Therapeutic management of the painful nerve: a narrative review of common rehabilitation interventions
Chelsey Kratter
Outpatient Hand Therapy, Stanford Medicine, Redwood City, CA 94063, USA.
Abstract There are many ways that rehabilitation therapists, including occupational and physical therapists, treat nerverelated pain.Commonly used interventions include neurodynamic treatment, pain neuroscience education,desensitization, exercise, physical agent modalities, mirror box therapy, and Kinesio taping.Despite common practice and anecdotal support, it can be challenging to determine the appropriate intervention for each patient.In this article, each of these treatment approaches is discussed, including indicated pain phenotypes and diagnoses,timing, efficacy, mechanism, contraindications, and limitations.
Keywords: Nerve, pain, physical therapy, occupational therapy, hand therapy
INTRODUCTION
Treating the painful nerve in a rehabilitation setting is often an elusive endeavor.This is due to a variety of factors including the patient’s pain phenotype and a broad choice of treatment interventions.This review aims to present several commonly used treatment interventions for nerve-related pain in the context of pain phenotypes and relevant diagnoses, as well as to provide referring surgeons with relevant clinical applications in the context of typical rehabilitation processes [Figure 1] and timeframes [Table 1].While this paper describes treatment interventions from the perspective of the rehabilitation therapist, there aremany other potential medical and surgical interventions that should be considered by the referring physician to ensure that the best care is being delivered.Any potential treatment or combination of treatments should be considered in the context of the patient's presentation.Further, the risks and benefits of any intervention must be weighed by all providers of the treatment team to ensure proper care is being delivered.

Table 1.A detailed summary of implementing the presented treatments and any applicable sub-types in both non-surgical and postoperative scenarios with time frames relevant to surgery
To appropriately treat nerve-related pain, one must understand the subtypes of pain.Also called pain phenotypes[1], there are three recognized categories of pain: nociceptive, neuropathic, and nociplastic[2].Nociceptive pain is pain derived from actual or threat of tissue damage, such as a broken bone, torn ligament, or laceration.In nociceptive pain, nociceptor neurons are activated, thus signaling warning and promoting protection of potential or actual injury[3].Neuropathic pai is a result of damage to the peripheral or central nervous system, such as carpal tunnel syndrome, radiculopathy, and spinal cord injury[4].Nociplastic pain is pain that is neither related to tissue damage nor nerve damage, but is due to central sensitization[2].Central sensitization involves changes at the level of the peripheral nerve, spinal cord, and brain that amplify pain signals and/or decrease inhibitory pain stimuli[2,5].Examples of nociplastic pain include complex regional pain syndrome type I (CRPS-I) and fibromyalgia.While often thought of as distinct experiences, it is suggested that pain phenotypes be conceptualized as a continuum with overlap between categories, lending to what is called mixed pain at overlapping symptoms and etiologies.For example, complex regional pain syndrome type II (CRPS-II) involves central sensitization that originates from a diagnosed nerve injury[2].Nerve-related pain, for the purposes of this review, encompasses neuropathic, nociplastic, and mixed pain phenotypes.

Figure 1.Rehabilitation considerations in nerve-related pain in the context of typical treatment processes between surgeons and rehabilitation therapists.In addition to the treatments presented in this review (shown in the green boxes) are other common rehabilitation interventions (shown in yellow boxes).
When evaluating a patient with nerve-related pain, the treating therapist has many options for interventions.These may include, but are not limited to, neurodynamic treatment, pain neuroscience education, desensitization, exercise, physical agent modalities, mirror box therapy, and Kinesio taping[Table 2].
NEURODYNAMIC TREATMENT
Neurodynamic treatment, also known as nerve gliding or nerve flossing, is the therapeutic movement of neural tissues relative to adjacent non-neural tissues with the goal of reducing pain by restoring normal intrinsic nerve pressures and thereby normal physiologic nerve function[6].These maneuvers can be performed manually on a patient by a trained therapist[7]or taught as an exercise for carryover at home[8].There are two main categories of neurodynamic treatment: those that promote a “flossing” motion of the neural tissues and those that stretch neural tissues[9], although stretching neural tissues has fallen out of favor due to elevated risk of nerve injury[10].
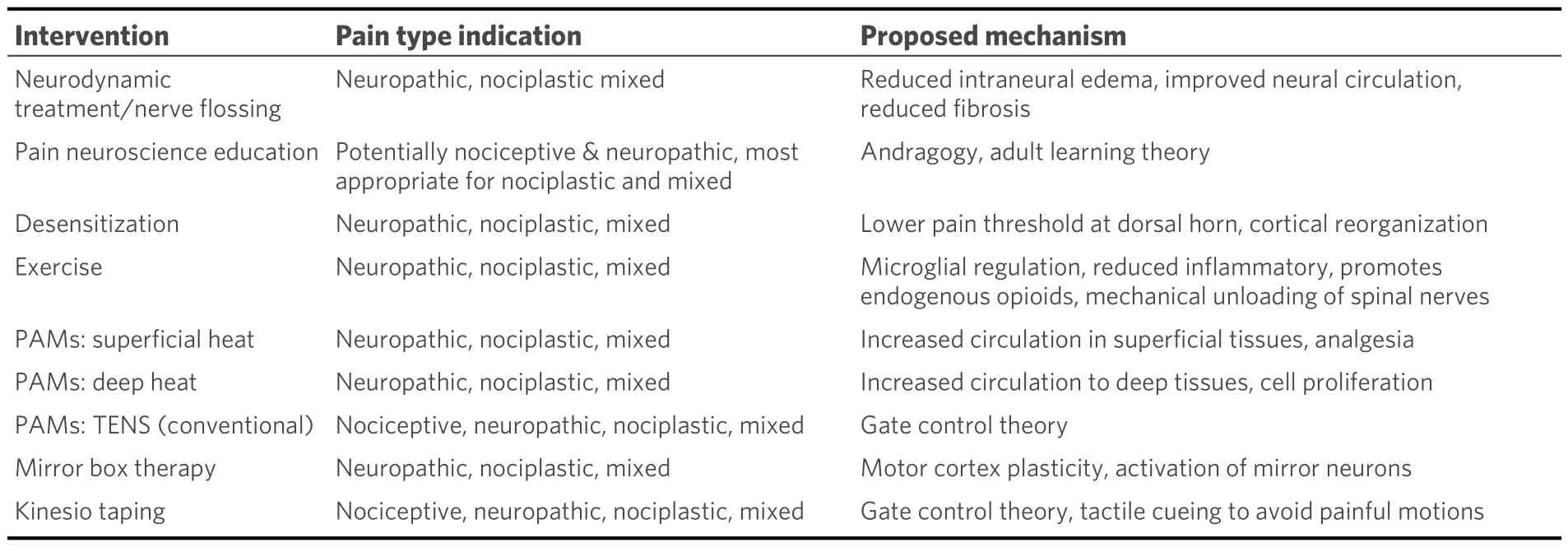
Table 2.Summary of interventions, pain phenotype indication and associated mechanisms
Neurodynamic treatment is most commonly used in neuropathic pain stemming from entrapment neuropathies such as carpal tunnel syndrome[7,11-14]and tarsal tunnel syndrome[8].However, neurodynamic treatment has also been proposed as a treatment modality for other conditions such as lateral epicondylalgia[15], multiple sclerosis[16], fibromyalgia[17], low back pain[18], chemotherapy-induced peripheral neuropathy[19], rheumatoid arthritis[20]and osteoarthritis[21].
For entrapment neuropathies, neurodynamic treatment is often included with some form of immobilization and activity modification in the initial course of conservative management[22].If conservative management fails and the patient’s nerve compression is worsening, surgical intervention should be expedited to avoid further nerve damage.If the entrapment is released surgically, neurodynamic treatment may also be used in the early postoperative period, as soon as the patient is allowed to start active movement.In the postoperative phase, neurodynamic treatment is thought to keep the nerve mobile and reduce the effects of scar adhesions.Symptomatic adhesions to the nerve, also called nerve tethering, can be influenced by other factors including incision location[23], and the extent to which nerve flossing mitigates tethering is unclear.The benefit of neurodynamic treatment after surgical release of an entrapment neuropathy, although considered common practice, is based on theoretical principles of nerve gliding, and more research is needed to fully understand its role in the postoperative population.After nerve grafting, transfer, or repair,neurodynamic treatment, in a modified and gentle form, may be started when the nerve is fully healed,around 6 weeks after surgery.
The effectiveness of neurodynamic treatment varies in the literature and across diagnoses.A systematic review and meta-analysis by Bassonet al.[24], reported reduced pain and disability in tarsal tunnel.The same study determined there was insufficient evidence to support the use of neurodynamic treatment in carpal tunnel syndrome and cubital tunnel syndrome[24].An umbrella review with meta-meta-analysis by Cuenca-Martinezet al.demonstrated an overall reduction in pain and disability scores after neurodynamic treatment; however, they found the literature was weak in study quality, lacked a description of treatment parameters, and did not explore long-term effects of treatment[9].A Cochrane review of exercise and neural mobilization treatment in carpal tunnel syndrome demonstrated limited and low-quality evidence for exercise and neural mobilization techniques in this condition[12].
The exact mechanism through which neurodynamic treatment works is not well understood.However, it is suspected to reduce intraneural edema, improve blood flow to the nerve, and reduce fibrosis, all of which contribute to restoration of nerve function[25].Rat models exploring mechanisms of neurodynamic treatment demonstrate decreased hyperalgesia and decreased dorsal root ganglion excitability after treatment[26].However, a study by Schmidet al.compared median nerve edema as measured on MRI between subjects who were prescribed a splinting program only and subjects prescribed a nerve and tendon gliding exercise program[26].They reported that compared to pre-intervention values, there was a significant decrease in signal intensity in both groups that correlated with patient-reported outcome measures of pain and symptom severity after 1 week of treatment[27], suggesting that both immobilization and neurodynamic treatment may provide similar benefit.Although promising, more high-quality research is needed to fully understand the biological mechanisms of neurodynamic treatment in human populations.
There are few published contraindications for “flossing” of a nerve, but it is generally considered unsafe in the acute postoperative phases of nerve repair, grafing, or transfer.Stretching of neural tissues carries higher risks and has accordingly fallen out of favor.Any provider considering adding neurodynamic treatment to a treatment plan should consider principles of tissue healing, the patient’s history and symptom status, as well as any surgical interventions to the nerve that may be negatively impacted, such as recent nerve grafting or nerve transfer.Further, other injuries in the targeted body part, such as fractures, grafts, and tendon lacerations, must be considered.If neurodynamic treatment is being used as a component of conservative management of entrapment neuropathy, close care should be taken to monitor for clinical signs of worsening nerve compression, including progressive sensory loss and muscle wasting.If there is concern for worsening nerve damage, the patient should be promptly referred to a surgeon for release to avoid further nerve damage.Neurodynamic treatment is a means of symptom reduction and does not necessarily address functional deficits.Thus, it should be used in conjunction with other interventions to help the patient achieve their goals.
PAIN NEUROSCIENCE EDUCATION
Pain Neuroscience Education (PNE) is a multidisciplinary, biopsychosocial approach that is an educational intervention for patients regarding the nature of persistent pain that is related to central sensitization, but can be used for all pain phenotypes[28].PNE represents a paradigm shift in how pain is best conceptualized to both providers and patients.This approach was developed to help patients with complex and persistent pain better understand their experience, avoid maladaptive behaviors, navigate treatment options, reduce costs, and improve overall function[28,29].Before completing PNE, the patient fully explains their story and symptoms, explains their beliefs about what the pain means, and details functional deficits.A full clinical evaluation is performed to rule out other causes of their symptoms and psychosocial factors are discussed.Once a thorough history has been taken, the clinician educates the patient on how pain is a multifactorial experience that is influenced by one’s activity level, social stressors, psychological health, and diet.Based on the patient’s history, the provider may suggest recommendations to modify lifestyle, activity, and cognitive distortions, but should also emphasize that long-term lifestyle changes are required for the most benefit[28].
PNE can be used with patients experiencing nociceptive, neuropathic, nociplastic pain, and mixed pain.However, because nociceptive and neuropathic pain are more logically understood by the general population, less intensive PNE is needed.People experiencing nociplastic and mixed pain, such as complex regional pain syndrome (CRPS), benefit the most from PNE due to the lack of general understanding of these conditions[30].PNE has been used in a variety of conditions including, but not limited to, carpal tunnel syndrome[31], CRPS types I and II[32,33], frozen shoulder[34], low back pain[35], and in pediatric populations[36].
PNE, even in an abbreviated version, may be used by surgeons prior to nerve surgery to help the patient understand their pain.Additionally, if postoperative nerve pain complications arise, such as scar hypersensitivity or pillar pain after carpal tunnel release[37], PNE may be implemented by the surgery team as well as the rehabilitation team to help the patient conceptualize nerve-related pain and move forward with their rehabilitation.
Pain is a complex experience that is influenced by many body systems, and the literature demonstrating the effectiveness of PNE on neuropathic pain is limited.A longitudinal study of 799 subjects by Leeet al.demonstrated that 12 months after PNE alone, increased pain biology knowledge was associated with lower pain intensity and catastrophization[38].Nonetheless, many argue that in isolation, the benefits of PNE are limited and must be accompanied by other interventions and/or physical activity under the guidance of a therapist for best results[39].A systematic review and meta-analysis by Ramet al.showed no benefit of PNE alone in kinesiophobia, pain intensity, or catastrophization ratings in the short term[40].Further, a small randomized controlled trial (n= 30) by Nunez-Corteset al.compared one single PNE session prior to carpal tunnel surgery plus exercise to standard treatment and did not find any significant differences in treatment groups[31].A case report by Shepherdet al.demonstrated significant improvement in function and catastrophization in a patient with foot CRPS after receiving PNE in addition to graded motor imagery therapy[41].More research is needed to demonstrate the effectiveness of PNE, both alone and in conjunction with other treatments, on specific populations and the best way to train providers to deliver PNE.
The notion of andragogy, an approach used across adult learning, Is the theoretical basis supporting PNE[42].Simply put, PNE assumes that the more a patient understands their pain experience and modifiable factors they can control, the better they can manage their symptoms.
There are no published contraindications for PNE, but the patient must have sufficient cognitive ability, be receptive, and take ownership of the information and suggestions provided for any benefit to occur[28].Thus,PNE is not universally applicable and should be used in select patients and situations.
For maximum benefit, PNE depends on a multidisciplinary approach to reinforce teaching concepts across specialty-specific recommendations[28].If providers deliver inconsistent messages about pain and how to manage it, it may result in less patient motivation and less directed patient action.Additionally, PNE is not a current educational standard for most healthcare professions; a biomedical model is still more commonly taught and used over a biopsychosocial model[29].Therefore, inconsistencies in pain education among providers are likely common.
DESENSITIZATION
Tactile desensitization, a form of sensory reeducation, is the method of cortical reorganization of the somatosensory complex to reduce pain and hypersensitivity after nerve injury through gradual and graded exposure to stimuli[43].Desensitization protocols vary, but commonly include the gentle but tolerably uncomfortable application of soft textures, such as fleece or cotton balls, either directly to the hypersensitive zone or near the area of most sensitivity[44].As hypersensitivity improves, the sensory input gradually focuses on areas of most discomfort and input texture progressively becomes more noxious and may include other sensory types such as vibration[44].
Desensitization has been described in treating both neuropathic and nociplastic pain phenotypes.Many studies have explored the use of desensitization programs in the treatment of CRPS[45,46].Additionally,desensitization has been described in the treatment of peripheral nerve injuries[44], nerves that have undergone primary repair[43,47], fingers that have undergone replantation[48], after thumb reconstruction[49],phantom limb pain[50], in hypersensitive surgical scars[51]and in areas having undergone skin grafts, flaps,and tissue transfers[52].
Desensitization for CRPS may begin immediately after diagnosis[53].After surgery, desensitization directly to a hypersensitive incision may begin once the incision has healed and can withstand light pressure without risk of dehiscence or infection[51].Also in the postoperative state, desensitization of hypersensitive areas adjacent to a healing incision may begin as early as postoperative dressing removal[51].In symptomatic neuroma, desensitization may be used before considering surgery, although it is unlikely to yield much benefit[54]and desensitization may be used in postoperative rehabilitation.Similarly, tactile desensitization of symptoms associated with nerve entrapment syndromes prior to surgery is not commonly performed as it is not helpful on nerves remaining under compression, but may be considered if needed after surgical release.
The efficacy of tactile desensitization has been researched in many diagnoses[55].An fMRI study of 6 patients with CRPS type I compared cortical map size before and after desensitization treatment over 6 months and demonstrated a positive correlation between map size and pain intensity, suggesting an objectively measurable benefit from desensitization[46].A scoping review by Zink and Phillip of interventions targeting cortical neuroplasticity in peripheral nerve injuries showed that desensitization programs were theoretically justified but more research is needed to support their use in specific diagnoses and to further investigate mechanisms[56].A study of 39 patients with hypersensitive scars after hand injury or surgery demonstrated decreased pain and improved function, although this study did not include a control group[51].A retrospective study comparing sensory outcomes in 31 patients after fingertip replant without nerve repair showed that the group undergoing desensitization and sensory reeducation had better function, better sensation, and less pain[57].While the general consensus in the literature seems to support the desensitization of painful nerves in certain scenarios, more research is needed to support specific treatment parameters and dosage across different diagnoses and procedures.
The mechanism through which tactile desensitization reduces pain is based on neuroplasticity.When a peripheral nerve is injured, there can be an “unmasking” of normally dormant synapses in the dorsal horn of the spinal cord that receive nerve signals from areas near the site of injury[58].Additionally, new afferent nerve endings can grow into areas that were previously innervated by the damaged nerve[58].Symptoms such as pain and paresthesia can be generated through abnormal afferent signals along these new or damaged pathways and, in certain circumstances, can cause central sensitization[59].The approach of inundating the hypersensitive area with input is thought to lower the pain threshold at the dorsal horn[60], whereas providing sensory input to areas adjacent to the injury is thought to positively affect cortical mapping,which is associated with reduced pain[46].
Desensitization is not indicated in patients with cognitive impairments that limit their ability to communicate their sensory experience and understand the rationale and directions from the therapist.Because desensitization is based on theories of neuroplasticity, it is not indicated on nociceptive pain that is from an acute inflammatory process or recent injury.Further, the role of the therapist is to educate the patient on the process of desensitization and explain that minor discomfort is to be expected and does not imply that tissue is being damaged.The therapist must also determine the appropriate dosage and type of desensitization for the patient so as not to over-sensitize a painful area.Doing so may further irritate a nerve, possibly weaken the therapist-patient relationship, perpetuate a patient’s guarding response, and foster sinister beliefs about the meaning of the pain[58].
Desensitization alone will not address functional deficits and must be used in conjunction with functional activity and exercise to improve the patient’s performance in daily activities.
EXERCISE
Physical activity, including strengthening and cardiovascular exercise, is an increasingly well-documented modality used to treat a variety of pain phenotypes, especially neuropathic pain[61].Exercise is a broad category and can include anything from non-resisted movement, tai chi, yoga, pilates, walking, targeted muscle strengthening to high-intensity interval training.There is a wide range of treatment protocols for dosage and frequency of exercise depending on the patient, type of exercise, and diagnosis.Generally speaking, cardiovascular exercise is the safest treatment for most types of nerve-related pain, and is recommended to last 30-60 min 3-7 days a week for 1-15 weeks[62].Strength training may be considered when treating deconditioning associated with central sensitization and after surgical release or repair of a peripheral nerve injury.However, in entrapment neuropathies that have not had surgery, the forceful and repetitive nature of strengthening exercise can mimic the very activities that caused nerve compression in the first place, and will likely worsen nerve compression[63-65].The patient should be educated that making physical activity part of lifestyle changes will likely yield the most benefit[28].Exercise may be initiated by the patient, guided by a therapist, and/or incorporated into a home exercise program.
Cardiovascular exercise, in particular, has been extensively studied in the treatment of neuropathic and nociplastic pain[62,66,67]and is commonly used in the treatment of both upper and lower extremity CRPS[68].Other studies have examined the effect of a mixed-exercise approach, combining cardiovascular and strengthening exercises for neuropathic pain[65].
Light and gradually progressive cardiovascular exercise after surgical release of entrapment neuropathies may begin once surgical incisions are fully healed and when tolerated by the patient.After nerve transfer or repair, light cardiovascular exercise may start as early as 5 days after surgery as it has been shown to help with regeneration and pain[69], but may be deferred depending on incision healing, movement-related risk to the transfer or repair, and patient tolerance to exercise.
Aerobic exercise has been shown to be effective in CRPS-I.A randomized controlled trial of upper-body aerobic exercise in subacute stroke showed a significant reduction in pain and CRPS-I symptoms[70].Sherryet al.demonstrated short- and long-term resolution of CRPS-I symptoms in 91% of 103 children who underwent an intensive aerobic exercise program guided by a therapist without the use of medications[71].Mouse models of intensive swimming aerobic exercise in CRPS-I demonstrated improved mechanical allodynia after intervention[66].There is less evidence in the literature for exercise in the treatment of peripheral neuropathies such as carpal tunnel syndrome.In a Cochrane Review, Pageet al.found limited and low-quality evidence for the use of targeted exercises for carpal tunnel syndrome[12].A meta-analysis comparing studies of rat models with neuropathic pain in peripheral nerves that were treated with exercise supported exercise as a treatment modality, but acknowledged heterogeneity of data and the need for more research to delineate type, dosage, and frequency guidelines for clinicians[72].
Research on mechanisms for the role of exercise, particularly aerobic exercise, in the treatment of pain is derived from preclinical studies using animal models.It has been found that exercise helps regulate overactive microglial activity after peripheral nerve injury, reduces pro-inflammatory cytokines and increases anti-inflammatory cytokines, and promotes the release of endogenous opioids[62,67].
Therapy-specific contraindications for physical activity in patients with nerve pain follow general guidelines for exercise.Exercise should be used with caution in patients with cardiovascular or pulmonary disease and requires physician clearance if patients have associated symptoms.When introducing a new exercise program, vitals should be monitored closely by the therapist to ensure safety.Strength training may be contraindicated in scenarios with acute inflammation, peripheral nerve entrapment, or injury[63,64,73].Inappropriately dosed or executed strengthening, even in scenarios where strengthening is indicated, may flare pain symptoms and effective communication between therapist and patient is necessary to determine tolerance to activity.
While exercise alone may be beneficial in the treatment of nerve pain, some authors argue that it will have a greater effect if it is delivered with pain neuroscience education[39,74].
PHYSICAL AGENT MODALITIES
Physical Agent Modalities (PAMs) are agents used to modify tissue to address symptoms for a desired effect[75].These treatments are often used in conjunction with other interventions to increase benefits and promote healing.While there are many examples of PAMs that are commonly used in rehabilitation, there are a few that stand out in the treatment of nerve-related pain, including superficial heat, deep heat, and electrotherapy.
Superficial heat application, which may be applied via a heat pack or paraffin bath, is a mainstay in treating nerve-related pain.Superficial heat increases circulation and reduces pain in tissues up to 1 cm deep in circumstances of subacute inflammation and neuropathic pain[75].Deep heat is commonly achieved through the application of therapeutic ultrasound.When used in a continuous setting, ultrasound provides deep heat up to 2.5-5 cm[76]and is indicated for increasing circulation to muscle, tendon, and ligament tissues as well as reducing pain[77].There are various types of electrotherapy, also known as electric stimulation, but the most commonly used form for treating nerve pain is Transcutaneous Electrical Nerve Stimulation(TENS).TENS may be indicated for all pain types, including nociceptive, neuropathic, nociplastic, and mixed phenotypes[75].
TENS is distinctly different from and should not be confused with intraoperative electrical nerve stimulation.Intraoperative electrical nerve stimulation is performed on a single occasion by a surgeon,where a stripped wire electrode is placed directly on the nerve of an anesthetized patient immediately after nerve repair or grafting surgery to promote axonal sprouting and nerve healing[78].Conversely, TENS units are commercially available, utilize reusable adhesive skin electrodes, and can be applied numerous times in the therapy setting or at home by the patient for the management of nerve pain.While TENS has been proposed to promote axonal regeneration after nerve injury, more research is needed to support this claim[79].
Superficial and deep heat may be used to treat nerve-related pain anytime after the patient is beyond the acute inflammatory phase of healing, typically after 1-2 weeks from injury or surgery[80].TENS may be used for nerve-related pain management as tolerated in both conservative management and in the acute postoperative phase[81].
The goal of PAMs application when treating nerve-related pain is to reduce pain symptoms.Because nerverelated pain may manifest differently among individuals, even with the same diagnosis, an effective modality for one individual may not work for another.For example, cold sensitivity and cold allodynia are common in neuropathic pain[59]and the application of cryotherapy may aggravate symptoms.However, a patient may prefer the feeling of cold to heat.Heat, however, is more commonly found to alleviate nerverelated pain.An randomized controlled study (RCT) by Ozcanet al.[82]studied 30 individuals with CRPS-I who received standard rehabilitation 5 days a week for 3 weeks.In addition to standard care, the treatment group received an additional 15 min of superficial heat therapy, also 5 days a week.They found the treatment group had significantly improved neuropathic pain compared to the control group[82].Therapeutic ultrasound has more mixed evidence supporting its role in the treatment of nerve pain.For example, an RCT using a thoracotomy rat model showed that the group receiving therapeutic ultrasound had less mechanical allodynia than the control group[77].However, a Cochrane review by Pageet al.found poor and limited evidence supporting the use of therapeutic ultrasound in the treatment of carpal tunnel syndrome[83].Finally, an RCT with 30 subjects diagnosed with upper extremity CRPS-I compared a treatment group, who received conventional TENS, and a control group, who received sham TENS.Both groups received standard physical therapy treatment.They found a significant improvement in pain severity in the treatment group[84].However, another RCT found that TENS was less effective than another type of electrical stimulation, interferential current, in treating carpal tunnel pain[85].
In addition to vasodilation in the outer layers of tissue, superficial heat has an inherent analgesic effect[86].Therapeutic ultrasound on a continuous setting has been shown to promote cell proliferation, angiogenesis,and collagen production[87], but the exact mechanism for its role in nerve-related pain reduction is unclear[75].Conventional TENS is thought to work through the gate control theory[88,89].
The following are contraindications for superficial heat application: acute musculoskeletal conditions,impaired circulation, peripheral vascular disease, skin anesthesia, open wounds, and infection[75,90].Contraindications for deep heat include all those listed for superficial heat.Moreover, deep heat application is contraindicated near nerve, brain, eyes, and reproductive organs, as well as on a pregnant uterus, near spine, in cases of malignancy, near a pacemaker, and on epiphysis implants containing plastic materials[75,90].TENS and other forms of electrotherapy should not be used near implanted or temporary stimulators such as pacemakers, near the carotid sinus or sympathetic ganglia, near a deep vein thrombosis, or near a pregnant uterus[75,88].TENS should be used with caution on skin with diminished sensation and with individuals with cognitive deficits[88].
PAMs may provide symptom relief but are not independently sufficient for comprehensive treatment of nerve-related pain.The American Occupational Therapy Association recommends that PAMs be used to minimize symptoms before or during activities, or as part of their health management within the context of skilled therapy services directed by a trained occupational therapist[91].The American Physical Therapy Association recommends a similar approach, noting that physical agents alone must be supplemented by the skilled treatment of a physical therapist[92].
MIRROR BOX THERAPY
Mirror box therapy, also called mirror therapy, is where a patient views a reflection of a body part in a mirror with the goal of pain reduction and cortical remapping.It was first described in 1996 by Ramachandran and Rogers-Ramachandran[93,94]as a treatment for phantom limb pain.During mirror box therapy, the patient places their affected limb inside a mirror box while performing activities or exercises under the guidance of a therapist.While inside the box, the vision of the affected limb is occluded and the patient instead visualizes the unaffected limb in the mirror.Once proficient, the patient may perform mirror box exercises as part of a home program[95].Treatment parameters vary, but often include daily practice for at least 20 min per session[96].Mirror boxes are low-cost to the clinic and the patient.
Mirror therapy has been used to treat phantom limb pain[94,97-99], CPRS[100,101], and chronic pain[95], as well as for sensory and motor reeducation[102].
Mirror box therapy is low-risk and can be used at any time in both conservative management and immediately after surgery.Because the patient is actually looking at and moving only the unaffected limb,this treatment may even be initiated when the affected limb is fully immobilized and even before movement is permitted.
A systematic review and meta-analysis by Boweringet al.[103]examined mirror therapy in conjunction with graded motor imagery, a cognitive retraining tool[104], and found that they were promising interventions for treating chronic pain but recommended more robust research[103].Moseleyet al.argued that based on the current evidence, mirror therapy is likely no better than graded motor imagery at treating pain[105], although an RCT published the following year by Cacchioet al.suggested otherwise[101].In their study, which included 48 stroke patients with CRPS-I, they found that the group receiving mirror therapy had significantly better function and less pain compared to the control group[100].Chanet al.compared mirror therapy to imagery and sham mirror therapy in phantom limb pain and found that the group receiving mirror therapy had significantly less pain than the other groups[106].The current evidence for the use of mirror therapy in many pain types is encouraging, but more research is needed to make more confident clinical recommendations across diagnoses.
The mechanism through which mirror therapy works is unclear; however, the two predominant supporting theories are motor cortex plasticity and mirror neuron activation[107].Studies that support the motor cortex theory demonstrate that the brain does not perceive the difference between a limb and its reflection[108]and that experiencing the affected limb in a different state may lead to downregulation of pain pathways[94].Other studies based on fMRI have demonstrated links to the mirror neuron system that suggest links between visual input and motor or sensory output, but the specific mechanism remains unknown[109].
There are no published contraindications for mirror box therapy[95]and there have been no published cases of mirror therapy worsening pain[106].However, the treating therapist must consider and be sensitive to the potential emotional effects of the patient “seeing” their injured or amputated limb in its uninjured form in the mirror, as a small portion of patients have reported transient feelings of grief upon viewing the reflected limb[106].
While promising, mirror therapy for pain reduction might also be used in conjunction with other interventions that incorporate movement and engagement in functional activities to reap the most functional gains[110].
KINESIO TAPING
Kinesio taping, also known as textile taping, was developed in the 1970s by Kenzo Kase, a Japanese chiropractor[111].The tape is thin, elastic, latex-free, and dries easily.It is relatively inexpensive and is readily available to the public for purchase.It gained public attention after many athletes displayed their colorful taping patterns during the Olympics[112].Depending on the desired effect, the tape is cut into the desired length and shape, and applied in varying degrees of tension in numerous patterns and configurations by a trained therapist or an educated patient or family member for carryover at home.Proponents of Kinesio taping claim it has four benefits: (1) normalizing muscle function and strength; (2) reducing pain; (3)promoting lymphatic drainage; and (4) promoting proper joint alignment[113].
Kinesio taping has been used to treat an extremely wide range of conditions across the continuum of pain phenotypes, including musculoskeletal disorders, pain, and neurological disorders[114].Many studies have examined the effect of Kinesio taping on edema reduction[115,116].
Kinesio taping may be used in conservative management of entrapment neuropathies and central sensitization if it is tolerated by the patient.It may be used after nerve surgery when the incision is healed.
In regards to subacute or chronic nerve-related pain, the evidence on Kinesio taping is mixed.A systematic review by Kalron and Bar-Sela found short-term benefits of general pain relief, including musculoskeletal pain, only while the tape was applied to the skin[114].Case reports describing the use of Kinesio taping for nerve-related pain in conjunction with many other common interventions suggest benefits, but are based on low-quality evidence[117,118].A prospective randomized study by Akgolet al.comparing the effects of Kinesio taping versus low-power laser in the treatment of carpal tunnel syndrome found improvement in both groups, with the most improvement in the low-power laser group[119].A systematic review by Artioli and Bertolini found that any pain benefit of Kinesio taping lasted 24 h to 1 week and was similar or slightly better than other nonpharmacologic interventions[112].
While still unclear, the mechanism through which Kinesio taping reduces pain is based on the gate control theory[89,112].Additionally, the gentle tug on the patient’s skin may remind the patient to avoid aggravating movements[120].
Contraindications for Kinesio taping include open wounds, cancer, active infections, and thrombosis[121].It should be used with caution if the patient has sensitive skin, renal disease, or diabetes and if the patient has difficulty communicating[122].
Kinesio taping alone is not enough to restore function and should be used for symptom management purposes for nerve-related pain to allow for patient engagement in meaningful activity and prescribed exercises.
CONCLUSION
Rehabilitation therapists and surgeons treating patients with nerve-related pain have many nonpharmacologic tools to help mitigate symptoms and allow patients to more effectively engage in meaningful and functional activities.The provider must choose interventions that are compatible with the patient’s pain phenotype, diagnosis, postoperative healing if relevant, and individual symptoms.While many commonly used interventions may have anecdotal support, more robust research is needed in this realm to confidently recommend each intervention for specific diagnoses and presentations.
DECLARATIONS
Acknowledgments
The author would like to thank Ian Kratter, Wendy Moore, Minnie Mau, and Carolyn Gordon for their thoughtful review and comments on the draft manuscript.
Author’s contributions
The author contributed solely to the article.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
The author declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
 Plastic and Aesthetic Research2024年1期
Plastic and Aesthetic Research2024年1期
- Plastic and Aesthetic Research的其它文章
- The mangled upper extremity: a principled approach to management
- Systematic review on the centrocentral anastomosis technique for the surgical treatment of traumatic neuromas
- Role of transoral robotic surgery in the salvage setting: pitfalls and challenges
- The emerging role of sentinel lymph node biopsy in oral cavity and oropharyngeal carcinomas
- Early lymphaticovenous anastomosis in lymphedema management: a pilot study
