水位变动对三峡水库消落带典型雌激素效应物质及活性的影响
周敏 邵迎 黄思瑜 陈忠礼


doi: 10.11835/j.issn.1000-582X.2023.253
收稿日期:2022-12-12
網络出版日期:2023-06-19
基金项目:国家自然科学基金资助项目(51909015)。
Foundation:Supported by National Natural Science Foundation of China(51909015).
作者简介:周敏(1998—),女,硕士研究生,主要从事环境毒理学方向的研究,(E-mail)mindy.zhou@cqu.edu.cn。
通信作者:邵迎,女,讲师,(E-mail)ying.shao@cqu.edu.cn。
摘要:以三峡库区消落带为研究对象,将化学分析与生物测试相结合,研究水位变动对消落带土壤(落干期)和沉积物(淹水期)雌激素效应物质种类和含量及雌激素活性的影响,探讨典型雌激素与雌激素效应的关联程度,以期为水库消落带生态安全和环境健康管理提供重要的数据支撑。借助超高效液相色谱-质谱(UPLC-MS)对8种典型雌激素的赋存与质量浓度进行靶向分析,并使用重组基因酵母筛选(YES)体系检测环境样品雌激素活性,通过相关性分析和浓度加和计算建立化学物质与生物效应间的相关联系。8种典型雌激素仅有雌酮(E1)和乙炔基雌二醇(EE2)被检出,含量在0.025~2.667 ng/g范围内。淹水期的沉积物具有明显的雌激素活性,其雌二醇当量(EEQ)值为0.637~6.987 ng/g。相关性分析结果显示,靶向分析的雌激素效应物质与雌激素效应间无明显相关性,仅能解释29.46%的雌激素活性。水位变动影响消落带雌激素效应物质的种类和质量浓度,淹水提高了沉积物雌激素活性。典型雌激素物质与库区消落带雌激素效应无显著关联。因此,需要开发新的方法与技术,以便更为精确地指导消落带雌激素效应的风险识别与管控。
关键词:沉积物;水位变动;三峡水库;雌激素活性;典型雌激素
中图分类号:X524 文献标志码:A 文章编号:1000-582X(2024)04-001-11
The effects of water fluctuation on typical estrogens and activities in the water level fluctuation zone of the Three Gorges Reservoir
ZHOU Min1, SHAO Ying1, HUANG Siyu2, CHEN Zhongli1
(1. Key Laboratory of the Three Gorges Reservoir Regions Eco-Environment, Chongqing University, Chongqing 400044, P. R. China; 2. Chongqing Institute for Food and Drug Control, Chongqing 401121, P. R. China)
Abstract: In this study, the water level fluctuation zone of the Three Gorges Reservoir (TGR) was selected for investigation. A combination of chemical analysis and biological testing was utilized to investigate the effects of water fluctuation on the compounds and concentrations of estrogenic effector substances, as well as the estrogenic activity in soil (during the non-flooding period) and sediment (during the flooding period). The correlation between typical estrogens and estrogenic activity was analyzed. The study aimed to provide crucial data support for reservoir ecological safety and environmental health management. The distribution and concentration of 8 typical estrogens were analyzed using an ultra-performance liquid chromatography-mass spectrometry (UPLC-MS), while estrogenic activity was determined via the yeast estrogen screening assay (YES). Correlation analysis and concentration summation calculations were used to establish the relationships between chemical substances and biological effects. Among the 8 studied estrogens, only estrone (E1) and ethinylestradiol (EE2) were detected, with concentrations ranging from 0.025 ng/g to 2.667 ng/g. Sediments during the flooding period showed significantly higher estrogenic activity, with 17β-estradiol equivalents (EEQ) ranging from 0.637 ng/g to 6.987 ng/g. However, correlation analysis did not reveal a distinct correlation between the target compounds and estrogenic effects, as the detected chemicals only accounted for about 29.46% of the estrogenic activity. The results suggest that water fluctuation can influence the type and the concentration of estrogens in the water level fluctuation zone of the TGR, leading to increased estrogenic activity during the flooding period. The direct linkage between typical estrogens and estrogenic activity was not identified. Therefore, new methods and techniques are required to enhance identification accuracy and improve the management of the risk of estrogenic effects in the water level fluctuation zone.
Keywords: sediments; water level fluctuation; Three Gorges Reservoir; estrogenic activity; typical estrogens
内分泌干扰物(EDCs)是一类在环境中广泛存在,可对动物和人类的甲状腺、皮质激素、神经系统、生殖系统产生不良影响的新污染物[1-2],已经被联合国环境规划署列为需要全球合作的环境问题[3]。其中,雌激素会影响生殖能力,降低精子数量,诱导产生卵黄素(VTG)并改变雄鱼的其他生殖特征,甚至还会增加妇女患乳腺癌的风险[4]。大量研究表明,典型雌激素物质在世界各地土壤和水体检出率、浓度均明显增加,在该环境浓度下会破坏鱼类、家畜、野生动物以及人类健康[5]。因此,环境中典型雌激素的含量及其风险效应逐渐引起关注。环境样品的雌激素效应通常是所有化合物综合作用的结果,基于效应的生物分析方法常被用于评估环境的雌激素效应风险。例如,通过荧光素酶实验评估丁基羟基茴香醚(BHA)、丁基羟基甲苯(BHT)、丙基棓酸盐(PG)和丁基苯甲酸酯(BP)的雌激素/抗雌激素作用[6]。2,3,7,8-四氯二苯并對二噁英(TCDD)染毒转基因小鼠的体内实验通过雌激素反应性报告检测雌激素/抗雌激素作用[7]。通过测量6种有机氯农药和这些农药的混合物在转染的HeLa细胞中调节雌激素反应报告基因的转录激活能力的体外实验,来测量这些物质的雌激素活性[8]。
在河流、水库和湖泊等天然与人工水体中,水文情势是污染物迁移转化的主要驱动因子。人工调控水位变动是水库区别于天然水体的重要特征之一,影响污染物在水环境中的迁移、分布、浓度及效应。与其他大型水库和湖泊相比,三峡水库具有独特的反季节水位调度运行方式,自2010年进入正常水位调控运行周期以来,三峡水库采用“蓄清排洪”的调度方式,在丰水期(夏季)以145 m低水位运行,枯水期(冬季)则以175 m高水位运行,在30 m水位变动范围内形成了与天然河流涨落季节相反的水库消落带,水位落差大、水淹时间长且具有显著的水淹梯度。水位波动使得三峡库区消落带物质交换和能量流动极为活跃[9]。水位变动下消落带处在淹水和裸露交替的动态变化过程中,影响污染物在沉积物中的吸附解吸过程,从而导致污染物空间分布格局发生变化[9]。水位变动还会引起水体流量和悬浮颗粒物明显变化,水体稀释作用以及悬浮颗粒物含量将对污染物浓度水平和污染效应产生重要影响[10]。此外,水位波动过程、库区气候变化、水体含氧量、氧化还原条件、温度以及微生物活动会形成复杂的综合效应,影响污染物的转化释放[11],改变污染物种类和毒性效应。因此,在水库及消落带生态系统中,水位变动与污染物分布及生物效应存在密切的响应关系。
研究表明,三峡水库运行初期,库区重庆段的沉积物有机萃取物已经表现出显著的雌激素效应[12- 13],雌激素及其效应可能对库区生态环境造成潜在风险。水库经过多年水文运行以来,典型雌激素在三峡库区消落带的环境赋存与分布情况如何?水位变动对消落带雌激素效应潜在风险的影响怎样?为了回答上述问题,以三峡水库消落带作为研究对象,在淹水期和落干期从水库干流和支流(共5个地点)消落带采集沉积物样品,采用液相色谱-质谱(LC-MS)分析雌三醇、17β-雌二醇、17α-雌二醇、17α-乙炔雌二醇、雌酚酮、己烯雌酚、双烯雌酚和己烯雌酚8种典型环境雌激素的分布,使用重组基因酵母筛选(YES)技术测试雌激素活性,分析三峡库区典型雌激素的污染情况以及水位波动对雌激素效应的影响,以期为水库消落带生态系统健康保护提供支撑。
1 材料与方法
1.1 试剂
雌三醇、17β-雌二醇、17α-雌二醇、17α-乙炔雌二醇、雌酚酮、己烯雌酚、双烯雌酚和己烯雌酚的混合标准储备溶液(纯度为99%)购自上海安谱璀世标准技术服务有限公司。17β-雌二醇(E2,Sigma-Aldrich)溶于二甲亚砜(DMSO,Sigma-Aldrich)作为阳性对照(PC)储备液。所有用于样品处理和分析的溶剂(正己烷、丙酮、乙腈和甲醇)均为HPLC级。
1.2 样品采集与处理
三峡水库位于长江中上游地区,涉及重庆市和湖北省共26个区县。占地面积54 061.5 km2,其中地表水面积1 864 km2,占整个水库面积的3.44%[14]。三峡库区的饮用水源为129个城镇和117万多人提供饮用水,对当地居民的生活和区域经济发展具有重要意义[15- 16]。2010年以来,三峡库区水位在夏季145 m和冬季175 m之间波动[17],在三峡库区形成了垂直高度为30 m、长度为662 km、总面积为349 km2的水库消落带[18]。
研究中,在三峡库区干流和典型支流共设置了5个采样点进行沉积物样品采集(见表1),分别是丰都(FD)、高阳(GY)、云阳(YY)、奉节(FJ)、巫山(WS)。样点布设包括库首峡谷地带、库区中部腹心地带、库尾区域,体现水库不同区域的水文特征。在2020年8月(落干期)和2021年4月(淹水期)分别于每个采样点用抓斗随机采取3个沉积物样品,并充分混合,作为该样点的代表沉积物样品,总共采集了10个代表样品。所有的沉积物样品都收集在锡箔袋中,低温保存运送至实验室,并保存于-80 ℃冰箱中。
沉积物样品的预处理流程如下[19-20]:经过冷冻干燥、研磨并过筛(60目)后,称取20 g样品,以正己烷:丙酮(1:1,V/V)混合液作为溶剂,使用索氏提取器(JPSXT-06,中国上海)进行为期18 h的索氏提取。提取液经旋转蒸发、氮吹至近干,并复溶于1 mL甲醇中,此后,提取液被平均分成2部分,分别用于分析化学成分和生物测定。生物测定样品将提取液再次氮吹近干,复溶于0.5 mL二甲亚砜(DMSO,Sigma-Aldrich)中,待分析。同时,用于化学分析的提取液过膜(0.22 μm)去除杂质。这两部分待分析提取液的最终沉积物当量(SEQ)质量浓度均为20 g/mL。
1.3 雌激素活性测定(YES)
为了避免细胞毒性对雌激素效应测定的干扰,首先根据下述方法使用H4IIE大鼠肝癌细胞进行了细胞毒性测定(MTT法)[21]。细胞在37 ℃、5%的二氧化碳和95%的湿度环境下,在含有10%胎牛血清(FBS,Gibco)和1%青霉素-链霉素溶液(PS,BI)的DMED培养基(Dulbeccos Modified Eagles Medium,BI)中培养。进行MTT测定前,将2×104 /mL细胞悬液接种于96孔板中,每孔100 μL,在37 ℃下培养24 h,使细胞贴壁,再将细胞暴露在每个样品的1:2系列稀释液中,每个质量浓度梯度设置3个平行[22]。暴露48 h后,去掉原培养液,每孔加入100 μL的MTT(3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四氮唑,0.5 mg/mL)溶液培养30 min。吸出孔内溶液,每孔加入200 μL DMSO,振荡并混匀。在酶标仪(Synergy LX,BioTek,美国)492 nm下测定吸光度进行细胞活力估计。细胞存活率用样品组与空白对照组细胞吸光度的比值來表示,结果为相对存活率并以百分比给出,相对存活率低于80%,表明具有潜在细胞毒性,每个样品独立重复实验3次,每次设置3个平行。
重组酵母菌细胞购自无锡中科水质环境技术有限公司,该菌株将人类雌激素受体基因、雌激素应答表达子质粒和编码了β-半乳糖苷酶的基因转入酵母中,通过测量β-半乳糖苷酶的活性来检测样品的雌激素激动剂活性[23]。酵母检测按以下方法进行[24]。简而言之,将酵母菌在30 ℃恒温振荡培养箱中培养24 h,使其到对数生长期,并调整到光密度(OD600)为0.75±0.005,将5 μL样品与995 μL菌液混合,取200 μL置于96孔板中,每个样品进行1:2的系列稀释,设3个平行孔,培养4 h后,用酶标仪测定OD600。随后进行β-半乳糖苷酶的活性测定,以加入Na2CO3终止酶活反应,用酶标仪测定OD420。根据细胞毒性测试结果确定测试样品的最高质量浓度,每次实验中包括17β-雌二醇(E2)的稀释系列(4、8、20、40、80、200、400、800、2 000 pM)作为阳性对照(PC)和0.5%DMSO作为阴性对照,所有的样品至少进行3次独立重复实验。β-半乳糖苷酶的活性根据式(1)计算[25]。
, (1)
式中:U为β-半乳糖苷酶活性,t、V、D分别为酶反应时间、体积和稀释系数。OD600是在600 nm处测量的吸光度,OD420和OD'420分别是样品组和阴性对照组在420 nm处的吸光度。每个样品有9个1:2的系列稀释质量浓度。
1.4 化学分析
使用超高效液相色谱(UPLC)串联三重四级杆质谱(Waters Xevo TQ-S,Waters公司,美国)进行典型雌激素的含量测定,该串联质谱仪在多重反应监测(MRM)模式下运行。液相色谱柱为ACQUITY UPLC HSS T3柱(2.1 mm × 50 mm,1.8 μm粒径,Waters公司,美国),进样量为5 ?L,柱温40 ℃。流动相由水(溶剂A)和乙腈(溶剂B)组成(V/V),流速400 μL/min。梯度洗脱模式按照以下条件进行:0~2 min,90%溶剂A,10%溶剂B;2~3 min,50%溶剂A,50%溶剂B;3~4.1 min:10%溶剂A,90%溶剂B;4.1~5 min,90%溶剂A,10%溶剂B。
1.5 数据分析
所有实验数据均使用SPSS和Origin 2019(美国微软)进行分析。采用单因素方差分析(ANOVA)进行统计学上的差异检验,再用皮尔逊相关分析确定化学物质与雌激素作用之间的相关性(*p≤0.05和**p≤0.01)。不同的大写字母表示淹水期不同采样点的提取物产生的β-半乳糖苷酶活性在p<0.05时有显著差异,不同的小写字母表示落干期不同采样点的提取物产生的β-半乳糖苷酶活性在p<0.05时有显著差异。
为了确定E2和环境样品在YES检测中的EC20和EC50,使用Graphpad Prism(8.0版,美国)绘制质量浓度与β-半乳糖苷酶活性的剂量反应曲线并进行拟合,生物测定的雌激素当量(EEQbio)用公式(2)计算[26]。
, (2)
式中:Ci指当样品雌激素活性等于E2的EC20对应的雌激素活性时样品的质量浓度。
环境样品的理论EEQ值根据浓度加和概念从目标化合物的化学分析中计算出来,理论雌激素当量(EEQchem)用式(3)计算[27]:
, (3)
式中:Ci和REPi是每个目标化学品的质量浓度和它在YES实验中的相对雌激素潜力。
2 结果与讨论
2.1 三峡库区消落带沉积物细胞毒性
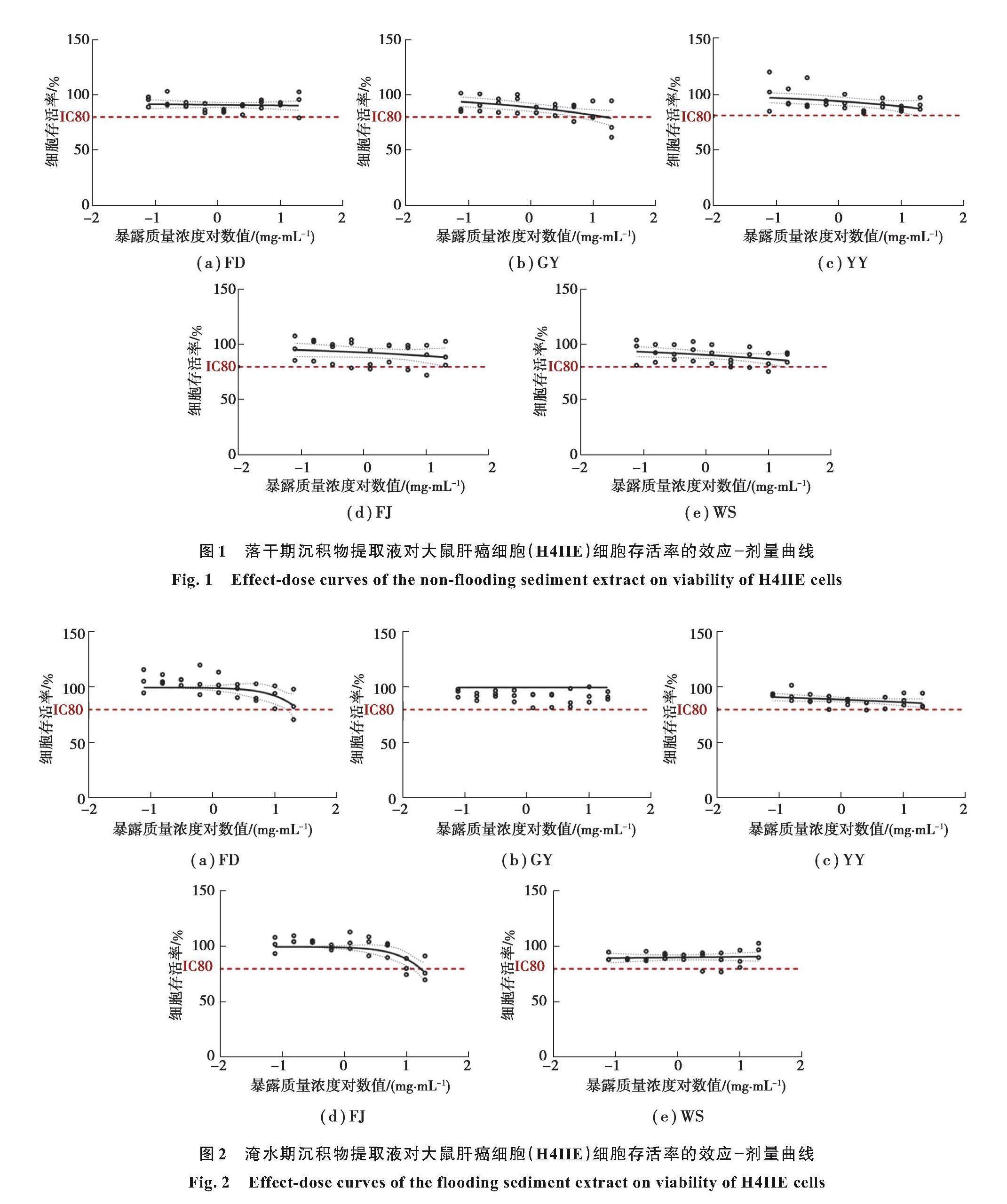
细胞毒性实验结果显示,在0.08~20 mg/mL范围内,大多数沉积物样品对H4IIE细胞没有诱发细胞毒性作用。沉积物提取液对H4IIE细胞暴露48 h后,细胞存活率如图1和图2所示,随着提取液质量浓度的增加,细胞活力略有下降。落干期位于库区支流高阳的沉积物提取液毒性最大:当暴露质量浓度为20 mg/mL时,细胞存活率最低,仅为76%(见图1)。分析原因是水库蓄水导致支流回水,一些污染物被输送到支流中[28]。同时,支流较低的流速和较长的水体停留时间可能会促进污染物在沉积物中的积累[29],类似情况已在三峡库区污染物的研究中被证实[30]。丰都的沉积物样品在最高暴露质量浓度(20 mg/mL)下,细胞存活率下降到79%(见图2)。其余8个提取液在0.08~20 mg/mL范围内细胞存活率均高于80%,因此,这些样品在测试质量浓度下无细胞毒性[22, 31]。
2.2 三峡库区消落带沉积物雌激素活性
使用YES实验测定了10个沉淀物样品提取物的雌激素活性(见图3)。结果显示,在0.39~100 g/L的范圍内,淹水期的5个沉积物提取液均会导致β-半乳糖苷酶活性对质量浓度依赖性升高,并呈现“S”型效应-剂量关系,其诱导产生的β-半乳糖苷酶活性比E2低大约108倍。其中,淹水期的高阳提取物表现出最强的雌激素活性,EC50值为(3.381±1.498)g/L,这与上述细胞毒性实验结果实验一致。巫山、丰都和奉节在淹水期的沉积物提取物雌激素效应紧随其后,EC50值分别为(4.461±2.277)g/L、(7.249±2.922)g/L和(11.61±3.763)g/L。淹水期云阳提取物的EC50值为(41.73±5.187)g/L,明显低于其他4个地区(p<0.05)。所有淹水期提取物雌二醇当量(EEQ)值在0.637~6.987 ng/g之间,略高于Wang等[12]的研究结果(EEQ在0.3~1.0 ng/g)。这一结果与其他地区的雌激素活性研究结果相当,例如辽河沉积物雌激素活性当量为未检出~6.04 ng/g [32]、珠江(未检出~7.24 ng/g)[33]、黄河(0.45~1.29 ng/g)[27],以及日本东京湾(2.07~12.1 ng/g)[34]。由于E2对淡水生物的长期预测无效应质量浓度(PNEC)为1 ng/L,这意味着水生环境中的EEQ值>1 ng/L时,可能会导致一些鱼类的生殖问题[32]。说明三峡库区消落带沉积物可能具有较强的雌激素活性,并且会对一些水生生物造成健康风险。
为明晰环境雌激素效应在三峡水库的分布规律,进一步分析了最大样品暴露质量浓度(100 g/L)下雌激素活性的时空特征(见图3)。消落带沉积物相同暴露质量浓度下,落干期样品的雌激素活性均明显低于淹水期(p<0.05,巫山样点除外)。在天然河流、湖泊等水体中,当水体稀释作用减弱时通常会出现雌激素活性增强的现象[35],本研究结果与之相反,其原因是落干期(8月份)的温度高于淹水期(4月),此时水环境中营养物质浓度通常也高于淹水期,因此微生物代谢活性也更强[36]。已有研究证明,微生物活性的增加能够加速EDCs降解,E2在夏季的降解速度比春季快,从而导致春季的雌激素活性可能比夏季高[37]。此外,随着淹水期水位升高水流速度减缓,水体自净能力减弱,促使雌激素活性物质在沉积物中的累积,从而使得淹水期沉积物具有较强的雌激素活性[38]。换言之,季节性水位变动也伴随着气候温度和水文条件的变化,是影响环境中雌激素活性的重要因素。尽管落干期沉积物未表现出明显的效应-剂量关系,但其仍然表现出一定的β-半乳糖苷酶诱导活性。巫山的提取物显示出最强的雌激素活性,其相对于E2的效应强度为34%,显著高于奉节和高阳(p < 0.05),其效应强度分别为5.2%和5.6%(见图3)。这一发现与他人研究一致,即在巫山采集的沉积物的雌激素活性高于库区上游的万州[12]。可能的原因是巫山位于水库的下游地区,由于三峡大坝的阻隔作用,导致颗粒物在库区下游沉积[30],一些具有雌激素活性的污染物吸附在颗粒上,随后在下游积累,从而产生更强的雌激素活性。
2.3 沉积物中典型环境雌激素(EEs)含量分布
在8种典型雌激素中,只有雌酮(E1)和乙炔基雌二醇(EE2)2种被检测到,分别为0.025~2.667 ng/g(EE2)和0.136 ng/g(E1)(见表2)。三峡库区是我国重要的山地农业区域,其中畜禽养殖在库区中分布广泛,由于缺乏污染控制设施,一些牲畜粪便未经充分处理甚至未经处理直排水体,导致在沉积物中检测到E1和EE2[39]。此外,这2种雌激素稳定性较高,尤其是EE2可以吸附在沉积物中并持续累积[40-41]。已有研究发现太湖中的E1为5.49~164 ng/g,EE2为4.32~184 ng/g [42]。在西班牙Cardener河沉积物中,E1的含量高达11.9 ng/g,EE2为 22.8 ng/g[43]。与其他地区的结果相比,本研究中三峡库区的E1和EE2含量处于较低水平。
笔者发现除高阳样点外,其余样点淹水期提取物中都检测到EE2,其中奉节的含量最高,为2.667 ng/g。空间分布上,各采样位点的EE2污染状况相差较大,并未表现出明显的流向变化趋势或干支流差异,可能与位点附近人口密度及工农产业分布差异相关[44],EE2是口服避孕药的常见成分,在人口密集地区的污水处理厂废水常常能检测到,并最终进入天然水环境[45],导致潜在的生态效应。有研究表明,环境中96%的EE2来源于畜牧业粪便及尿液[46],奉节采样点位于码头附近,人类活动对水环境干扰较大,生活废水可能是水环境中EE2的主要来源。落干期提取物均未检出典型雌激素,这与雌激素效应生物实验结果一致。本研究结果与三峡库区嘉陵江段类固醇类雌激素的时间分布特性一致[47-48],落干期随着水位降低,沉积物经历淹水-裸露的动态过程,导致吸附于沉积物上的污染物发生解吸,释放到其他环境介质中[49]。此外,冬季大坝蓄水使得消落带处于淹没状态,流速较慢和水力停留时间长,有利于细颗粒物沉积,淹没期沉积物含量比落干期高[50]。多数雌激素活性物质疏水性较强,容易吸附在细微颗粒物上,导致淹水期沉积物中雌激素物质的含量高于落干期[51]。
2.4 典型雌激素对雌激素效应的贡献
为了确定三峡库区中雌激素效应的来源,通过皮尔逊相关测试分析了三峡库区沉积物样品基于1/EC50值的雌激素效应与每种测试雌激素效应物质质量浓度之间的相关性(见图4)。
典型雌激素通常被认为是造成环境雌激素效应的主要物质,在低浓度下也可能产生较强的雌激素活性[52]。因此,通常雌激素活性随着典型雌激素浓度的增加而增强[53-54]。然而,本研究中沉积物样品所检测到的2种典型雌激素与雌激素活性之间不存在明显相关性,表明典型的环境雌激素不是三峡库区沉积物中雌激素活性的主要来源。类似的结果也在底特律河沉积物中观察到[55]。
本研究发现典型雌激素并非三峡库区沉积物雌激素活性的主要贡献物质,为进一步明晰典型雌激素对环境样品雌激素效应解析的程度,根据检测到的可疑雌激素的含量及其相对雌激素效力(REP)值计算了每个样点的EEQchem,并与生物测定的EEQbio进行对比(见表3)。E1和EE2在YES体系中的相对雌激素效力(REP)分别为0.053和0.17[56],所有样品的理论雌激素活性(EEQchem)低于YES测定的雌激素活性(EEQbio),研究结果印证了非典型雌激素对其活性存在较大贡献的可能[27]。EEQchem解释了0%~29.46%的雌激素活性(见表3),在荷兰的一项研究中,也发现已知雌激素的计算效力仅解释了污水中20%的雌激素活性[57]。然而,也有研究报道检测到的6种典型雌激素物质可以解释80%的雌激素效应[58],一项有关沉积物的研究也发现大约67%的雌激素活性来源于已确定的化合物[59]。因此,典型雌激素物质对三峡库区沉积物的雌激素活性贡献较低,其活性来源是未关注的非典型雌激素物质。
典型雌激素对三峡库区沉积物的雌激素活性贡献很小,而雌激素活性的主要贡献者仍然未知。有研究表明,由于污染物的类型越来越复杂,已知的内分泌活性物质可能无法解释雌激素活性的来源[60]。对于大多数沉积物样品,用生物测定法确定的EEQ值高于基于典型物质的计算效应值,表明一些未知的化学物质对样品雌激素活性贡献的普遍性[27]。因此,仅根据已知的化学品来估计环境样品雌激素活性,将可能低估潜在环境雌激素污染风险。此外,目标物质靶向分析只能关注已知的典型环境雌激素,而多氯联苯,包括苯并[α]芘(BaP)和苯并[α]蒽(BaA)在内的几种多环芳烃以及烷基酚等都显示出一定的雌激素活性[61],其混合效应不容忽视。笔者发现,水位变动等环境变化下,消落带雌激素效应和物质差异显著,这为水库可持续管理策略提供了重要的数据支撑。
3 结 论
1)三峡库区中8种典型雌激素仅有雌酮(E1)和乙炔基雌二醇(EE2)被检出,含量在0.025~2.667 ng/g范围内,处于较低水平。
2)沉积物的雌激素活性在不同的水位时期,具有较大差异。淹水期的沉积物具有明显的雌激素活性,其雌二醇当量(EEQ)值为0.637~6.987 ng/g。落干期的沉積物样品,均未表现出明显的雌激素活性。水位波动和环境变化可能会影响水环境中污染物的雌激素活性。
3)典型雌激素效应物质与雌激素活性间不存在明显相关性,最多仅解释了29.46%的雌激素活性,可能还存在尚未知的雌激素活性物质。
参考文献
[1] Liew Z, Guo P F. Human health effects of chemical mixtures[J]. Science, 2022, 375(6582): 720-721.
[2] Kabir E R, Rahman M S, Rahman I. A review on endocrine disruptors and their possible impacts on human health[J]. Environmental Toxicology and Pharmacology, 2015, 40(1): 241-258.
[3] Tan J S, Liu L H, Li F X, et al. Screening of endocrine disrupting potential of surface waters via an affinity-based biosensor in a rural community in the Yellow River Basin, China[J]. Environmental Science & Technology, 2022, 56(20): 14350-14360.
[4] Adeel M, Song X M, Wang Y Y, et al. Environmental impact of estrogens on human, animal and plant life: a critical review[J]. Environment International, 2017, 99: 107-119.
[5] Tran T K A, Yu R M K, Islam R, et al. The utility of vitellogenin as a biomarker of estrogenic endocrine disrupting chemicals in molluscs[J]. Environmental Pollution, 2019, 248: 1067-1078.
[6] Pop A, Drugan T, Loghin F, et al. In vitro androgenic/anti-antiandrogenic effects of certain food additives and cosmetic preservatives[J]. Toxicology Letters, 2014, 229: S181.
[7] Yoshida I, Ishida K, Yoshikawa H, et al. In vivo profiling of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced estrogenic/anti-estrogenic effects in female estrogen-responsive reporter transgenic mice[J]. Journal of Hazardous Materials, 2020, 385: 121526.
[8] Tully D B, Cox V T, Mumtaz M M, et al. Six high-priority organochlorine pesticides, either singly or in combination, are nonestrogenic in transfected HeLa cells[J]. Reproductive Toxicology, 2000, 14(2): 95-102.
[9] Pei S X, Jian Z J, Guo Q S, et al. Temporal and spatial variation and risk assessment of soil heavy metal concentrations for water-level-fluctuating zones of the Three Gorges Reservoir[J]. Journal of Soils and Sediments, 2018, 18(9): 2924-2934.
[10] Wu Y H, Wang X X, Zhou J, et al. The fate of phosphorus in sediments after the full operation of the Three Gorges Reservoir, China[J]. Environmental Pollution, 2016, 214: 282-289.
[11] Zhu L, Li X, Zhang C, et al. Pollutants release, redistribution and remediation of black smelly river sediment based on re-suspension and deep aeration of sediment[J]. International Journal of Environmental Research and Public Health, 2017, 14(4): 374.
[12] Wang J X, Bovee T F H, Bi Y H, et al. Aryl hydrocarbon receptor (AhR) inducers and estrogen receptor (ER) activities in surface sediments of Three Gorges Reservoir, China evaluated with in vitro cell bioassays[J]. Environmental Science and Pollution Research, 2014, 21(4): 3145-3155.
[13] 朱毅, 田懷军, 舒为群, 等. 长江、嘉陵江(重庆段)源水有机提取物的类雌激素活性评价[J]. 环境污染与防治, 2003, 25(2): 65-67.
Zhu Y, Tian H J, Shu W Q, et al. Evaluation of the estrogenic activity of organic extracts from source water of Yangtze River and Jialing River in Chongqing section[J]. Environmental Pollution & Control, 2003, 25(2): 65-67.(in Chinese)
[14] Li Y H, Huang S J, Qu X X. Water pollution prediction in the Three Gorges Reservoir area and countermeasures for sustainable development of the water environment[J]. International Journal of Environmental Research and Public Health, 2017, 14(11): 1307.
[15] Ding X W, Zhang J J, Jiang G H, et al. Early warning and forecasting system of water quality safety for drinking water source areas in Three Gorges Reservoir area, China[J]. Water, 2017, 9(7): 465.
[16] Wang J X, Bi Y H, Pfister G, et al. Determination of PAH, PCB, and OCP in water from the Three Gorges Reservoir accumulated by semipermeable membrane devices (SPMD)[J]. Chemosphere, 2009, 75(8): 1119-1127.
[17] Han C N, Zheng B H, Qin Y W, et al. Impact of upstream river inputs and reservoir operation on phosphorus fractions in water-particulate phases in the Three Gorges Reservoir[J]. Science of the Total Environment, 2018, 610/611: 1546-1556.
[18] Bao Y H, Gao P, He X B. The water-level fluctuation zone of Three Gorges Reservoir: a unique geomorphological unit[J]. Earth-Science Reviews, 2015, 150: 14-24.
[19] Hong S, Khim J S, Ryu J, et al. Two years after the Hebei spirit oil spill: residual crude-derived hydrocarbons and potential AhR-mediated activities in coastal sediments[J]. Environmental Science & Technology, 2012, 46(3): 1406-1414.
[20] Cha J, Hong S, Kim J, et al. Major AhR-active chemicals in sediments of Lake Sihwa, South Korea: application of effect-directed analysis combined with full-scan screening analysis[J]. Environment International, 2019, 133: 105199.
[21] Shao Y, Zhu L Y, Chen Z L, et al. Evidence of increased estrogenicity upon metabolism of Bisphenol F: elucidation of the key metabolites[J]. Science of the Total Environment, 2021, 787: 147669.
[22] Mennillo E, Cappelli F, Arukwe A. Biotransformation and oxidative stress responses in rat hepatic cell-line (H4IIE) exposed to organophosphate esters (OPEs)[J]. Toxicology and Applied Pharmacology, 2019, 371: 84-94.
[23] Lei B L, Xu J, Peng W, et al. In vitro profiling of toxicity and endocrine disrupting effects of bisphenol analogues by employing MCF-7 cells and two-hybrid yeast bioassay[J]. Environmental Toxicology, 2017, 32(1): 278-289.
[24] Ma M, Li J, Wang Z J. Assessing the detoxication efficiencies of wastewater treatment processes using a battery of bioassays/biomarkers[J]. Archives of Environmental Contamination and Toxicology, 2005, 49(4): 480-487.
[25] Wang J Y, Wang J P, Liu J S, et al. The evaluation of endocrine disrupting effects of tert-butylphenols towards estrogenic receptor α, androgen receptor and thyroid hormone receptor β and aquatic toxicities towards freshwater organisms[J]. Environmental Pollution, 2018, 240: 396-402.
[26] Prochazkova T, Sychrova E, Vecerkova J, et al. Estrogenic activity and contributing compounds in stagnant water bodies with massive occurrence of phytoplankton[J]. Water Research, 2018, 136: 12-21.
[27] Wang L, Ying G G, Chen F, et al. Monitoring of selected estrogenic compounds and estrogenic activity in surface water and sediment of the Yellow River in China using combined chemical and biological tools[J]. Environmental Pollution, 2012, 165: 241-249.
[28] Holbach A, Norra S, Wang L J, et al. Three gorges reservoir: density pump amplification of pollutant transport into tributaries[J]. Environmental Science & Technology, 2014, 48(14): 7798-7806.
[29] Yin W P, Ji D B, Hu N S, et al. Three-dimensional water temperature and hydrodynamic simulation of Xiangxi River Estuary[J]. Advanced Materials Research, 2013, 726/727/728/729/730/731: 3212-3221.
[30] Xu D Y, Gao B, Peng W Q, et al. Thallium pollution in sediments response to consecutive water seasons in Three Gorges Reservoir using geochemical baseline concentrations[J]. Journal of Hydrology, 2018, 564: 740-747.
[31] Li J, Wang Z J, Ma M, et al. Analysis of environmental endocrine disrupting activities using recombinant yeast assay in wastewater treatment plant effluents[J]. Bulletin of Environmental Contamination and Toxicology, 2010, 84(5): 529-535.
[32] Wang L, Ying G G, Zhao J L, et al. Assessing estrogenic activity in surface water and sediment of the Liao River system in northeast China using combined chemical and biological tools[J]. Environmental Pollution, 2011, 159(1): 148-156.
[33] Zhao J L, Ying G G, Yang B, et al. Screening of multiple hormonal activities in surface water and sediment from the Pearl River system, South China, using effect-directed in vitro bioassays[J]. Environmental Toxicology and Chemistry, 2011, 30(10): 2208-2215.
[34] Hashimoto S, Horiuchi A, Yoshimoto T, et al. Horizontal and vertical distribution of estrogenic activities in sediments and waters from Tokyo Bay, Japan[J]. Archives of Environmental Contamination and Toxicology, 2005, 48(2): 209-216.
[35] Yan C X, Yang Y, Zhou J L, et al. Antibiotics in the surface water of the Yangtze Estuary: occurrence, distribution and risk assessment[J]. Environmental Pollution, 2013, 175: 22-29.
[36] Chen Z B, Zhou Z Y, Peng X, et al. Effects of wet and dry seasons on the aquatic bacterial community structure of the Three Gorges Reservoir[J]. World Journal of Microbiology and Biotechnology, 2013, 29(5): 841-853.
[37] Jürgens M D, Holthaus K I E, Johnson A C, et al. The potential for estradiol and ethinylestradiol degradation in English Rivers[J]. Environmental Toxicology and Chemistry, 2002, 21(3): 480-488.
[38] Lei K, Lin C Y, Zhu Y, et al. Estrogens in municipal wastewater and receiving waters in the Beijing-Tianjin-Hebei region, China: occurrence and risk assessment of mixtures[J]. Journal of Hazardous Materials, 2020, 389: 121891.
[39] Zhang T, Ni J P, Xie D T. Severe situation of rural nonpoint source pollution and efficient utilization of agricultural wastes in the Three Gorges Reservoir Area[J]. Environmental Science and Pollution Research, 2015, 22(21): 16453-16462.
[40] Zhang C, Li Y, Wang C, et al. Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: a review[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(1): 1-59.
[41] Aris A Z, Shamsuddin A S, Praveena S M. Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review[J]. Environment International, 2014, 69: 104-119.
[42] Wang Y H, Wang Q Y, Hu L F, et al. Occurrence of estrogens in water, sediment and biota and their ecological risk in Northern Taihu Lake in China[J]. Environmental Geochemistry and Health, 2015, 37(1): 147-156.
[43] de Alda M J L, Gil A, Paz E, et al. Occurrence and analysis of estrogens and progestogens in river sediments by liquid chromatography-electrospray-mass spectrometry[J]. Analyst, 2002, 127(10): 1299-1304.
[44] 卓麗, 许榕发, 石运刚, 等. 重庆长江流域水体中8种典型环境雌激素污染特征[J]. 生态毒理学报, 2020, 15(3): 149-157.
Zhuo L, Xu R F, Shi Y G, et al. Estrogens in surface water of the Yangtze River in Chongqing section[J]. Asian Journal of Ecotoxicology, 2020, 15(3): 149-157.(in Chinese)
[45] Wang W F, Ndungu A W, Wang J. Monitoring of endocrine-disrupting compounds in surface water and sediments of the Three Gorges Reservoir region, China[J]. Archives of Environmental Contamination and Toxicology, 2016, 71(4): 509-517.
[46] 李宁健, 张庆华, 张曙琳, 等. 环境内分泌干扰物雌激素的微生物降解研究进展[J]. 微生物学通报, 2023, 50(4): 1591-1606.
Li N J, Zhang Q H, Zhang S L, et al. Microbial degradation of estrogens in environmental endocrine disruptors[J]. Microbiology China, 2023, 50(4): 1591-1606.(in Chinese)
[47] 李显芳, 印成, 万巧玲, 等. 三峡库区重庆段水体中有机污染物的研究进展[J]. 环境与健康杂志, 2019, 36(7): 649-654.
Li X F, Yin C, Wan Q L, et al. Research progress on organic contaminants in Chongqing reach of Three Gorges Reservoir region[J]. Journal of Environment and Health, 2019, 36(7): 649-654.(in Chinese)
[48] 胡碧波, 阳春, 张智, 等. 嘉陵江典型城市江段的类固醇雌激素分布特性[J]. 中国给水排水, 2011, 27(21): 54-58.
Hu B B, Yang C, Zhang Z, et al. Distribution characteristics of steroid estrogens in a typical urban section of Jialing River[J]. China Water & Wastewater, 2011, 27(21): 54-58.(in Chinese)
[49] 胡莺. 三峡水库消落带水—土体系中典型环境雌激素的迁移转化研究[D]. 重庆: 重庆交通大学, 2019.
Hu Y. Study on migration and transformation of typical environmental estrogen in water-soil system in water-level-fluctuating zone of three gorges reservoir[D].Chongqing: Chongqing Jiaotong University, 2019. (in Chinese)
[50] 赵岱寅, 蔡茂雪, 张代钧, 等. 三峡库区消落带表层沉积物生物标志物时空变化与来源分析[J]. 中国环境科学, 2022, 42(6): 2810-2820.
Zhao D Y, Cai M X, Zhang D J, et al. Temporal and spatial variation and source analysis of biomarkers in surface sediments in the water-level-fluctuating zone of the Three Gorges Reservoir Region[J]. China Environmental Science, 2022, 42(6): 2810-2820.(in Chinese)
[51] 陈斌, 马伟芳, 曾凡刚, 等. 类固醇雌激素在土壤与沉积物中的污染水平及其吸附研究进展[J]. 环境工程, 2014, 32(7): 131-137.
Chen B, Ma W F, Zeng F G, et al. Review of steroid estrogens contamination and sorption in soil and sediment[J]. Environmental Engineering, 2014, 32(7): 131-137. (in Chinese)
[52] Duong C N, Ra J S, Cho J, et al. Estrogenic chemicals and estrogenicity in river waters of South Korea and seven Asian countries[J]. Chemosphere, 2010, 78(3): 286-293.
[53] Chou P H, Lin Y L, Liu T C, et al. Exploring potential contributors to endocrine disrupting activities in Taiwans surface waters using yeast assays and chemical analysis[J]. Chemosphere, 2015, 138: 814-820.
[54] Müller A K, Leser K, K?mpfer D, et al. Bioavailability of estrogenic compounds from sediment in the context of flood events evaluated by passive sampling[J]. Water Research, 2019, 161: 540-548.
[55] Michallet-Ferrier P, A?t-A?ssa S, Balaguer P, et al. Assessment of estrogen (ER) and aryl hydrocarbon receptor (AhR) mediated activities in organic sediment extracts of the Detroit River, using in vitro bioassays based on human MELN and teleost PLHC-1 cell lines[J]. Journal of Great Lakes Research, 2004, 30(1): 82-92.
[56] Luo J P, Lei B L, Ma M, et al. Identification of estrogen receptor agonists in sediments from Wenyu River, Beijing, China[J]. Water Research, 2011, 45(13): 3908-3914.
[57] Murk A J, Legler J, van Lipzig M M H, et al. Detection of estrogenic potency in wastewater and surface water with three in vitro bioassays[J]. Environmental Toxicology and Chemistry, 2002, 21(1): 16-23.
[58] Neale P A, Ait-Aissa S, Brack W, et al. Linking in vitro effects and detected organic micropollutants in surface water using mixture-toxicity modeling[J]. Environmental Science & Technology, 2015, 49(24): 14614-14624.
[59] Schmitt S, Reifferscheid G, Claus E, et al. Effect directed analysis and mixture effects of estrogenic compounds in a sediment of the River Elbe[J]. Environmental Science and Pollution Research, 2012, 19(8): 3350-3361.
[60] Kirchnawy C, Hager F, Osorio Piniella V, et al. Potential endocrine disrupting properties of toys for babies and infants[J]. PLoS One, 2020, 15(4): e0231171.
[61] Song M Y, Xu Y, Jiang Q T, et al. Measurement of estrogenic activity in sediments from Haihe and Dagu River, China[J]. Environment International, 2006, 32(5): 676-681.
(編辑 郑洁)

