果,筛选出位列最前的无性系19个。其苗高、地径、侧枝数和最长侧枝长度均值,分别高于群体均值的14.06%、18.81%、20.39%和17.86%。这批材料既为下一步多地点造林测定和长期观察奠定了遗传材料基础,同时又有效降低了造林比较试验林规模和性状测定工作量,显著节约了无性系选育综合成本。说明利用圃地开展杉木无性系的超短期测定,进行大量试验材料的初筛是可行的。
本试验结果还表明,杉木无性系性状的遗传-环境互作效应对表型性状的影响是值得重视的。在4个观察性状的总遗传方差中,遗传-环境互作方差比例达到35%左右,说明遗传-环境互作效应对杉木无性系的表现型值有显著的影响。有关遗传-环境互作对杨树无性系生根性状变异的报道中,发现美洲黑杨×青杨(Populus deltoides × P. euramericana)无性系水培生根试验中,无性系的所有生根性状均明显受到可传递遗传-环境互作效应的影响[22]。本试验以杉木无性系地径生长量排序去劣留优,逐步淘汰地径生长量小的无性系,发现选择强度与遗传-环境互作的联动关系。当入选率降低到40%以下时,随着淘汰无性系数量的增加,杉木无性系的苗高、侧枝数量和最长侧枝长度3个性状的遗传-环境互作方差比例分别达到41.18%~48.61%、37.82%~40.13%和39.61%~54.37%,但地径的遗传-环境互作方差比例由45.91%快速上升至94.33%。这表明在圃地超短期测定中,杉木无性系苗高生长和分枝性状受遗传-环境互作效应影响相对较小,而地径生长对圃地微环境变化或不明因素扰动较为敏感,因而该性状可能易受遗传-互作效应影响。所以在杉木无性系超短期测定时,将苗高和地径性状综合起来进行选择可能更为理想。
优良基因型的无性繁殖,通常认为是一种可充分利用该基因型的加性和非加性遗传效应,保持无性系原株优良特性的繁殖方法[23]。但对于待测无性系来说,既有不同基因型对生存环境波动反应规范的不同,也有诸如C-效应(C-effects)对无性系表现的附加效应等因素,影响到对基因型的客观评价。杉木遗传早期的遗传测定结果发现,试验中某性状遗传-环境互作方差分量虽然达到统计的显著或极显著水准,但并不意味着所有参试材料、所有性状上都是遗传-环境互作敏感基因型[24]。就高生长和材积生长量而言,一般1/3左右基因型为“稳定速生型”,另外1/3左右的基因型是属于“遗传-环境互作敏感型”(生长波动型或不稳定型),其余1/3左右为“持续慢生型”。显然,从生长量性状来考虑,“持续慢生型”无性系无疑是淘汰对象,但往往“遗传-环境敏感型”无性系常因其性状不稳定而被忽视。实际上,杉木无性系测定群体中“遗传-环境敏感型”无性系,最大的特点是具较强的逆境感知和自我保护能力,一旦逆境解除即可恢复生长,是适应特殊逆境定向栽培品种候选材料来源。对于固定“遗传-环境敏感型”无性系中遗传-环境互作效应的可行性还有待深入研究。
除了基因型与环境的互作效应对无性系的影响,还有一类广义地被称作“C-效应”的非典型的遗传效应(atypical genetic effects)的影响,如位置效应(topophysis)、成熟效应(cyclophysis)、环境预调节效应(environmental pre-conditioning effects)、母体效应(maternal effects)和细胞核外(如线粒体和叶绿体的)DNA遗传物质导致的效应。据报道这类非DNA序列变异导致的效应,能在遗传材料的有性或无性繁殖世代间多代传递,形成表型性状的附加修饰,使得基因型效应估计偏高或偏低于根据经典遗传学理论和方法计算得到的估值[19]。国内也有在不同树种上发现C-效应的存在对无性系表现的不良影响的报道,国内报道有涉及杉木、杨树(Populus spp.)、青海云杉(Picea crassifolia)等树种的无性系繁殖时,插穗的位置效应、成熟效应导致的无性系分株间性状的不稳定性和生长量下降等问题[25-27]。为了在源头上控制待测无性系苗木繁育阶段C-效应的前置影响,本研究严格执行《中华人民共和国林业行业标准:杉木无性系扦插育苗技术规程》(LY/T 1885—2010)[28],从无性系原株选择、采穗圃营建管理、穗条采集调制、扦插和苗圃管理等每个关键环节都严格把关,有效控制了位置效应、成熟效应等C-效应对无性系苗的不利影响,利用圃地顺利开展了超短期杉木无性系测定,从67个无性系材料中初筛出19个表现优良的无性系开展多地点长期测定,不仅显著降低了无性系测定的综合成本,而且也为杉木无性系多地点长期测定材料来源提供了保障。
参考文献(reference):
[1]韩刚,黄少伟.无性系林业与林业可持续发展[J].福建林业科技,2003,30(4):89-92.HAN G,HUANG S W.Clone forest and the sustainable development of forestry[J].J Fujian For Sci Technol,2003,30(4):89-92.DOI: 10.13428/j.cnki.fjlk.2003.04.026.
[2]康向阳.关于无性系林业若干问题的认识和建议:以杨树为例[J].北京林业大学学报,2017,39(9):1-7.KANG X Y.Cognition and suggestions on some issues related to clonal forestry:taking poplar as an example[J].J Beijing For Univ,2017,39(9):1-7.DOI: 10.13332/j.1000-1522.20170019.
[3]彭万喜,吴义强,张仲凤,等.中国的杉木研究现状与发展途径[J].世界林业研究,2006,19(5):54-58.PENG W X,WU Y Q,ZHANG Z F,et al.Situation and developing trends of Chinese Fir[J].World For Res,2006,19(5):54-58.DOI: 10.13348/j.cnki.sjlyyj.2006.05.010.
[4]马常耕.杉木近期良种选育的基本策略[J].广东林业科技,1992,8(4):1-5.MA C G.Basic strategy of Chinese fir breeding in the near future[J].Guangdong For Sci Technol,1992,8(4):1-5.
[5]王港,陈骏,侯娜,等.杉木无性系规模化组培繁育技术研究[J].湖北林业科技,2014,43(5):7-9,63.WANG G,CHEN J,HOU N,et al.Study on large-scale tissue culture propagation technology of Cunninghamia lanceolata[J].Hubei For Sci Technol,2014,43(5):7-9,63.DOI: 10.3969/j.issn.1004-3020.2014.05.003.
[6]欧阳磊,郑仁华,翁玉榛,等.杉木优良无性系组培快繁技术体系的建立[J].南京林业大学学报(自然科学版),2007,31(3):47-51.OUYANG L,ZHENG R H,WENG Y Z,et al.Establishment of technique system of tissue culture on Chinese fir superior clones[J].J Nanjing For Univ (Nat Sci Ed),2007,31(3):47-51.DOI: 10.3969/j.issn.1000-2006.2007.03.010.
[7]吴擢溪,李振问,吴大忠.杉木组织培养繁殖体系建立的研究[J].福建林学院学报,1991,11(1):67-74.WU Z X,LI Z W,WU D Z.Study on the establishment for breeding system Chinese fir tissue culture[J].J Fujian Coll For,1991,11(1):67-74.
[8]贾茹,孙海燕,王玉荣,等.杉木无性系新品种‘洋020’和‘洋061’10年生幼龄材微观结构与力学性能的相关性[J].林业科学,2021,57(5):165-175.JIA R,SUN H Y,WANG Y R, et al.Relativity of microstructures and mechanical properties of juvenile woods of 10-year-old new Chinese fir clones ‘Yang 020’ and ‘Yang 061’[J].Sci Silvae Sin,2021,57(5):165-175.DOI: 10.11707/j.1001-7488.20210516.
[9]李荣丽,黄寿先,梁机,等.杉木无性系生长和木材品质性状遗传变异研究[J].南方农业学报,2014,45(9):1626-1631.LI R L,HUANG S X,LIANG J,et al.Genetic variation of growth traits and wood properties in Chinese fir clones[J].J South Agric,2014,45(9):1626-1631.DOI: 10.3969/j.issn.2095-1191.2014.9.1626.
[10]彭华贵,李兆佳,周志平,等.4个杉木品系在广东省天井山林场的生长比较[J].林业与环境科学,2017,33(4):25-28.PENG H G,LI Z J,ZHOU Z P,et al.The comparison of growth performance of four provenances Cunninghamia lanceolata in Guangdong Tianjingshan Forest Farm[J].For Environ Sci,2017,33(4):25-28.DOI: 10.3969/j.issn.1006-4427.2017.04.005.
[11]孙云,李鑫,李勇,等.幼树阶段杉木不同无性系生长与形态性状分析[J].中南林业科技大学学报,2019,39(3):34-39.SUN Y,LI X,LI Y,et al.Analysis of growth traits and morphological characters among different clones of Cunninghamia lanceolata in young tree stage[J].J Cent South Univ For Technol,2019,39(3):34-39.DOI: 10.14067/j.cnki.1673-923x.2019.03.006.
[12]胡德活,林绪平,阮梓材,等.杉木无性系早-晚龄生长性状的相关性及早期选择的研究[J].林业科学研究,2001,14(2):168-175.HU D H,LIN X P,RUAN Z C,et al.Study on the growth character correlation of Chinese fir clone and early selection[J].For Res,2001,14(2):168-175.DOI: 10.3321/j.issn:1001-1498.2001.02.008.
[13]何贵平,陈益泰,关志山,等.杉木无性系生长及分枝习性的遗传变异[J].林业科学研究,1997,10(5):556-559.HE G P,CHEN Y T,GUAN Z S,et al.Genetic variation of growth and branching habits of Chinese fir clones[J].For Res,1997,10(5):556-559.
[14]段红静,曹森,郑会全,等.杉木不同无性系主要经济性状变异分析[J].西南林业大学学报,2016,36(2):78-83.DUAN H J,CAO S,ZHENG H Q,et al.Variation analysis on the main economic characters of Chinese fir clones[J].J Southwest For Univ (Nat Sci),2016,36(2):78-83.DOI: 10.11929/j.issn.2095-1914.2016.02.013.
[15]饶显生,程书建,刘化桐,等.杉木无性系苗期选择可靠性分析[J].福建林学院学报,2002,22(1):82-85.RAO X S,CHENG S J,LIU H T,et al.Study on reliability of clones selection in Chinese fir on seedling stage[J].J Fujian Coll For,2002,22(1):82-85.
[16]齐明,何贵平,曹高铨,等.杉木耐贫瘠优良无性系苗期初选[J].林业科学研究,2013,26(3):379-383.QI M,HE G P,CAO G Q,et al.Preliminary evaluation on fine clones of Chinese fir based on sexual progeny tests[J].For Res,2013,26(3):379-383.DOI: 10.13275/j.cnki.lykxyj.2013.03.019.
[17]王明庥.林木遗传育种学[M].北京:中国林业出版社,2001.WANG M X.Forest tree genetics and breeding[M].Beijing:China Forestry Publishing House,2001.
[18]陈岳武,施季森.杉木遗传改良中的若干基本问题[J].南京林业大学学报(自然科学版),1983,7(4):5-19.CHEN Y W,SHI J S.Some fundamental problems in genetic improvement of Chinese fir[J].J Nanjing ForUniv,1983,7(4):5-19.
[19]WHITE T L, ADAMS W T, NEALE D B. Forest Genetics[M]. Massachusetts, USA: CABI Publishing, 2007.
[20]林元震.R与ASReml-R统计学[M].北京:中国林业出版社,2017.LIN Y Z.R and ASReml-R statistics[M].Beijing:China Forestry Publishing House,2017.
[21]朱之悌.林木遗传学基础[M].北京:中国林业出版社,1990.ZHU Z T.Basis of forest genetics[M].Beijing:China Forestry Publishing House,1990.
[22]李火根,黄敏仁,陈道明.美洲黑杨×青杨F1无性系生根性状的遗传变异及C效应[J].东北林业大学学报,1998,26(3):12-15.LI H G,HUANG M R,CHEN D M.Genetic variation and ceffect of rooting characters of Populus deltoides× Populus euramericana F1 clone[J].J Northeast For Univ,1998,26(3):12-15.
[22]李火根,黄敏仁,陈道明.美洲黑杨×青杨F_1无性系生根性状的遗传变异及C效应[J].东北林业大学学报,1998,26(3):12-15.LI H G,HUANG M R,CHEN D M.Genetic variation and C-effectsin rooting characteristicsof Populus deltoides × P.cathayana F1clones[J].J Northeast For Univ,1998,26(3):12-15.
[23]NGUYEN H T,CHEN Z Q,FRIESA,et al.Effect of additive,dominant and epistatic variances on breeding and deployment strategy in Norway spruce[J].Forestry,2022,95(3):416-427.DOI: 10.1093/forestry/cpab052.
[24]杨米娇. 杉木半同胞家系种批和空间重复对育种值估计的影响[D]. 南京:南京林业大学,2016.YANG M J. Different planting time and the space repeat effects on the estimation of the breeding value for the Half-sib families of Chinese fir[D]. Nanjing: Nanjing Forestry University,2016.
[25]平文丽,杨铁钊.体细胞无性系变异及其在作物育种中的应用[J].西北农业学报,2005,14(5):23-31.PING W L,YANG T Z.Somaclonal variation and it’s application in crop breeding[J].Acta Agric Boreali Occidentalis Sin,2005,14(5):23-31.DOI: 10.3969/j.issn.1004-1389.2005.05.006.
[26]王军辉,张建国,张守攻,等.青海云杉硬枝扦插的激素、年龄和位置效应研究[J].西北农林科技大学学报(自然科学版),2006,34(7):65-71.WANG J H,ZHANG J G,ZHANG S G,et al.Research of hormone,age and position effect of hardwood cutting in Picea crassifolia Kom[J].J Northwest Sci Tech Univ Agric For (Nat Sci Ed),2006,34(7):65-71.DOI: 10.13207/j.cnki.jnwafu.2006.07.015.
[27]郭长花.白杨年龄与位置效应的生理生化机制研究[D].北京:北京林业大学,2008.GUO C H.Study on physiological and biochemical mechanism of age effect and position effect in white poplar[D].Beijing:Beijing Forestry University,2008.
[28]国家林业局.杉木无性系扦插育苗技术规程:LY/T 1885—2010[S].北京:中国标准出版社,2010.State Forestry Administration of the People’s Republic of China.Technical regulation of cutting propagation for Cunninghamia lanceolata clones:LY/T 1885—2010[S].Beijing:Standards Press of China,2010.
(责任编辑 吴祝华)

