Ellagic acid inhibits gastric cancer cells by modulating oxidative stress and inducing apoptosis
Jian Zheng, Chun-Feng Li
1Department of Diagnostic Radiology Division, Harbin Medical University Cancer Hospital, Heilongjiang, China
2Department of Gastrointestinal Surgical Ward, Harbin Medical University Cancer Hospital, Heilongjiang, China
ABSTRACT Objective: To evaluate the anticancer effect of ellagic acid on gastric cancer cells.Methods: MTT assay was used to evaluate the effect of ellagic acid at different concentrations (0.5-100 μg/mL) on gastric cancer AGS cells.RT-qPCR and Western blot analyses were applied to assess apoptosis (BCL-2, CASP-3, and BAX) and autophagy (LC3, ATG5,and BECN1) in AGS cells treated with ellagic acid.The expression of invasion-related markers including TP53, CDKN2A, and PTEN was determined.In addition, cell cycle markers including cyclin A, B,D, and E were measured by ELISA.Oxidative stress markers were evaluated using spectrophotometry.Results: Ellagic acid inhibited the proliferation of AGS cells in a concentration- and time-dependent manner.The expression of BCL-2 was significantly decreased (P<0.05) and CASP-3 and BAX were markedly increased (P<0.01) in AGS cells treated with ellagic acid.However, this compound induced no significant changes in the expression levels of LC3, ATG5, and BECN1 (P>0.05).Moreover,the oxidative stress markers including SOD, TAC, and MDA were increased by ellagic acid (P<0.01).Conclusions: Ellagic acid can inhibit cell proliferation, induce apoptosis, and modulate oxidative stress in AGS cells.However,further in vivo and molecular studies are needed to verify its anticancer efficacy.
KEYWORDS: Ellagic acid; Gastric cancer; Apoptosis; Autophagy;Metastasis; Proliferation
1.Introduction
Globally, gastrointestinal cancers pose significant challenges in both medical and economic spheres, representing a substantial burden[1].The fourth most common type of cancer in the world is gastric cancer which is considered the second cause of cancer-related deaths[2].The public health burden of this malignancy is estimated to be remarkably high even though its incidence has reduced in recent years[3].The identification of standard-of-care therapy according to the international clinical trial is complicated due to the biological differences between tumors originating from Eastern and Western communities[4].Although multidisciplinary treatment including systemic chemotherapy, immunotherapy, radiotherapy, targeted therapy, and surgery is paramount to treatment selection, limitations such as undesired adverse effects remain[4].Systemic chemotherapy,for example, could be followed by both genders infertility, peripheral neuropathy, hypertension,etc[5].Therefore, great efforts are being made to find safe treatment approaches[6-11].
Ellagic acid (EA), a polyphenolic herbal compound that abundantly exists in pomegranate, berries, pecans, walnuts, cranberries, and longan, can scavenge oxidantsviaits antioxidant properties[12].Interestingly, previous evidence has shown that EA is a highly safe compound as even high doses of EA did not cause any remarkable toxicity to normal human and other mammalian cells[13].Moreover,the anticancer and anticarcinogenic properties of EA towards a variety of malignancies such as colorectal, breast, oral, prostate,ovarian, pancreatic, bladder, neuroblastoma, lymphoma, and melanoma have been evidenced[14].Furthermore, a recent study has revealed the potency of EA to treat the diseaseviabioinformatics analysis and network pharmacology[15].Nevertheless, the antigastric cancer efficacy of EA and the exact molecular mechanism by which EA inhibits gastric cancer are not elucidated.
Despite the high mortality rate caused by gastric cancer, current treatment strategies at the clinic face serious limitations.Therefore,the present study aimed to clarify the possible therapeutic effects of EA against gastric cancer and elucidate the underlying mechanisms.
2.Materials and methods
2.1.Chemicals
Roswell Park Memorial Institute (RPMI) media 1640,L-glutamine, trypsin, fetal bovine serum (FBS), minimum essential medium, and antibiotics were obtained from Gibco Ltd.(Paisley,UK).Proteinase inhibitor cocktail, 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), EA,tetramethylethylenediamine (TEMED), sodium fluoride, sodium orthovanadate, ammonia persulfate, sodium pyrophosphate, Triton X-100, acridine orange, bicinchoninic acid (BCA) protein assay reagent, and Tween 20 were purchased from Sigma (St.Louis,MO).Acrylamide and polyvinylidene fluoride (PVDF) membrane(Immobilon-P) were obtained from Millipore (Bedford, MA).SYBR Green RT-PCR reagents were purchased from Ampliqon, Denmark.β-actin (Cat: NB600-501, dilution: 1∶5 000), BCL-2 (Cat: NB100-56098, dilution: 1∶1 000), BECN1 (Cat: NB500-249, dilution:1∶500), and TP53 (Cat: NBP2-93452, dilution: 1∶1 000) antibodies were purchased from Novus Biologicals, USA.
2.2.Cell culture
The human gastric cancer cell line (AGS) was obtained from the American Type Culture Collection (ATCC).Briefly, cells were cultured at 37 ℃ in the presence of 5% CO2in RPMI 1640 medium supplemented with 10% (v/v) FBS and 1% penicillin-streptomycin.Valproic acid was reconstituted in RPMI 1640 medium as a 50-mM stock solution.A 0.22 μm filter was used for sterilization and stored as aliquots at -20 ℃.
2.3.Cell viability assay
MTT assay was performed in order to determine the effect of EA on the proliferation of cultured cancer cells, according to a previously published study[16].In brief, EA (0.5-100 μg/mL) was exposed to AGS cells for 24, 48, and 72 h.The absorbance was measured at 545 nm using an ELISA reader (MultiscanGo; Thermo).The concentration that inhibited 50% of the cell growth (IC50)was specified and 96-well culture plates were used to analyze 6 replicates/dilutions in 2 measurements.The cultured cells without EA treatment were considered negative controls.
2.4.RNA extraction and cDNA synthesis
TRIzol reagent was used for total RNA extraction according to the manufacturer’s instructions.The integrity and purity of extracted RNA were analyzed using 1.5% gel electrophoresis and nanodrop,respectively.EURX One-Step RT-PCR Kit (Gdansk, Poland) with random hexamers was used for the synthesis of complementary DNA (cDNA) based on the manufacturer's instructions.
2.5.Real-time quantified polymerase chain reaction (RTqPCR)
Specific primers were designed by AlleleID software and the NCBI database and RT-qPCR was performed to quantify the gene expression (Table 1).In this regard, SYBR Green RT-PCR reagents were utilized.An initial denaturation step(95 ℃, 10 min) was performed, followed by 40 amplification cycles including denaturation (95 ℃, 15 s), annealing at different annealing temperatures (Table 1) for 30 s, and extension (72 ℃, 20 s).A standard system (7500 system, Applied Biosystems, USA) was used to measure the levels of gene expression.β-actinwas used as an internal control and the expression levels of genes involved in the apoptosis (BCL-2, BAX,andCASP-3), autophagy (LC3,ATG5, andBECN1), and cell invasion (TP53,CDKN2A, andPTEN) processes were evaluated.The validation of measurement was analyzed by the construction of the melting curves.Moreover, samples were assessed in triplicate by using the 2-ΔΔCTmethod with SD of CT (threshold cycle) not exceeding 0.5 on a within-run basis[17].

Table 1.Sequence of primers in RT-qPCR analysis.
2.6.Western blot analysis
The effects of EA on BCL-2, BECN1, and TP53 protein levels in AGS cells after 48 hours of incubation were measured by Western blot analysis.Briefly, 1×105AGS cells/mL were cultured in 6 well plates.Following AGS attachment, cells were treated with IC50concentration of EA.Cells were harvested in ice-cold PBS and centrifuged at 1 500 ×gfor 4 min.Next, AGS cells were resuspended in RIPA lysis buffer that contained a protease inhibitor cocktail.The total protein concentration was determined with the BCA protein assay kit.Subsequently, equivalent quantities of proteins were separated by the 10% SDS/PAGE gel and were transferred onto a nitrocellulose membrane.The incubation of membranes was performed in BSA and then blocked for 1 h.After blocking, the membranes were maintained overnight in the primary antibody at 4 ℃.After washing, the secondary antibody was incubated with the membranes.Protein bands were visualized using the ECL chemiluminescent kit.
2.7.Enzyme-linked immunosorbent assay (ELISA)
In order to determine the cell cycle status, the level of relevant markers was measured.Human cyclin A (Cat: MBS8804448), B(Cat: MBS103343), D (Cat: MBS723526), and E (Cat: MBS722435)ELISA kits containing monoclonal antibodies were purchased(MyBioSource, Inc., San Diego, CA, USA) and the level of markers was measured according to the manufacturer's instructions.
2.8.Spectrophotometry
The activity of catalase (CAT, Cat: MBS2021346) and superoxide dismutase (SOD, Cat: MBS036924), the level of malondialdehyde(MDA, Cat: MBS2000071), and total antioxidant capacity (TAC,Cat: MBS2540515) were measured using commercially available kits and according to the manufacturer's instructions.
2.9.Statistical analysis
All experiments were replicated at least three times, and data are expressed as mean ± standard deviation (SD).The Statistical Package for Social Sciences (IBM SPSS Statistics for Windows,version 24) was used for statistical analyses and all the graphs were analyzed by GraphPad Prism 8.0.2.263.The statistical significance was determined using the Student’st-test and two-way ANOVA analysis.P<0.05 was considered statistically significant.
3.Results
3.1.Cytotoxic effects of EA in AGS cells
EA inhibited AGS cell viability in a dose- and time-dependent manner (P<0.01) (Figure 1).The highest cell viability was achieved in cells that were treated for 24 h, and the cell viability decreased gradually after 48 h and 72 h treatments.Based on the results of the present study as well as a similar study that was conducted previously[18], 48-hour treatment was considered for further investigations.Moreover, EA in concentrations over 5 μg/mL induced a more significant inhibitory effect on AGS cell viability.IC50values were 50 μg/mL, 20 μg/mL, and 7 μg/mL after 24 h, 48 h,and 72 h treatments, respectively.According to the findings, the IC50value (20 μg/mL) at 48 h was used for further experiments.
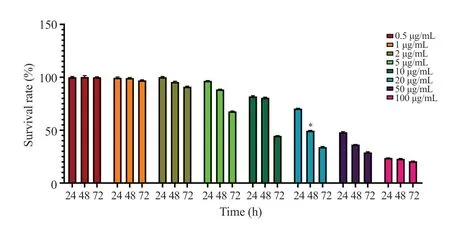
Figure 1.Cell viability after treatment with ellagic acid for 24-72 h by MTT assay.The two-way ANOVA was performed to determine the concentration of EA (IC50) and treatment duration for further investigations.Six replicates in two measurements were analyzed.*indicates a significant difference at P<0.05.
3.2.Effect of EA on apoptosis in AGS cells
The expression of theBCL-2gene was decreased remarkably in cells treated with the 20 μg/mL EA (P<0.01), whereas the expression ofBAX(P<0.01) andCASP-3(P<0.01) genes was increased significantly.In addition, 20 μg/mL EA resulted in a significant decrease in BCL-2 protein levels (P<0.01) (Figure 2).
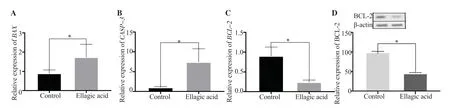
Figure 2.Ellagic acid induces apoptosis in AGS cells.(A) BAX gene, (B) CASP-3 gene, (C-D) BCL-2 gene and protein expression.The data are expressed as mean ± SD and analyzed by Student’s t-test.*P<0.05 compared with the control group.
3.3.Effect of EA on autophagy in AGS cells
EA did not induce significant changes in the expression levels ofLC3,ATG5, andBECN1(P>0.05, Figure 3) in AGS cells.However,the BECN1 protein level was significantly decreased in EA-treated AGS cells (P<0.05).
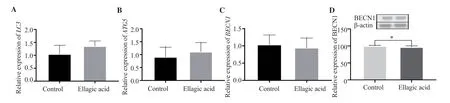
Figure 3.Effect of ellagic acid on autophagy in AGS cells.(A-C) The gene expressions of LC3, ATG5, and BECN1 were determined by RT-qPCR analysis, and β-actin was used as an internal control.(D) The expression of BECN1 protein was measured by Western blot analysis and normalized against β-actin.The data are expressed as mean ± SD and analyzed by Student’s t-test.*P<0.05 compared with the control.
3.4.Effect of EA on invasion-related markers in AGS cells
As shown in Figure 4, treatment with EA caused a 4.35-fold and 2.85-fold increase in the expression levels ofTP53andPTENgenes,respectively (P<0.01).WhereasCDKN2Agene expression level was reduced markedly after treatment with the EA (P<0.01).In addition,EA treatment resulted in a 2.19-fold increase in TP53 protein levels when compared to the control group (P<0.01).
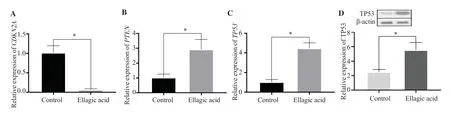
Figure 4.Effect of ellagic acid on AGS cell invasion.The gene expressions of CDKN2A, PTEN, and TP53 were determined by RT-qPCR analysis, and β-actin was used as an internal control.(D) The expression of TP53 was measured by Western blot analysis and normalized against β-actin.The data are expressed as mean ± SD and analyzed by Student’s t-test.*P<0.05 compared with the control.
3.5.Effect of EA on cell cycle arrest in AGS cells
As shown in Figure 5, the level of cyclin D was decreased by 26.36% in AGS cells treated with the EA (P<0.01).Similarly,treatment with EA caused a prominent decrease of 20.81% and 21.22% in the level of cyclins E and B compared to the control group.Meanwhile, a slight decrease in cyclin A level was observed in EA-treated AGS cells (P>0.05).

Figure 5.The level of cyclins after treatment of AGS cells with ellagic acid.The data are expressed as mean ± SD and analyzed by Student’s t-test.*P<0.05 compared with the control.
3.6.Effect of EA on oxidative stress in AGS cells
Figure 6 demonstrates that EA markedly increased SOD activity and TAC level (P<0.01).However, the slightly increased CAT activity caused by treatment with EA was not significantly different from that in the control group (P>0.05).In addition, a significant increase in MDA level was found after EA treatment (P<0.01).

Figure 6.Effect of ellagic acid on oxidative stress in AGS cells.The data are expressed as mean ± SD and analyzed by Student’s t-test.*P<0.05 compared with the control.
4.Discussion
Continuous studies have elucidated an increase in new cases of gastrointestinal cancers especially gastric cancer patients, leading to a significantly high mortality rate worldwide[19].Despite extraordinary advances in cancer diagnosis and treatment in the last two decades[18,19], especially chemotherapy, limitations such as undesired adverse effects in nontargeted normal tissues and reduced quality of life of cancer survivors have raised great concerns.Herbal compounds with anti-inflammatory, anti-proliferative, antioxidant,and anti-tumorigenesis properties as well as a safety profile have led many studies conducted to propose efficient herbal medicine in cancer treatment[20].Accumulative evidence has shown that EA, abundantly found in fruits, exhibits anti-proliferative, anticarcinogenic, and antioxidant properties and could contribute to the management of a variety of malignancies[21].Nevertheless,its efficacy in gastric cancer has not been clarified.Therefore, the present study measured the effect of EA on gastric cancer cells.
The findings of the present study demonstrated that gene and protein expressions of BCL-2 were decreased significantly after treatment with the EA.BCL-2 is considered an anti-apoptotic factor that prevents apoptosis in cells[22,23].However, with the downregulation of this protein, an apoptotic cascade will be established in cells, which ultimately leads to cell death[22].In addition, the present study revealed that treatment with EA caused the overexpression ofCASP-3andBAXgenes in gastric cancer cells.Caspases are described as crucial mediators of programmed cell death, among which CASP-3 is a frequently activated death protease that catalyzes the specific cleavage of several major cellular proteins,facilitating cell death[24].In addition, BAX is considered a member of the BCL-2 family and a core regulator of the intrinsic pathway of apoptosis that is activated upon apoptotic stimuli, oligomerizes with BAK at the mitochondrial outer membrane, and mediates mitochondrial outer membrane permeabilization, which eventually ends in programmed cell death[25].A previous study by Edderkaouiet al.suggested that EA can stimulate the mitochondrial pathway of apoptosis in human pancreatic adenocarcinoma cells associated with mitochondrial depolarization and downstream caspase activation[26].Similarly, other studies have determined that EA is capable of inducing cancer cell death by altering the BCL-2/BAX signaling pathway and triggering the CASP-3-dependent cascade[27].According to the findings, EA may inhibit the proliferation of cancer cells through the activation of apoptosis.
Autophagy, a highly conserved eukaryotic cellular recycling process, has recently been emphasized as a non-apoptotic regulated cell death program[28].The activation of the autophagic flux, which occurs through several intermediate proteins, causes the transfer of dysfunctional proteins or cell organelles to a structure called autolysosome, consisting of autophagosome and lysosome, finally leading to the destruction of the cargo[28].Usually, autophagy is considered a mechanism involved in the survival of cells due to its participation in removing unfunctional components and providing blocks/energy.However, its dual role is documented by many studies[28].BECN1 contributes to autophagic flux and membrane trafficking processes by interacting with a variety of other proteins[29].LC3 mediates the elongation step of the isolation membrane of autophagosome formation and ATG5 is considered a major autophagy protein as an important component of the ATG5-ATG12-ATG16L1 complex that contributes to autophagosomeslysosomes fusion[30].Interestingly, the present findings demonstrated that the expression of the genes encoding BECN1, LC3, and ATG5 in AGS cells was not altered significantly after treatment with EA.Previous studies have published conflicting findings about the EA's effect on autophagy in cancer cells.Some studies have acknowledged that EA treatment induces autophagy in lung and colorectal cancer[31].Chunget al.found EA exerted an inhibitory effect on the autophagy pathway in ovarian carcinoma cells[32].In addition, there are many contradictions in the role of autophagy in cancer, where it can inhibit normal to cancerous transition, but in advanced stages, it increases the survival of cancer cells[33].Also, the findings of the present study showed that although theBECN1gene expression did not change significantly, the protein level decreased significantly after treatment with EA in AGS cells.The other autophagy markers that are not significantly changed, along with the downregulation of BECN1 level, may be due to the participation of this protein in other cellular mechanisms such as intracellular trafficking[34].However, further investigations are required to clarify this issue.
The migration of cells within a tissue, known as cell invasion, is considered a pivotal process in tissue development and immune surveillance, however, the aberrant regulation of this process in cancer cells leads to malignant invasion either in local tissues or systematically in blood and lymphatic vessels[35].Cell migration is regulated by several signaling pathways, the dysregulation of which in cancer cells is associated with disease progression.Mutations in theTP53are extremely prevalent in human cancers leading to the loss of tumor suppressive activities and promotion of chemoresistance and invasion of cancer cells[36].In addition,PTENexerts tumor suppressive functions and is mainly involved in several signaling pathways (e.g.PTEN/ERK,PTEN/PI3K/AKT, andPTEN/FAK/P130cas), all of which contribute to cell cycle arrest as well as the inhibition of cancerous cell metastasis and tumor angiogenesis[37].Therefore, the findings of the present study on the ability of EA to significantly induce overexpression ofTP53andPTENand also to remarkably suppress the expression ofCDKN2Acould be assumed to be an indicator of the capacity of the compound to inhibit gastric cancer cell invasion.Consistent with this result, a number of studies have stated that EA may be involved in suppressing the invasion of cancer cells such as pancreatic,prostate, endometrial, and glioblastoma.through the regulation of the expression of the studied genes[38].
The results obtained in the present research showed that EA treatment caused a significant decrease in the levels of all cyclins except cyclin A in AGS cells.A review study recently published by Javedet al.comprehensively described the function of cyclins and cyclin-dependent kinases in gastric cancer pathoetiology and considered the increased levels of cyclins to be a manifestation of increased proliferation and invasion of cancer cells[39].Cyclins can be considered a diagnostic tool to detect the disease, determine disease progression, as well as evaluate the prognosis and how it responds to the treatment, and choose the appropriate treatment option[39].In addition, the high levels of cyclins in patients with gastric cancer and the direct association with the progression of the disease indicate the potential of targeting cyclins for therapeutic purposes[40].In fact, the EA's efficiency in reducing the levels of cyclins is an indicator of the therapeutic potential of this compound by inhibiting the proliferation and invasion of cancer cells and inducing AGS cell death.Similarly, other studies have suggested the EA's ability to modulate the levels of cyclins as a strategy in cancer management[41].
Induction of oxidative stress is an indicator of a cellular pathological state, which may be an advantage for the establishment and proliferation of cancer cells by increasing angiogenesis[42] and/or may lead to tumor inhibition by activating cell death mechanisms[43].The present study revealed that treatment with EA can increase SOD activity and TAC within AGS cells while inducing no significant effect on CAT activity.This evidence may suggest that EA improved the oxidative stress of cancer cells, which is contrary to the performance of chemotherapeutic agents such as doxorubicin[17,20].On the contrary, the level of MDA, the end product of oxidative stress and lipid deposition[20], in cancer cells after treatment with EA was increased significantly, which may be involved in the activation of regulated cell death, especially ferroptosis[44].However, further studies are needed to clarify its effect on oxidative stress.In conclusion, EA can inhibit the proliferation of AGS cells, induce cell apoptosis, and modulate oxidative stress.However, there are some limitations in the study.The effect of EA on other cancer cell lines is not determined andin vivoand clinical studies are required to verify its anticancer efficacy.
Conflict of interest statement
The authors report no conflict of interest.
Funding
This study was supported by the Heilongjiang Provincial Natural Science Foundation of China (LH2022H063).
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’contributions
CL contributed to the conception of the study.CL and JZ performed the experiments and data interpretation, and JZ wrote the manuscript.All authors read and approved the manuscript for submission and publication.
 Asian Pacific Journal of Tropical Biomedicine2024年4期
Asian Pacific Journal of Tropical Biomedicine2024年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Pachymic acid exerts antitumor activities by modulating the Wnt/β-catenin signaling pathway via targeting PTP1B
- Methanolic extract of Ephedra alata inhibits breast cancer cells in vitro and in vivo
- Isoimperatorin alleviates acetic acid-induced colitis in rats
- Ethyl acetate fraction of Sargassum pallidum extract attenuates particulate matterinduced oxidative stress and inflammation in keratinocytes and zebrafish
