Early lymphaticovenous anastomosis in lymphedema management: a pilot study
Fumio Onishi, Hayato Nagashima, Nanae Okuda, Toshiharu Minabe
1Department of plastic surgery, Saitama Medical Center, Saitama Medical University, Kawagoe, Saitama 350-8550, Japan.
2Division of plastic and reconstructive surgery, Tochigi Cancer Center, Utsunomiya, Tochigi 320-0834, Japan.
3Nursing department, Tochigi Cancer Center, Utsunomiya, Tochigi 320-0834, Japan.
Abstract Aim: Lymphedema is a progressive degenerative disease that can cause severe swelling and recurrent infections.Conservative and surgical treatments, such as lymphaticovenous anastomosis (LVA), are available; however, the optimal timing for LVA after the initiation of complex decongestive therapy (CDT) remains unclear.This study aimed to evaluate the effect of CDT duration prior to LVA on the treatment outcomes of upper extremity lymphedema.
Keywords: Lymphedema, complex decongestive therapy, CDT, lymphaticovenular anastomosis, LVA, early intervention, early surgical indication
INTRODUCTION
Lymphedema is a progressive degenerative disease that can develop in severe cases with massive swelling,elephantiasis, and repeated infection.Conservative and surgical treatments can be offered to patients with lymphedema, with conservative treatment usually preceding surgery.CDT, a conservative treatment,comprises compression therapy, manual lymph drainage, remedial exercises, and skin care.Delay in the initiation of therapeutic intervention may cause resistance to treatment, which is one of the worst problems associated with lymphedema.
Over the past decade, LVA has evolved as a surgical treatment for lymphedema.LVA is indicated as an adjunct to CDT or when CDT fails to eliminate edema[1].In general, LVA is best suited for the treatment of early-stage lymphedema.Campisiet al.reported that lymphatic reconstruction in the early stages of disease,when tissue changes are minimal, can offer excellent outcomes with complete restoration of lymphatic flow[2].However, the dilemma of LVA becoming less effective arises when long-term CDT is not sufficient for improvement due to the progression of tissue fibrosis[3,4].Therefore, LVA should be performed prior to disease progression[5].
However, the proper timing for LVA after CDT initiation remains unclear.Since the deterioration of lymphatic function due to tissue fibrosis would not cease even with CDT unless the edema disappeared completely, we hypothesized that earlier surgical indications should be considered and that early LVA would improve treatment outcomes.
This study aimed to test this hypothesis by evaluating whether the time from the start of CDT to LVA affects treatment outcomes, including conservative and surgical treatments.
METHODS
We retrospectively studied 50 consecutive patients with upper extremity lymphedema of the arm with clinical stage II (International Society of Lymphology[1]) who underwent LVA between September 2015 and November 2022.All of them presented with secondary lymphedema after breast cancer treatment.
The patients were divided into two groups based on the duration of CDT before LVA: patients with CDT < 6 months (early group) and those with CDT > 6 months (non-early group).We compared the PEV between the two groups using the propensity score weighting method to adjust for confounding factors.
CDT
Patients were fitted with elastic sleeves of compression classes 1-3, depending on their condition, under the supervision of lymphedema therapists.Some patients ceased compression therapy or continued light compression of less than compression class 1 because of intolerance or adverse effects.In this case, the compression class was classified as Class 0 for analysis.All patients received complex decongestive therapy(CDT) postoperatively during their hospital stay, which included:
- Compression therapy: Patients were fitted with individualized elastic compression garments before discharge.
- Remedial exercise: All patients participated in exercises to stimulate lymph drainage.
- Manual lymph drainage: Massage was performed by trained therapists to aid drainage.
After discharge, patients continued wearing compression garments and performing self-massage and exercises at home.They were followed up at 1 week, 1 month, 3 months, 6 months, and 12 months postoperatively to monitor limb volume and receive additional CDT as needed.
Surgical indication
1.Edema plateaued and remained despite reduction by proper CDT
2.Subjective symptoms such as heaviness, fatigue, and tightness remained despite proper CDT
3.Intolerance of CDT due to physical or mental difficulties
Surgical procedure
Prior to surgery, ICG lymphangiography was performed to visualize the lymphatic drainage pattern and plan the anastomotic sites, usually three sites [Figure 1].Patent blue was additionally used in a subset of patients whose lymphatic vessels were anticipated to be challenging to identify under the surgical microscope with ICG alone.The collective lymphatic vessels and subcutaneous veins were located after skin incisions at each of the three surgical sites.The lymphatic and subcutaneous veins were anastomosed in a side-to-end fashion, where a side-wall incision was made on a lymphatic vessel to join it to a nearby subcutaneous vein using 11-0 nylon sutures under a surgical microscope.
Data collection
We collected clinical data, including age, body mass index (BMI), number of irradiated areas, previous chemotherapy (taxane), previous LVA, clinical stage, history of lymphangitis, the period from onset of lymphedema to CDT, duration of CDT before LVA surgery, compression class of the elastic garment, and measured girth.The clinical stage was based on the classification of lymphedematous limbs set forth by the International Society of Lymphology.Girth measurements were performed at five points on the upper extremities: hand (Ch), wrist (Cw), forearm (Cf), elbow (Ce), and upper arm (Ca).Girth measurement data were collected at the first visit, one month before, and one year after LVA surgery.Extremity volume was estimated using the upper extremity lymphedema index (UEL index)[6]which is given by the formula: UEL index = (Ch2+ Cw2+ Cf2+ Ce2+ Ca2)/BMI.PEV was defined as the percentage difference between the UEL index of the affected and healthy limbs.
Statistical analysis
Overlap weighting is a propensity scoring method that aims to mimic important attributes of randomized clinical trials, such as a clinically relevant target population, covariate balance, and precision.We applied overlap weighting to adjust for confounding factors and improve the robustness of our findings.We estimated propensity scores (PS) using logistic regression and calculated the overlap weights for each participant based on their propensity scores.The propensity score was calculated using a logistic regression model with the following variables: age, BMI, number of irradiated areas, previous chemotherapy (taxane),previous LVA, history of lymphangitis, duration from the onset of lymphedema to CDT, PEV at the first visit, and compression classes of compression garments during the preoperative CDT period.Overlap weights were computed as 1-PSi when participant i is from the target population, otherwise, PSi.After applying overlap weighting, we compared the PEV at 1 year postoperatively and the reduction rate between the early and non-early indication groups.
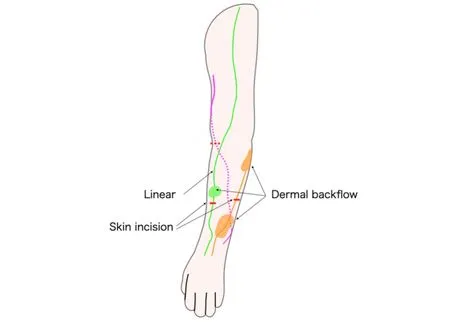
Figure 1.Schematic drawing of the surgical planning of LVA for upper extremity lymphedema.LVA targets the linear-visualized lymphatic vessels or those with dermal backflow, if present.LVA: lymphaticovenous anastomosis.
To compare the two groups, the Wilcoxon rank-sum test was performed for continuous data, and the chisquare or Fisher's exact test for categorical data.All tests were two-tailed, and statistical significance was defined asP< 0.05.Standardized mean differences were calculated.All analyses were performed using EZR[7], a graphical user interface software for R (version 3.6.3; R Foundation for Statistical Computing,Vienna, Austria).
RESULTS
All 50 patients were clinically classified as having stage II disease.Of these, 28 patients underwent LVA within 6 months of CDT initiation, whereas 22 patients underwent LVA > 6 months after CDT initiation[Table 1].The stratified number of LVA cases per 2-year period over the study duration is shown in Figure 2.
All patients received CDT from the same trained therapist.Postoperative compression was individualized.Preoperatively, 10 Early Group patients (36%) wore Class 0 compression garments, 2 (7%) Class 1, and 16(57%) Class 2.Among Non-early Group, 4 (18%) used Class 0, 1 (7.1%) Class 1, and 17 (77.3%) Class 2.Postoperatively, 6 Early Group patients (21.4%) wore Class 0, 4 (14.3%) Class 1, and 18 (64.3%) Class 2 compression.In the Non-early group postoperatively, 6 (27.3%) wore Class 0, 1 (4.5%) Class 1, and 15(68.2%) Class 2.No significant differences were seen between the two groups both preoperatively and postoperatively [Table 2].The primary outcomes were postoperative PEV and the reduction rate at 12 months.The results showed that, after applying overlap weighting, the baseline characteristics and demographics of the treatment and control groups were balanced [Table 2].At the 12-month postoperative follow-up, the early treatment group had a significantly lower PEV of 4% compared with 10% in the delayed treatment group (P= 0.02) [Figure 3].This difference corresponds to a medium effect size, with a standardized mean difference (SMD) of 0.42.Additionally, the early treatment group had a significantly greater reduction rate of 56% compared to 25% in the delayed treatment group (P= 0.03) [Figure 4].The reduction rate difference also represented a medium effect size,with an SMD of 0.59 [Table 2].
Representative cases
Case 1: A 52-year-old woman presented with lymphedema of the left upper extremity [Figure 5A].CDT started 1 month after the onset of lymphedema, and LVA surgery was performed after four months of CDT management.The postoperative picture showed marked improvement of the edema in the affected limb[Figure 5B].
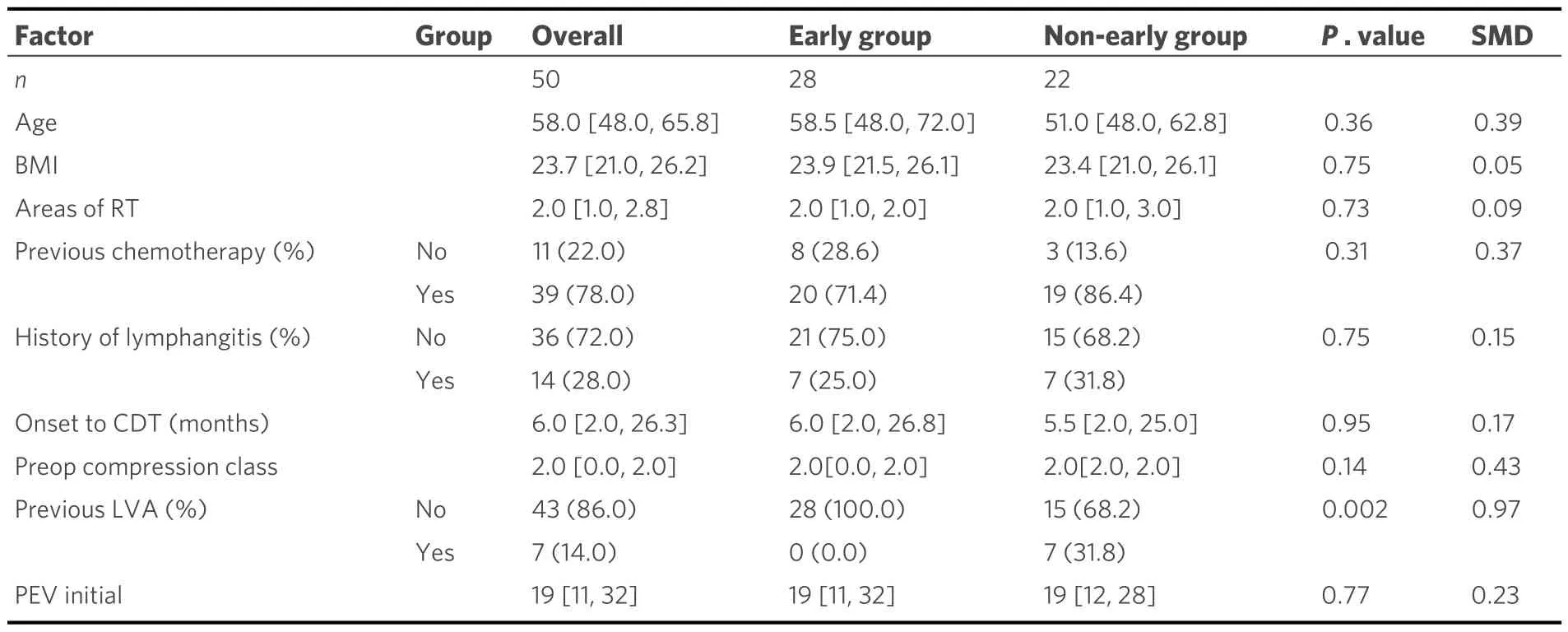
Table 1.Characteristics and demographics

Table 2.Comparison between early and non-early groups after overlap weighting
Case 2: A 60-year-old woman presented with lymphedema of the right upper extremity [Figure 6A].CDT started 27 months after the onset of lymphedema, and LVA surgery was performed after eight months of CDT.Despite mild improvement, apparent edema remained [Figure 6B].
DISCUSSION
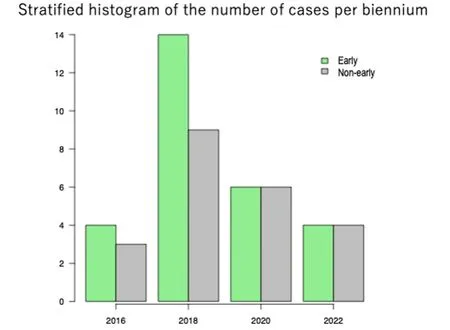
Figure 2.Stratified histogram of the number of cases per 2-year period over the study duration.
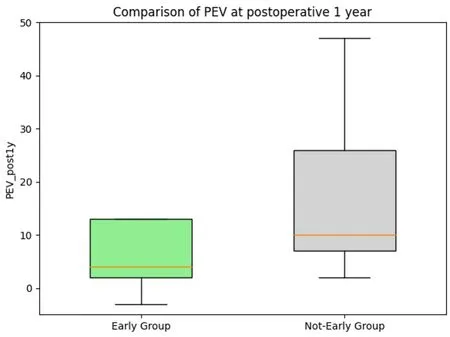
Figure 3.PEV comparison at 1 year postoperatively.
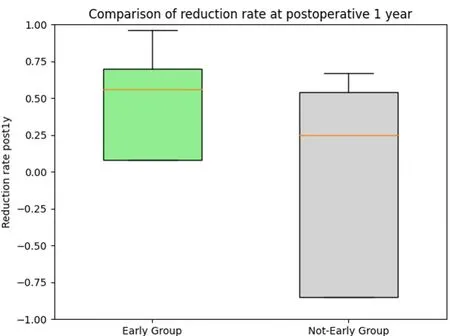
Figure 4.Comparison of the volume reduction rate at 1 year postoperatively.
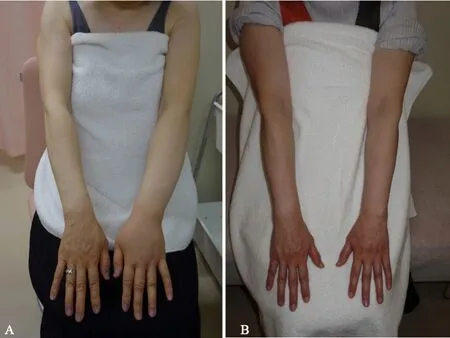
Figure 5.A patient in the early group before (A) and after (B) LVA.PEV for the affected limb reduced from 16.6 to 6.4 after LVA surgery.LVA: lymphaticovenous anastomosis; PEV: percent excess volume.
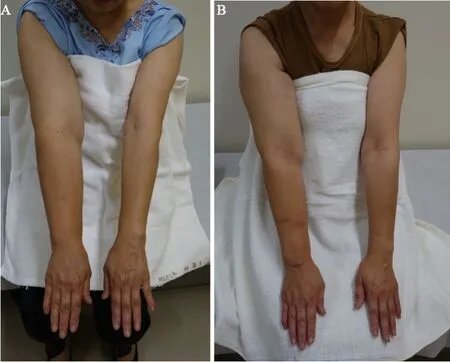
Figure 6.A patient in the non-early group before (A) and after (B) LVA.PEV of the affected limb decreased from 50.7 to 37.5 after LVA, showing a constant decrease, but still not less edema.LVA: lymphaticovenous anastomosis; PEV: percent excess volume.
This study compared the early and non-early groups in terms of the timing of LVA.The early group showed significantly better outcomes in PEV and volume reduction rate 1 year after LVA, adjusting for patient background as much as possible using the propensity score with an overlap weighting method.These results suggest the benefit of early indication of LVA after the initiation of CDT in patients with upper extremity lymphedema.This supports previous literature[4], which argues that delaying reconstructive surgery beyond 1 year may compromise its efficacy because of irreversible damage to the lymphatic system.This is the first study to show how early LVA should be indicated for a better treatment prognosis of upper extremity lymphedema.
CDT is considered more beneficial when it begins as an early intervention[8].The mechanisms behind successful CDT are the remaining lymphatic drainage routes in the affected extremity and stimulation of smooth muscle contraction of lymphatic vessels, which is a major premise for the existence of functional lymphatic vessels[9].Namely, the efficacy of CDT depends on the residual lymphatic route.However,residual lymphatic pathways exhibit alterations in drainage patterns as early as stage 0/I[10], which indicates most patients who are starting CDT already have a functional loss to some extent, potentially affecting the efficacy of CDT.Since microsurgical treatment such as LVA increases lymphatic return[1]and relieves lymphatic hypertension[4], it should work complementarily as an adjunct to CDT to improve treatment efficiency and outcomes.
叙利亚已成为地缘政治冲突的焦点地区,不适合开发新的长期投资项目。因战争破坏而中断、在局势缓和后需要重启的项目,要视再投资风险及经济评估结果再进行决策。中国石油企业可寻找技术服务和油田工程建设周期短的项目,规避地缘政治变化造成的潜在投资风险。
The efficacy of LVA is influenced by residual lymphatic function.To obtain a sufficient LVA outcome,functional lymphatic vessels that suit the anastomosis must remain in the affected extremity[11].Leeet al.argued that it would be ideal to perform reconstructive surgery while the function of lymphatic transport is salvageable before damage to the lymphatic vessels becomes irreversible[4].In terms of residual lymphatic function, in future studies, it would be informative to directly compare ICG findings between earlier versus delayed LVA groups.This analysis could determine if preoperative lymphatic imaging characteristics correlate with surgical outcomes.Additionally, as the mechanisms of LVA are based on a pressure gradient through the bypass[12], the benefit of LVA would be greater in the presence of lymphatic hypertension.Considering that lymphatic pressure rises at the beginning of lymphatic obstruction[13], whereas it declines due to lymphatic pump failure from chronic excessive afterload to lymphatic smooth muscle cells[14], LVA should be performed in the early phase of the disease.
For how long should preoperative CDT be performed? CDT has been effective in reducing edema and improving the patients’ quality of life; however, it does not address the cause of the disease and remains incomplete[5].Studies have demonstrated the long-term efficacy of CDT, with an edema reduction rate of approximately 60%[15].The remaining chronic edema continued to damage the lymphatic system, causing further tissue fibrosis and fat accumulation[16].Given the dilemma of LVA, the earliest evaluation of the efficacy of CDT and indications for reconstructive microsurgery is paramount.According to Hwanget al.,the greatest decrease in percent excess volume was observed at 6 months after CDT, suggesting that evaluation for the indication of LVA can be performed at 6 months after the initiation of CDT[17].Moreover,another study stated that surgical intervention is considered after 3-6 months of the CDT protocol[18].Based on our results, it may be reasonable to consider LVA as early as 6 months or less to maximize treatment outcomes.
Our study has some limitations.First, our cohort had a limited sample size (n= 50), accrued over 7 years with an average of 7 cases annually, which may limit the generalizability of our findings.Additionally, upper extremity cases are less common than lower extremity cases in many Japanese institutions, further impacting external validity.However, we argue that our cohort of breast cancer-related lymphedema represents an appropriate population for evaluating this intervention’s efficacy.Still, caution is warranted when extrapolating these findings to other populations and settings.Further research is necessary to validate and expand on our observations.Second, even a propensity score analysis with overlap weighting or inverse probability weighting cannot be fully adjusted for potential biases.Randomized controlled trials are needed to determine the true effects of the early indications for LVA in the management of lymphedema.Third, the observed results include not only the LVA but also the CDT, which we should be aware of.The treatment effect should always be combined because these modalities are complementary.
CONCLUSION
LVA would be more beneficial if performed less than 6 months compared to if performed more than 6 months after CDT initiation for stage II upper extremity lymphedema.The present study suggests a potential improvement in the treatment prognosis of lymphedema by considering the early indication of LVA while impaired lymphatic function remains reversible and fibroadipose tissue accumulation is reduced.
DECLARATIONS
Authors’ contributions
Conception and design of the study: Onishi F, Nagashima H, Minabe T, Okuda N
Manuscript writing: Onishi F, Minabe T,
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
All study participants provided informed consent, and this study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board.
Consent for publication
Obtained publishing license from the participant.
Copyright
© The Author(s) 2024.
 Plastic and Aesthetic Research2024年1期
Plastic and Aesthetic Research2024年1期
- Plastic and Aesthetic Research的其它文章
- The mangled upper extremity: a principled approach to management
- Systematic review on the centrocentral anastomosis technique for the surgical treatment of traumatic neuromas
- Role of transoral robotic surgery in the salvage setting: pitfalls and challenges
- Therapeutic management of the painful nerve: a narrative review of common rehabilitation interventions
- The emerging role of sentinel lymph node biopsy in oral cavity and oropharyngeal carcinomas
