Isoimperatorin alleviates acetic acid-induced colitis in rats
Saied Goodarzi, Amir Hossein Abdolghaffari, Behnaz Najafi, Mostafa Pirali Hamedani, Saeed Tavakoli, Mahshad Marvi, Maryam Baeeri, Narguess Yassa, Abbas Hadjiakhoondi,, Mohammad Abdollahi,7, Zahra Tofighi,✉
1Medicinal Plants Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
2Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
3Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
4Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Karaj, Iran
5International Campus School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
6Toxicology and Diseases group, The Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences, Tehran, Iran
7Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
ABSTRACT Objective: To investigate the effect of isoimperatorin on histopathological and biochemical changes in acetic acid-induced colitis rats.Methods: Colitis was induced by intracolonic administration of acetic acid solution (4% v/v) in rats.Rats were divided into six groups including the sham group, the negative control group,the dexamethasone-treated group, and the groups treated with isoimperatorin (0.1, 1, and 10 mg/kg/d by gavage).The treatments were administered for three days and then colonic status was assessed by macroscopic, histopathological, and biochemical analyses.Results: Isoimperatorin significantly alleviated colonic damage in a dose-dependent manner and improved histological changes in rats with acetic acid-induced colitis.It also significantly reduced myeloperoxidase, TNF-α, IL-1β, and malodialdehyde levels.Conclusions: Isoimperatorin alleviates acetic acid-induced colitis in rats and may be a potential therapeutic agent for the treatment of colitis.
KEYWORDS: Isoimperatorin; Inflammatory bowel disease; Acetic acid; Colitis; TNF-α; IL-1β
1.Introduction
Inflammatory bowel disease (IBD) is a chronic disorder of the digestive tract that includes two major idiopathic types; Crohn’s disease and ulcerative colitis.Abdominal pain, diarrhea, existence of mucus and blood in stool, fever, and weight loss are clinical characteristics of IBD[1].The etiology and pathogenesis of IBD are unknown, but innate and adaptive immune dysregulation, changes in normal gut microbiota, malfunction of intestinal epithelial barrier, and oxidative stress play important roles in this disorder[2,3].Intestinal bacteria and dietary antigen-induced inflammatory cytokines like interleukins (IL-1, IL-2, IL-6), and interferon-γ(INF-γ) at focal lesions, and tumor necrosis factor-α (TNF-α) were secreted by epithelial cells in IBD[4].According to these findings, the modulators of the immune system are effective for managing IBD signs and symptoms as many as different immunosuppressants and immune modulators used for IBD like methotrexate, prednisone, azathioprine, infliximab,etc.Despite using available methods of pharmacotherapy, results are not satisfying and patients also complain about adverse drug reactions[5].Therefore, finding new compounds with more efficiency and fewer side effects is necessary.Isoimperatorin is a candidate compound to improve IBD.Different studies showed the anti-inflammatory effects of isoimperatorin.It can play a key role in the inflammatory process,for example, it reduces prostaglandin D2 production by inhibiting cyclooxygenase-1 (COX-1) and COX-2 in bone marrow-derived mast cells[6].In another study, isoimperatorin that was isolated from Cimicifugae rhizome (synonym ofActaearhizome) demonstrated an anti-inflammatory effect by preventing the expression of vascular cell adhesion molecule-1 (VCAM-1) in human endothelial cells induced by TNF-α which can be a trigger for pro-inflammatory signal pathways and adhesion of endothelium cells[7].Isoimperatorin and the plants that contain this compound have been used for alleviating inflammatory diseases like asthma, arthritis and also as antipyretic and analgesic medicines in different countries for a long time[8].
Considering the abovementioned benefits of isoimperatorin, this study aimed to evaluate the effect of isoimperatorin isolated fromFerulago trifidain an animal model of IBD.
2.Materials and methods
2.1.Plant materials
TheFerulago trifidaBoiss.fruits were collected from Alamut,Qazvin Province, Iran, in July 2020.The plant was identified by Dr.Y.Ajani (as a botanist) and a specimen (TEH-6562) was deposited in herbarium of Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
2.2.Elucidation of isoimperatorin
The used method to isolate isoimperatorin was described by Tavakoliet al.[9].The dried fruits (300 g) were powdered and macerated with chloroform to obtain a 60 g extract.According to the mentioned method of isolation, 17 g of extract was subjected to normal silica gel column chromatography (10 cm×30 cm) eluted with petroleum ether/ethyl acetate to yield 14 fractions.Fraction 9(1 g) was re-chromatographed on a reversed phased C18column (1.5 cm×20 cm) washed out with a gradient solvent phase of MeOH/H2O (3∶7 to 7∶3) to obtain isoimperatorin (16 mg).The structure was confirmed with1H-NMR and compared with a previous study[9].
2.3.Animals
Male albino rats (Wistar strain, 180-200 g) were obtained from the animal service of Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.The rats were maintained under standard environmental conditions: temperature (25±2) ℃, humidity(55±10)%, and 12 h light-dark cycles.Rats were given free access to water and feeding.
2.4.Induction of colitis
Colitis was induced by acetic acid according to the previously described method[4].Briefly, rats were fasted for 24 h before the induction of colitis and allowed free access to water.Then, they were slightly anesthetized with ketamine/xylazine.For enteral feeding, a medical-grade polyurethane canal with an external diameter of 2 mm was inserted into the anus in such a way that its tip was advanced to 7 cm proximal to anal verge.After that, 2 mL of acetic acid 4%v/v(dissolved in 0.9% saline) was introduced intracolonically through a cannula and the fluid was withdrawn after 30 s.For spillage prevention of solution from the rectums, they were placed in a supine Trendelenburg position for 1-2 min.
2.5.Experimental groups
The rats were randomly assigned to 6 groups containing 6 animals in each.GroupⅠserved as the sham group which underwent cannulation procedure but received normal saline instead of acetic acid.The five remaining groups had colitis induced by receiving acetic acid.Group Ⅱ and Ⅲ were negative and positive control groups that received normal saline (no treatment) and dexamethasone(1 mg/kg/day), respectively.Group Ⅳ-Ⅵ were treated with isoimperatorin at 0.1, 1, and 10 mg/kg, respectively[10].All treatment groups were administered 1 h after induction of colitis (on the first day)by gavage in a 0.4 mL volume and continued for 3 d.
2.6.Assessment of colonic damages
On the third day of treatment, animals were anesthetized with ketamine (40 mg/kg) and xylazine (7 mg/kg).Then, the abdomen was cut open, the distal colons were removed.At the end of procedure, all animals were sacrificed.Rat colons were placed in an ice bath, and cleaned gently in physiological saline to remove fat,mesentery, and fecal residue.Subsequently, they were divided into two pieces, one for macroscopic and histopathological assessment(maintained in 5 mL of 10% formalin as a fixator) and the other for determination of biochemical markers (kept at -20 ℃).
2.7.Macroscopic scoring
Macroscopic inflammation scores were used to describe visual severity of colonic damage based on the following scales: 0 (normal macroscopic appearance with no changes), 1 (localized mucosal erythema and hyperemia without ulceration), 2 (linear ulceration including mild mucosal edema, small erosions or slight bleeding without significant inflammation), 3 (linear ulceration including moderate mucosal edema, slight bleeding erosions or ulcer with inflammation at one site), 4 (two or more sites of severe ulceration,edema, and tissue necrosis which extended along the length of the colon more than 1 cm), 5-8 (for damage extended more than 2 cm along the length of the colon, the score was increased by 1 for each increased cm of involvement)[11].
2.8.Histopathological studies
The specimens were fixed in 10% neutral buffered formalin(pH=7.26) for 48 h.Then, tissues were embedded in paraffin,sectioned at 5 μm thickness, and stained with hematoxylin and eosin (H&E).The histological characteristics were evaluated by an expert blinded pathologist using light microscopy (Olympus BX51; Olympus, Tokyo, Japan) for digital imaging of any severity of the inflammation including damage to tissue or nucleus, mucosal necrosis, or infiltration of cells.Microscopic scores were graded according to the following criteria: 0 (normal villi), 1 (capillary congestion), 2 (moderate lifting of the epithelial layer), 3 (massive epithelial lifting), 4 (denuded villi, dilated capillaries), 5 (digestion and disintegration of lamina propria, hemorrhage, and ulceration)[12].
2.9.Biochemical assay
Other pieces of the colon were homogenized in 50 mM icecold potassium phosphate buffer (pH=7.4).The samples were sonicated and centrifuged at 3 500gfor 30 min.The supernatants were transferred into microtubes for biochemical assays and were maintained at -80 ℃ until further analyses[13].
2.10.Inflammatory mediators
Quantitative determination of the colonic TNF-α and IL-1β levels was performed using an enzyme-linked immunosorbent assay ELISA rat-specific kits (Rat TNF-α ELISA and Rat IL-1β ELISA Bender medSystems).The absorbance of samples was measured at 450 nm and 620 nm as primary and reference wavelengths.TNF-α and IL-1β levels were reported as pg/mg of protein of tissue/wet tissue.
2.11.Myeloperoxidase (MPO) activity
MPO activity was measured according to the previously described method[14] by the addition of 0.5% hexadecyltrimethylammonium bromide in a phosphate buffer (pH=6.0) for homogenization of tissue and o-dianisidine hydrochloride (0.167 mg/mL) as chromogen.The absorbance of orange complex was measured by a UV spectrophotometer at 460 nm after 3 min as indices of MPO activity and reported as U/mg of protein of tissue.
2.12.Total protein measurement
The total protein content of colon tissue was quantitated by Lowry method according to a standard curve of bovine serum albumin(BSA).Results were expressed as mg/mL of homogenized tissue[15].
2.13.Lipid peroxidation assay
Byproduct of oxidation of polyunsaturated fatty acid and subsequent cellular damage including malondialdehyde (MDA) were determined using a thiobarbituric acid reactive substance (TBARS)assay[16].The absorbance of pink complex was measured by a UV spectrophotometer at 532 nm and the results were reported as μmol/mg protein.
2.14.Statistical analysis
The data were expressed as mean ± standard error of mean (SEM).One-way ANOVA followed by Tukey’spost hocwas used to compare the data andP<0.05 was considered statistically significant using GraphPad Prism 8 software.
2.15.Ethical statement
The whole experiments were carried out according to the international rules for the care and use of laboratory animals, and the study protocol was approved by Ethic Committee of Tehran University of Medical Sciences (IR.TUMS.PSRC.REC.1395.1802).
3.Results
3.1.Macroscopic and histopathological assessment of colonic damages
The results of macroscopic and microscopic data are demonstrated in Table 1.In macroscopic evaluation, the groups received 1 and 10 mg/kg of isoimperatorin and 1 mg/kg dexamethasone showed significantly improved appearance compared to the negative control group (P<0.01).

Table 1.Macroscopic and microscopic scores of colonic inflammation in rats.
The normal control group showed the normal mucosal layer, tunica submucosa, and muscularis layer (grade 0).In the negative control group, gangrene was evident, with a typical line of demarcation.Severe hemorrhage, hyperemia, capillary congestion, edema in the lamina propria, infiltration of polymorphonuclear cells (PMNCs),and massive mucosal layer necrosis were observed (grade 5).In the group treated with 0.1 mg/kg isoimperatorin, a massive necrotic lesion (gangrene) was seen with extensive hyperemia, severe edema of the submucosal layer, and infiltration of PMNCs (grade 4).The group treated with 1 mg/kg isoimperatorin showed reduced injury to the mucosal layer when compared to 0.1 mg/kg isoimperatorin(grade 3).The best results were seen in the group treated with 10 mg/kg isoimperatorin, the necrotic area and the number of PMNCs were markedly decreased in comparison to other doses (grade 2)(Figure 1).
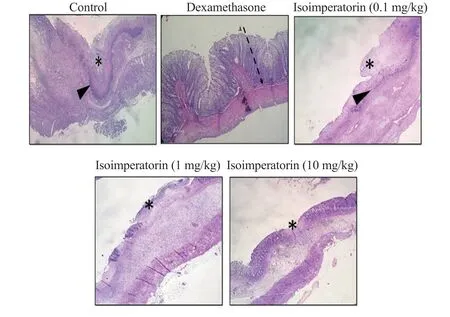
Figure 1.Histopathological sections of colon tissue in different groups (×40).Thin arrows: infiltration of inflammatory cells, asteroid: necrotic area (mucosal layer), arrow heads: line of demarcation (H&E stain).
3.2.Results of biochemical assays
An increase in TNF-α level was found in the control group compared to the sham group (P<0.01).Isoimperatorin (0.1, 1, and 10 mg/kg)significantly reduced TNF-α level (P<0.01).The control group showed higher IL-1β levels compared with the sham group (P<0.05).Isoimperatorin at 0.1 mg/kg did not induce any noticeable effect on IL-1β level.However, 1 and 10 mg/kg of isoimperatorin pronouncedly decreased IL-1β levels compared to the control group (P<0.05) (Figure 2).

Figure 2.Effect of isoimperatorin on TNF-α (A) and IL-1β (B) levels in rats with acetic acid-induced colonic damage.The data are expressed as mean ±standard error of mean (SEM) and analyzed by one-way ANOVA followed by Tukey’s post hoc test.#P<0.05, ##P<0.01 compared with the sham group; *P<0.05,**P<0.01 compared with the control group.Dex: dexamethasone (1 mg/kg).
3.3.MPO activity
As shown in Figure 3A, the activity of MPO in the control group was significantly increased in comparison with the sham group(P<0.01).Administration of 0.1 and 1 mg/kg of isoimperatorin did not significantly reduce the activity of MPO while 10 mg/kg of isoimperatorin significantly decreased MPO activity (P<0.05).

Figure 3.Effect of isoimperatorin on myeloperoxidase (A) and lipid peroxidation (B) levels in rats with acetic acid-induced colonic damage.The data are expressed as mean ± standard error of mean (SEM) and analyzed by one-way ANOVA followed by Tukey’s post hoc test.##P<0.01 compared with the sham group; *P<0.05, **P<0.01 compared with the control group.
3.4.Lipid peroxidation assay
According to Figure 3B, the induction of colitis caused an increase in lipid peroxidation in the control group compared to the sham group (P<0.01).The amount of TBARS was diminished in all treated groups and the group that received dexamethasone, 1 and 10 mg/kg of isoimperatorin showed more significant improvement compared with the 0.1 mg/kg isoimperatorin group (P<0.01).
3.5.Total protein result
Figure 4 demonstrates no significant difference in total protein content between the treatment groups (0.1, 1, and 10 mg/kg of isoimperatorin) and the control group at any doses (P>0.05).
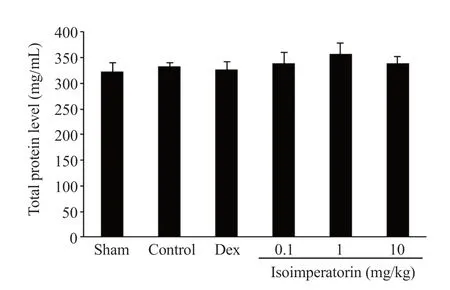
Figure 4.Effect of isoimperatorin on total protein contents in colonic tissue of acetic acid-induced colitis rats.
4.Discussion
Although the etiology and pathology of IBD are not known, the most plausible cause is a combination of two or more factors,immune system dysfunction, abnormal gastrointestinal factors,changed intestinal flora,etc.[17,18].
Among the different causes of IBD, immune system dysfunction and changes in inflammatory mediators are more important.Even non-inflammatory factors like gut microflora or genetic factors lead to inflammation.Gastrointestinal microbiota could induce inflammation by different mechanisms, for example, detection of special receptors named pattern recognition receptors by the immune system and activated NF-κB which causes production of pro-inflammatory cytokines[19,20].In the case of genetic factors, the mutation of special genes like nucleotide-binding oligomerization domain 2 (NOD2) could promote gastrointestinal inflammation and the rate of ulcerative colitis incidence was higher in people with NOD2 mutation compared to healthy individuals[21].
IL and TNF-α are among the main inflammatory factors in IBD development.In addition, oxidative stress due to the destructive effects of reactive oxygen species (ROS) is one of the major causes of IBD which promotes inflammation.In patients with IBD, the level of antioxidants decreases and the level of ROS increases[22,23].
Exploring the medicinal activity of herbal compounds and their mechanisms is always a way to design new medicines.Natural and synthetic coumarins with a wide range of biological properties have been of interest to scientists for many years.Moreover, the anti-inflammatory activities of coumarins have been investigated in different studies.Houltet al.evaluated the anti-inflammatory activities of 16 natural and synthetic coumarins by modifying leukotriene B4and thromboxane B2synthesis.They found that five of them inhibited production of leukotriene B4, and the cyclooxygenase pathway was inhibited by 5,7-dihydroxy-4-methylcoumarin[24].In another study, the anti-inflammatory effect of esculin, a plantderived coumarin, was assessed using three different models of inflammation.Esculin successfully attenuated the inflammation in carrageenan-induced rat paw edema, xylene-induced mouse ear edema, and carrageenan-induced mouse pleurisy[25].Daphnetin, a coumarin isolated fromDaphne odora, alleviated collagen-induced arthritis, as evidenced by significantly reduced arthritis scores,decreased levels of Th17, Th2-type, and Th1-type cytokines in serum, and improved inflammatory cell infiltration[26].
According to Singhet al., bergapten ameliorates the vincristineinduced peripheral neuropathy by reducing TNF-α and IL-1β levels and the expression of NF-κB, COX-2 and iNOS[27].Moreover,the production of TNF-α, IL-1β and IL-6 in macrophages was downregulated by imperatorin.It could inhibit expression of p38 and JunN-terminal kinase phosphorylation which are key factors for inflammatory mediators’ production[28].
Liet al.showed the effect of isoimperatorin on other inflammatory diseases such as rhinitis, asthma, and conjunctivitis.This compound also inhibited the secretion of TNF-α, IL-1β, and IL-4 and ameliorated NF-κB activity in RBL-2H3 cells[29].
In this study, isoimperatorin as a member of coumarin class prevented necrotic lesions, hyperemia, submucosal layer edema,and infiltration of PMNCs in a dose-dependent manner.It was also confirmed that isoimperatorin administration reduced inflammatory cytokines such as IL-1β and TNF-α, as well as MPO and lipid peroxidation.
The role of TNF-α in IBD development has been stated in different studies.Cumulative evidence showed that TNF levels increased in stool, blood, and mucus samples of IBD patients.It also directly influenced the clinical disease activity in patients with Crohn’s disease.The effect of anti-TNF monoclonal antibodies was demonstrated by promoting the activated T-cells apoptosis and preventing apoptosis and tight junction in gastrointestinal epithelial cells[30].It was also reported that TNF-like ligand 1A (TL1A)triggered some inflammatory signal pathways which finally resulted in IBD development[31], therefore the medical treatments that inhibited TNF-α could be effective in IBD management.
In addition to increasing TNF-α, IL-1 has an important role in IBD.IL-1β induces neutrophilia and neutrophil migration.IL-1β can trigger activation and survival of T cells[32].CD4+Th17 cell differentiation can also be promoted by IL-1β in association with other pro-inflammatory cytokines[33].
Many studies showed the high level of IL-1 in patients with inflammation-associated disease[34].IL-1 blocking agents decreased the risk of failure during anti-TNF-α therapy.IL-1 increases in the absence of TNF-α and causes IL-1-related inflammatory responses subsequently[35].Therefore, modulating both TNF-α and IL-1 is more reliable in the treatment of inflammation-associated diseases.
However, there are some limitations in this study.The effects of isoimperatorin on other inflammation-associated parameters are not determined and the molecular mechanisms of its anti-inflammatory activity are not elucidated.Further study is needed to verify its efficacy.In conclusion, isoimperatorin shows anti-inflammatory activity and alleviates acetic acid-induced colitis.It may be a therapeutic agent for the treatment of colitis, which needs further investigation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
The authors received no extramural funding for the study.
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’contributions
Both SG and BN drafted article and SG designed the study.AHA interpreted data.BN and MM collected data.MPH, ST, and MB analyzed data in different sections.Also, MPH revised the article.NY, AH, and MA advised and monitored the study.ZT was corresponding author and ideated and approved the final version of article.
 Asian Pacific Journal of Tropical Biomedicine2024年4期
Asian Pacific Journal of Tropical Biomedicine2024年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Pachymic acid exerts antitumor activities by modulating the Wnt/β-catenin signaling pathway via targeting PTP1B
- Ellagic acid inhibits gastric cancer cells by modulating oxidative stress and inducing apoptosis
- Methanolic extract of Ephedra alata inhibits breast cancer cells in vitro and in vivo
- Ethyl acetate fraction of Sargassum pallidum extract attenuates particulate matterinduced oxidative stress and inflammation in keratinocytes and zebrafish
