Associations between physical activity and risk of liver cancer: results from a population-based cohort study in Chinese women
Jing-Yu Tan, Zhuo-Ying Li, Qiu-Ming Shen, Yan Zhang1,, Jia-Yi Tuo1,, Da-Ke Liu, Yu-Ting Tan,Hong-Lan Li, Yong-Bing Xiang1,2,
1School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
2School of Public Health, Fudan University, Shanghai 200032, China.
3State Key Laboratory of Systems Medicine for Cancer & Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital,Shanghai Jiao Tong University School of Medicine, Shanghai 200032, China.
Abstract Aims: Epidemiological evidence has revealed varying degrees of relationship between physical activity and risk of most cancers, but the association between physical activity and risk for primary liver cancer in Chinese has seldom been reported.This study aims to characterize the associations between different physical activity types and liver cancer risk in Chinese women.
Keywords: Liver cancer, physical activity, lifestyle, prospective study, female
INTRODUCTION
Causes of cancer are complex and are mostly related to genetic, environmental and lifestyle factors, as well as their interactions[1].In recent years, there has been a growing awareness that lifestyle changes can contribute to cancer prevention; thus, there exists a significant imperative and opportunity to underscore the relevance of lifestyle choices in tumor progression[2].The evidence implied that, in general, the more physical activity people have, the lower their risk of some cancers.Strong evidence showed that physical activity can decrease the risk of colorectal[3], breast (post-menopause)[4], and endometrial cancers[5]and prevent excessive weight gain as well.Vigorous physical activity, such as running or fast cycling, decreases the risk of breast cancer[6].The evidence on physical activity and other cancers was limited, either in amount or by methodological flaws, but was suggestive of a decreased risk of esophagus, lung and liver cancers.
It is well known that liver cancer is the sixth most common cancer in both sexes worldwide.The agestandardized incidence rate stands at 14.1 per 100,000 population(ASIR) for males and 5.2 per 100,000 population for females[7].In most countries, the incidence rate and mortality rate in men are 2 to 3 times higher than in women.This difference is associated with risk factors for liver cancer.In addition to chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) and aflatoxin contamination, the main risk factors also include heavy alcohol intake and smoking, which are more common in men.However,excess body weight and type 2 diabetes have become more major risk factors than the hepatitis virus, with the changing prevalence in women.An incidence analysis of cancer registration data showed that liver cancer became one of the leading five cancer types in Chinese women, with 103,025 incidences and 107,440 new cases in 2020[8].With the increasing cases of liver cancer, research is being conducted constantly on the findings of risk or protective factors of liver cancer, including exercise and physical activity.National Institutes of Health-American Association of Retired Persons (NIH-AARP) study in America revealed that vigorous physical activity was significantly related to liver cancer risk, and the physically active group had a 36% risk reduction compared to the participants with the lowest level of physical activity[9].The results of several Japanese studies suggest that higher levels of physical activity were associated with a lower risk of liver cancer, although the association is more pronounced in men and needs further investigation in women[10-12].European and American cohort studies also found similar associations that increasing levels of physical activity can decrease the risk of liver cancer[9,13-17].However, few studies have focused on the Chinese population, and the results of these prospective studies did not yet indicate an obvious relationship between physical activity and liver cancer risk in Chinese[18,19].
Epidemiological evidence on the relationship between physical activity and liver cancer has not been quantitatively summarized, with previous studies mostly focusing on mortality rather than morbidity or concentrating on European and American populations.Therefore, we referred to previous definitions of physical activity and included several particular types of physical activity to better assess the association between physical activity and liver cancer risk.Considering the differences in occupation, diet and lifestyles between China and other regions, as well as variations in exercise habits in both sexes, we conducted this study based on the Shanghai Women's Health Study (SWHS).With the aim of understanding the causes and prevention of liver cancer, it is important to characterize the relationships between physical activity and the risk of female liver cancer in the Chinese population.
METHODS
Study population
SWHS is a population-based, prospective cohort study conducted in an urban district of Shanghai between 1997 and 2000.The design and detailed implementation of SWHS can be found in the previous report[20].Study participants consisted of female residents aged 40-70 years who were registered as households in Shanghai.Of the eligible women approached for the study, 74,940 completed a baseline interview, with a response rate of 92.3%.A uniformly designed questionnaire was used to collect information by trained staff in face-to-face interviews and by subjects completed the self-administered & structured questionnaire.We included the baseline information of sociodemographic characteristics, medical history, personal habits and lifestyles, dietary habits, physical activity, self-reported family history of cancer, occupational history, and reproductive history.
In the current study, participants who met the following criteria were excluded: (1) diagnosis of cancer in situ during follow-up (n= 135); (2) deaths from unconfirmed diagnosis of cancer (n= 310); (3) cancer at baseline (n= 1595); (4) loss to follow-up shortly after enrollment (n= 3); (5) extreme values for total energy intake (< 500 kcal/day or > 3500 kcal/day) (n= 125); (6) with missing data for any covariates of interest (n=98).After these exclusions, a total of 72,674 participants were finally included in the study.The flow diagram for participant inclusion is attached in Figure 1.
Exposure assessment
We asked each participant about their smoking and drinking habits, with smoking defined as at least one cigarette per day for more than six months continuously and drinking defined as at least three times per week for more than six months continuously[20].The total energy intake (kcal/day) of subjects was examined by the food frequency questionnaire (FFQ), which was tested for reliability and validity[21], and its calculation depended on theChinese food composition table.
The PAQ, which was also tested for reproducibility and validity, was used to assess the physical activity of the study participants[22].In our questionnaire, TPA was classified into two main categories: LTPA in the past 5 years and non-exercise DPA in the past 1 year.In the LTPA section, we recorded the frequency(hours/week) and duration (years) of up to 3 types of exercise/sports in the past 5 years.We also investigated the exercise participation of the study participants during their adolescence (13-19 years old).DPA includes stair climbing, housework participation, walking, and cycling.Housework mainly includes cooking, childcare, laundry, shopping and cleaning.
We used the standard metabolic equivalent values (MET) to estimate the intensity and the energy expenditure of physical activities.Energy expenditure of LTPA was estimated by using a weighted average of energy expended in all types of exercise reported in the past 5 years (MET-hour/week).The total energy expended in DPA was calculated by summing up the energy expended in each of the daily activities.The following MET values were used according to the physical activity compendium: stair climbing, 9.0 METs;housework, 2.0 METs; walking, 3.3 METs; and cycling, 4.0 METs[23].Considering the distribution of different exposure variables, we used three categorization methods to best characterize their distribution.For example, TPA and DPA were categorized by quartiles; LTPA and stair climbing were categorized as “none + tertiles” because some of the study participants reported that they did not participate in these activities; housework and walking were manually categorized into four groups according to their distribution.

Figure 1.The flow chart of the study design.
Follow-up and identification of outcome
All cohort participants were followed up to identify the outcome of incident liver cancer through in-person surveys every 3-4 years.In addition to the active follow-up surveys, the cohort members were regularly linked to incidence data from the Shanghai Cancer Registry and death data from the Vital Statistics Department to supplement data on new cancer cases and all deaths[24].Cancers are coded according to the International Classification of Diseases, Ninth Revision (ICD-9).Primary liver cancer is a malignant tumor that originates in the liver and coded as 155 in the ICD-9.
Statistical analyses
Baseline characteristics were described as numbers with frequencies for categorical variables and median(IQR) for continuous variables.The characteristics of participants with and without liver cancer were compared using χ2tests for categorical variables and Mann-Whitney tests for continuous variables.Personyears for every participant were calculated as the time interval between study entry and exit.We defined age at study enrollment as entry time, and the exit time was defined as age at liver cancer diagnosis or age at censoring due to death, loss to follow-up, or December 31, 2016, whichever came first[25].
The associations between physical activity and liver cancer risk were examined using the Cox regression models.Chronological age was taken as the underlying time scale and models were stratified by birth cohorts (1926-1935, 1936-1945, 1946-1960)[26].The proportional hazard assumption was examined by checking the correlations of Schoenfeld residuals of each covariate with three functions of follow-up time (t,log(t), and t2)[27].No evidence of a departure from the assumption was detected in our study.HRs and their 95%CIs were calculated in two multivariable-adjusted models[25].Potential confounders were determined based on the literature review and the findings from our previous studies.Model 1 was adjusted for education (elementary school or less, secondary school, college or above), income (low, middle, high),postmenopausal (yes, no), employment status (employed, retired), cigarette smoking (ever, never), alcohol drinking (ever, never), history of chronic hepatitis (yes, no), history of cholelithiasis (yes, no), family history of liver cancer (yes, no) and total energy intake (kcal/day, continuous).Model 2 was further adjusted for body mass index (BMI) (kg/m2, continuous) and history of type 2 diabetes mellitus (T2DM) (yes, no).Linear trends across categories were evaluated by assigning a series of ordinal scores to each category (0 for the reference category and 1, 2, and 3 for each of the three exposure categories) and entering that score into the model as a continuous variable.Considering the possible changes in physical activity following the diagnosis of chronic diseases, sensitivity analyses were conducted by excluding participants with preexisting chronic conditions (T2DM, hypertension, coronary heart disease, myocardial infarction and stroke)at baseline to examine the robustness of our main results.
A two-sidedPvalue of less than 0.05 was considered statistically significant.Analyses of data were performed in SAS 9.4 (SAS Inc., Cary, N.C., USA).
RESULTS
A total of 255 new cases of primary liver cancer in our cohort were identified during over 1.26 million person-years from 1997 to the end of 2016, with a median follow-up time of 18.12 years.The incidence density of female liver cancer was 20.12/100,000 person-years during the follow-up time.
Table 1 shows the baseline characteristics relevant to this study and the specifics of physical activity by liver cancer status.Older cohort members who had a medical history of chronic hepatitis, cholelithiasis, and type 2 diabetes, and a family history of liver cancer were more likely to develop liver cancer.We observed the differences in education background and family income in two groups of non-cases and liver cancer cases.In addition, compared to study participants who were not diagnosed with liver cancer, women diagnosed with liver cancer tended to have a higher BMI and engage in less exercise and sports tournaments in adolescence, with more exercise and less cycling in adulthood.Null associations were detected in the rest of the variables [Table 1].
The results of two multivariable-adjusted Cox regression models for the relationship between physical activity and the risk of liver cancer are presented in Table 2.Null associations were found in the relationships between TPA, LTPA, DPA and liver cancer risks in both multivariable-adjusted regression models.The highestvs.lowest quartile of HRs (95%CIs) were 0.82 (0.58, 1.17) for TPA, and 0.80 (0.56, 1.15)for DPA after further adjusting for multiple confounders, respectively.Taking the adult LTPA (MET-hour/week), adult LTPA (hour/week), adolescent LTPA and stair climbing into consideration, no significance was observed in both multivariable-adjusted models; the highest tertilevs.none of HRs (95%CIs) were 1.15(0.82, 1.61) for adult LTPA (MET-hour/week), 1.15 (0.82, 1.61) for adult LTPA (hour/week), 0.99 (0.60,1.62) for adolescent LTPA and 0.77 (0.55, 1.08) for stair climbing after further adjustment for confounders.Notably, after adjusting for confounding factors, the risk of liver cancer was reduced by 3%, 28%, and 25%among study participants who climbed stairs less than or equal to 8, 14 and greater than14 times per week,respectively, compared to those without participation in stairs climbing (Ptrend= 0.042).It suggests that the liver cancer risk of participants may have decreased gradually with increasing stair climbing, although this trend was not statistically significant.Furthermore, null associations were found in relation to the DPA.The highestvs.lowest levels of HRs (95% CIs) were 0.78 (0.53, 1.15) for housework and 0.87 (0.59, 1.30) for walking [Table 2].
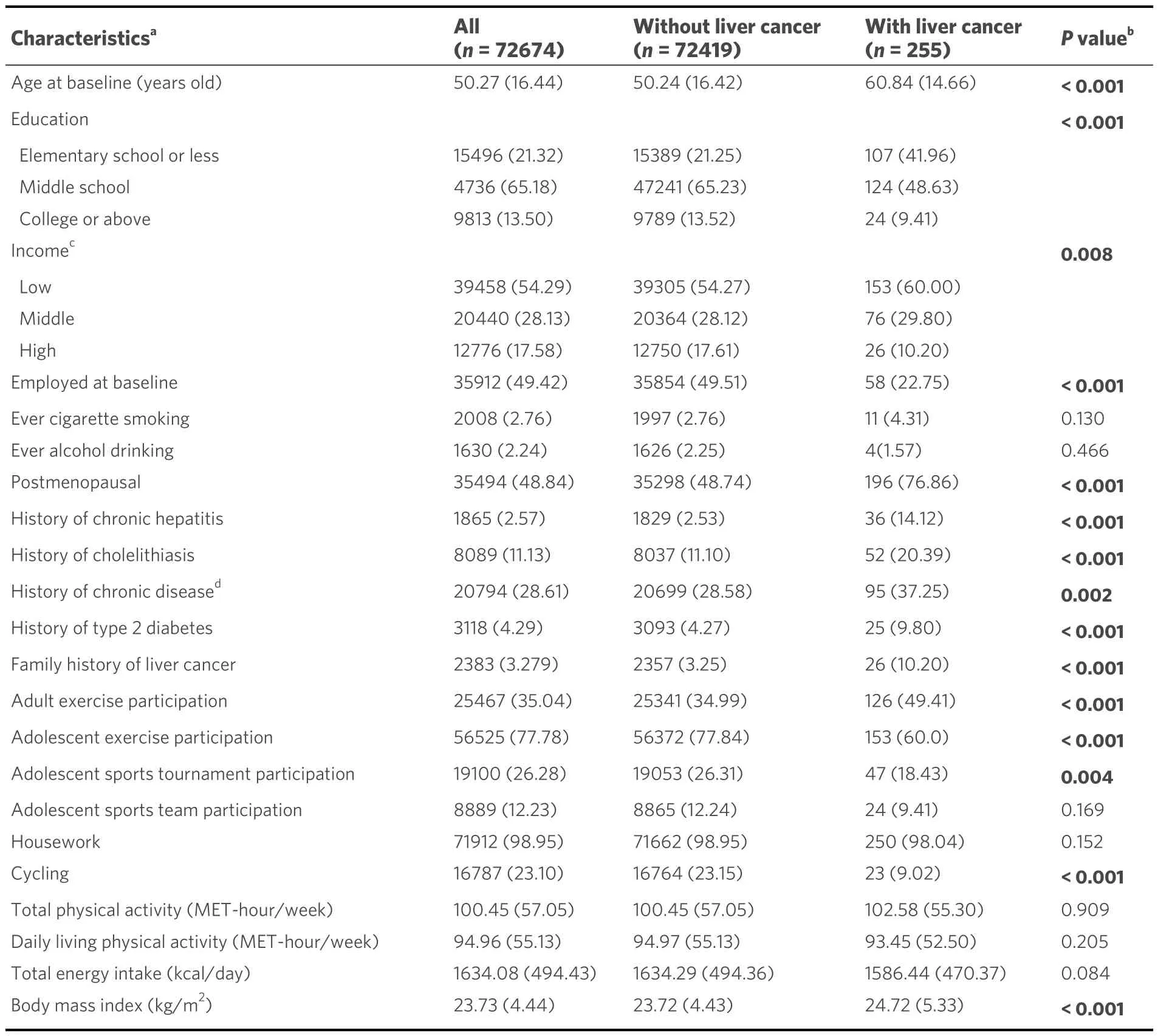
Table 1.Baseline characteristics and physical activity habits by liver cancer status (SWHS,1996-2016)
Finally, we made sensitivity analyses by excluding participants with pre-existing chronic conditions at baseline.We found results similar to the main analysis: the relationship between physical activity and liver cancer risk was not observed except for the presence or absence of housework participation.Compared to the study participants who did not participate in housework (n= 3), the HR (95%CI) of the women who participated in housework (n= 157) was 0.28 (0.09, 0.88) after further adjusting for BMI and T2DM.Statistical significance was not found in the results for other types of physical activity [Table 3].

Table 2.Associations between physical activities and liver cancer risk in all participants (SWHS,1996-2016)
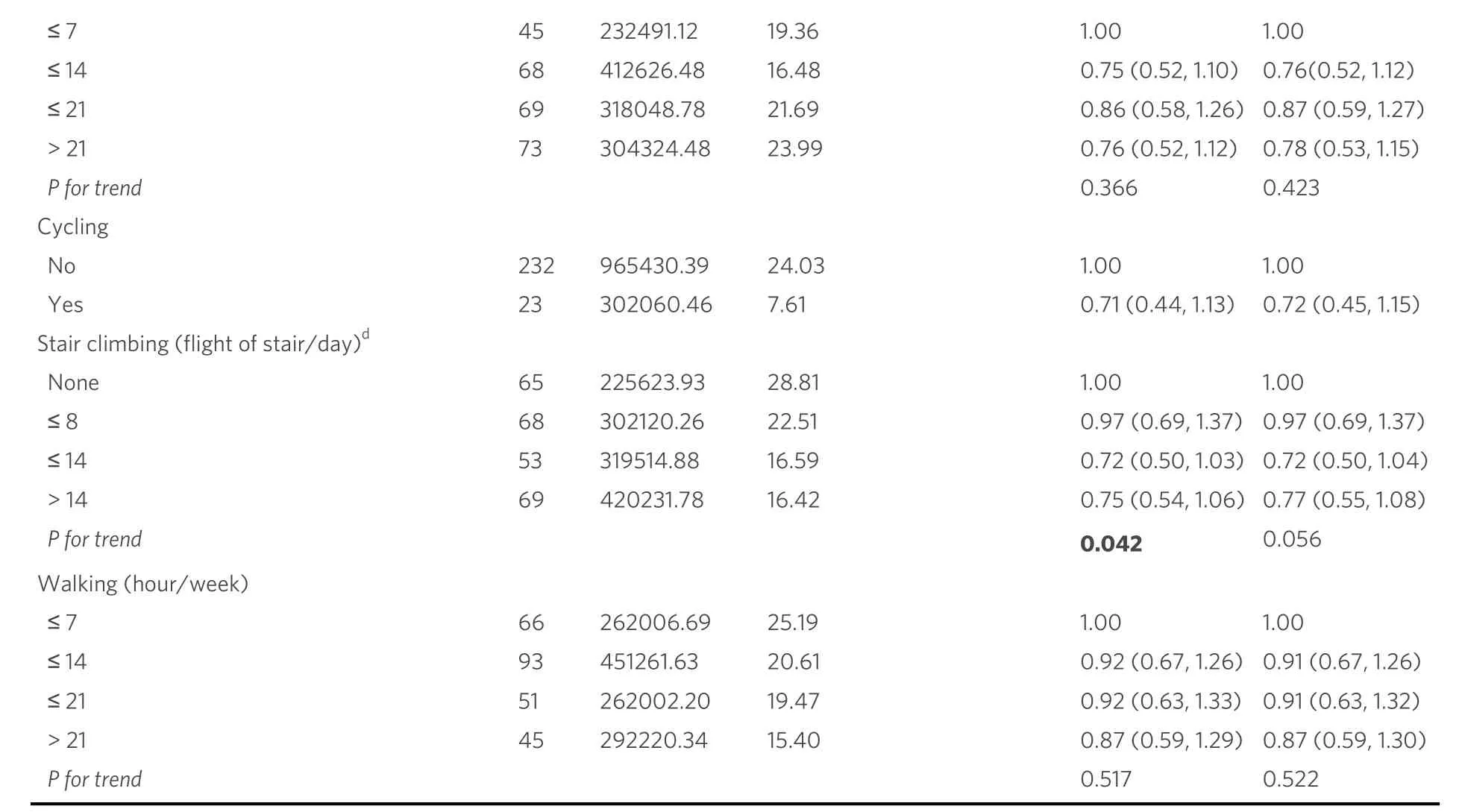
a: adjusted for education, income, employment status, menopause status, ever cigarette smoking, ever alcohol drinking, history of chronic hepatitis, history of cholelithiasis, family history of liver cancer and total energy intake; b: adjusted for education, income, employment status,menopause status, ever cigarette smoking, ever alcohol drinking, history of chronic hepatitis, history of cholelithiasis, family history of liver cancer and total energy intake, history of T2DM and BMI; c: categorized by quartiles; d: “none” referred to subjects who did not participate in this physical activity, and those who did participate in the activity were categorized by tertiles.BMI: body mass index; T2DM: type 2 diabetes mellitus.
DISCUSSION
We assessed the relationship between physical activity and female liver cancer incidence in this prospective population-based cohort study.Among 72,674 participants with 1.26 million person-years of follow-up, 255 women were newly diagnosed with primary liver cancer.No significant association between physical activity and risks of female liver cancer was detected, and even similar results were observed in the sensitivity analyses.However, there was a statistically significant relationship between participation in housework and the reduced risk of liver cancer, and the results of the trend test partly suggested a possible association between increased activity in stair climbing and reduced risk of liver cancer.
The role of physical activity in relation to the risk of cancer has received much speculation and found inconsistent results.Strong evidence reveals that higher levels of physical activity reduce the risks of bladder,breast, colon, endometrial, esophageal adenocarcinoma, and gastric cancers by 10%-25%[28,29].Moderate evidence exists that higher levels of physical activity could reduce the incidence of renal, ovarian, pancreatic and lung cancers[28].Nevertheless, there is insufficient evidence for the association between physical activity and liver cancer, particularly in women.Previous studies in the NIH-AARP and European prospective investigation into cancer and nutrition (EPIC) cohort showed that compared to inactive people, those who maintained activity levels over time had a decreased risk of liver cancer of about 25%-45%[14,15], and a statistically significant 36% lower liver cancer risk comparing high to a low frequency of vigorous physical activity[9].However, our study did not find a significant association between high levels of physical activity and liver cancer risk (HR = 0.82, 95%CI = 0.58-1.17), which is essentially inconsistent with the results of previous studies.In addition, our study also found that activities related to housework were negatively associated with reduced liver cancer risk after adjustment for chronic medical history at baseline (HR = 0.28,95%CI = 0.09-0.88).

Table 3.Associations between physical activities and liver cancer risk in participants without chronic diseasesa at baseline(SWHS,1996-2016)
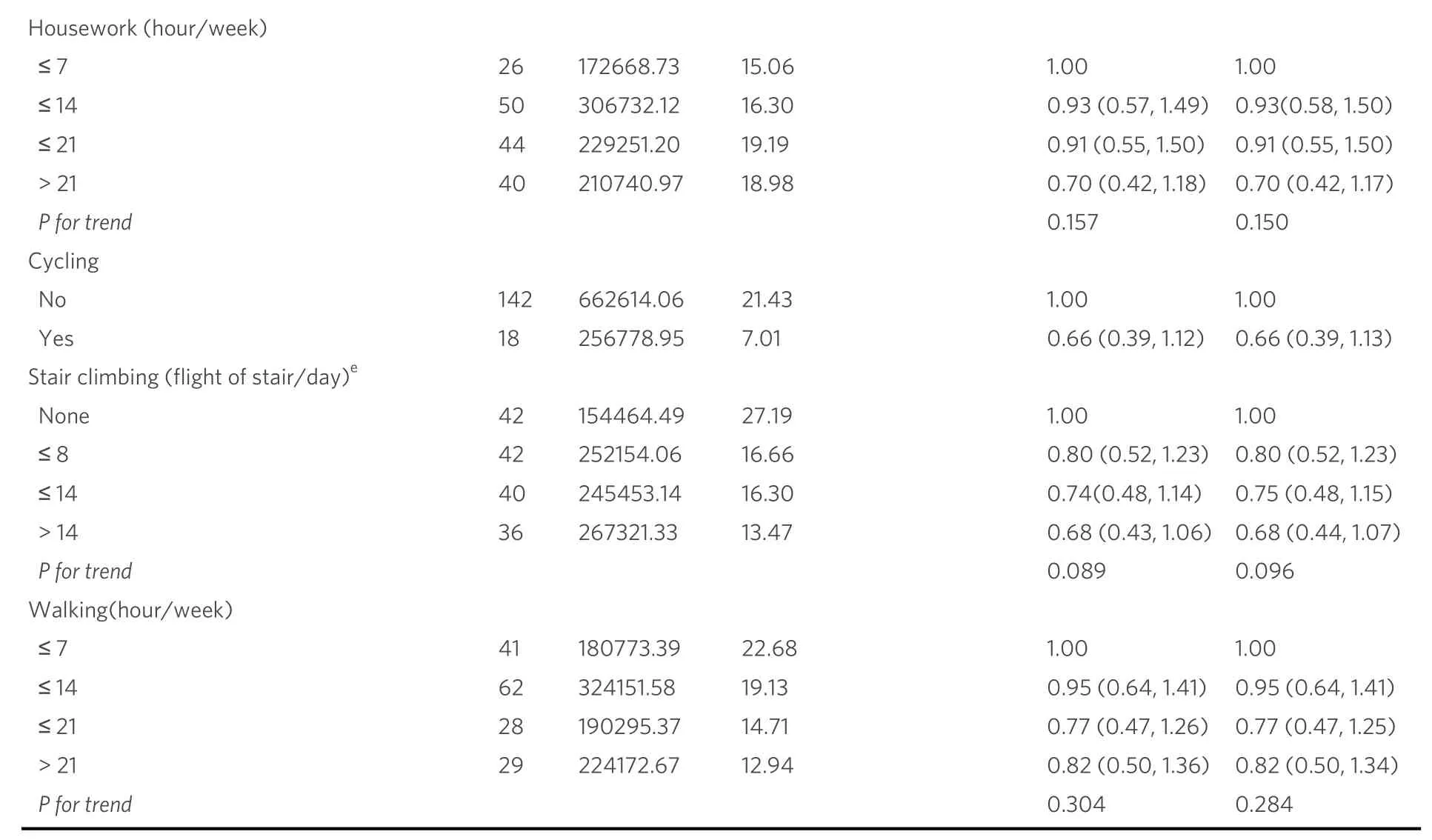
a: Chronic diseases including diabetes, hypertension, coronary heart disease, myocardial infarction and stroke; b: adjusted for education, income,employment status, menopause status, ever cigarette smoking, ever alcohol drinking, history of chronic hepatitis, history of cholelithiasis, family history of liver cancer and total energy intake; c: adjusted for education, income, employment status, menopause status, ever cigarette smoking,ever alcohol drinking, history of chronic hepatitis, history of cholelithiasis, family history of liver cancer, total energy intake, history of T2DM and BMI; d: categorized by quartiles; e: “none” referred to subjects who did not participate in this physical activity, and those who did participate in the activity were categorized by tertiles.BMI: body mass index; T2DM: type 2 diabetes mellitus.
The association was not significant in the results of our study, which indicated that the effect of physical activity might not be enough or a different pattern from that in other study populations.One explanation is the gender difference, because the previous studies are mostly male-dominated, both in European and American cohorts and Asian countries, with few reports among women[10,30].Another possible explanation is the ethnic difference.Most previous studies are from European and American populations, while only few studies in Chinese populations were also male-dominated, and the study outcome was cancer mortality rather than incidence, thus contradicting the results of the present study[18,19].Differences in the adjusted confounding factors and disease outcome could also lead to different results.We also took into account the differences between the methods for measuring and categorizing physical activity levels across epidemiologic studies, as well as changes in physical activity over the life course that may influence the results of the study.Additionally, the finding of significant association in the housework suggests that different physical activity types may have a different effect on the risk of liver cancer.
Excess body fatness might be a mediating factor linked to physical activity.The accumulation of ectopic fat tissue can interfere with normal function in tissue and organs, and then increase the risk of chronic diseases,especially cancer[31,32].Previous studies showed that excess body fat was positively correlated with insulin resistance and excess insulin increases circulating estrogens and androgens, thus promoting tumorigenesis[33].It is well known that chronic low-grade inflammation is crucial for cancer development.In some previous studies, adipocytes were also involved in inflammatory processes in the body by releasing pro-inflammatory adipokines and cytokines, which in turn lead to tumorigenesis[34,35].Based on the above physiological mechanisms, physical activity was associated with the reduction of liver cancer risk by acting on body fat and reducing the accumulation of excess fat, which in turn reduces systemic inflammatory markers and lowers insulin and insulin growth factor-1 (IGF-1) levels[36,37], along with the secretion of antiinflammatory muscle factors by skeletal muscle[38].In addition to indirect, excess fat-dependent mechanisms(described above), there is a growing appreciation that physical activity may also reduce the risk of cancer development in part through biological mechanisms other than body fat-related pathways (e.g., distribution of actin, distribution of key immune cell populations,etc.)[39].Significant differences were observed in the types of physical activity that women in China and Western countries engaged in decades ago, with the Western population participating in more varied and specialized physical activities, which may have affected the risk of liver cancer[40].Differences in dietary habits due to economic and cultural differences between regions could also explain the differences between the findings of previous and current studies[41,42].
Our study is one of the few population-based cohort studies in China that comprehensively examines multiple types of physical activity and the risk of liver cancer in women who were from a large and ongoing population-based cohort study in urban Shanghai.The study, in contrast to most previously published studies, took physical activity and sports competition during adolescence into consideration and assessed common forms of daily activity such as housework, cycling, stair climbing and walking separately.In addition, all potential confounders were adjusted in this study, such as ever cigarette smoking and alcohol drinking, personal medical histories of chronic hepatitis, cholelithiasis, type 2 diabetes and family history of liver cancer.However, some additional limitations still exist in this study.Firstly, we did not consider physical activity alteration during follow-up, and the measurement bias of physical activity from the baseline PAQ surveys adopted for the analyses may lead to biased results.Secondly, the information on physical activity in adolescents depended on the self-report of participants at study enrollment, thus resulting in recall bias of results.Thirdly, a small number of cases in the reference group of housework led to limited statistical power and imprecise results; thus, we need a larger sample for further statistical analysis.Furthermore, we lacked the record of HBV or HCV assays as the major causes of liver cancer.However, we adjusted the self-reported history of chronic hepatitis in the multivariable model, which could reduce this bias to some extent.
CONCLUSIONS
In conclusion, our findings showed no significant associations between TPA, DPA and LTPA and the risk of liver cancer in Chinese women, though a negative association was observed in the activities related to housework and stair climbing.Due to the limited number of observational studies, more epidemiological evidence or a large number of liver cancer cases in the cohort design are warranted to further evaluate the role of physical activity in female liver cancer.
DECLARATIONS
Acknowledgment
We would like to thank all participants and staff from the Shanghai Women’s Health Study for their contribution to this research.
Authors’ contributions
Designed research and obtained funding: Xiang YB
Conducted the study: Tan JY, Li ZY, Shen QM, Zhang Y, Tuo JY, Liu DK, Tan YT, Li HL, Xiang YB
Analyzed the data and interpreted the results: Tan JY, Li ZY, Xiang YB
Wrote the first draft: Tan JY, Li ZY, Xiang YB
All authors reviewed and approved the final version of the paper, and Xiang YB has primary
responsibility for final content.
Availability of data and materials
The data will be available on request pending approval by the scientific committee of the relevant institutes.Further details that support the findings of this study are available from the corresponding authors upon request.
Financial support and sponsorship
This work was supported by the National Key Project of Research and Development Program of China[2021YFC2500404, 2021YFC2500405]; the parent cohort was supported by a subcontract of the grant from the US National Institutes of Health [UM1 CA182910].All funders had no role in the design, analysis, and writing of this article.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The cohort protocol has been approved by the Institutional Review Boards of the Shanghai Cancer Institute and Vanderbilt University.Informed consent has been obtained from all participants.The current study has been approved by the Renji Hospital Ethics Committee of Shanghai Jiao Tong University School of Medicine (KY2019-197).Our study was based on the ongoing cohort for data analysis.The manuscript did not contain individual details, images or videos, and no cases of children, their parents or legal guardians.
Consent for publication
Not applicable
Copyright
© The Author(s) 2024.
- Hepatoma Research的其它文章
- Introduction of the Chinese expert consensus on postoperative adjuvant therapy for hepatocellular carcinoma (2023 Edition)
- Precise staging of advanced HCC promotes higher quality of personalized treatment management:Chinese experts consensus on precision diagnosis and management of advanced hepatocellular carcinoma (2023)
- Crosstalk between cancer cell plasticity and immune microenvironment in cholangiocarcinoma
- Interpretation of Chinese expert consensus on the whole-course management of hepatocellular carcinoma (2023 edition)
- Racial, ethnic, and socioeconomic differences in hepatocellular carcinoma across the United States
- Mutation-based therapies for intrahepatic cholangiocarcinoma: new options on the horizon